Abstract
The mangroves on the Red Sea coast, home to the most economically valuable plants, are currently experiencing significant spatio-temporal changes. A few previous studies used satellite data to monitor the mangroves in Saudi Arabia. As an extension of these studies, we aim to detect the differences in the density and coverage of the mangroves that grow between Allith and Alqahma on the Red Sea coast, produce a digital map for the region, and determine the natural and anthropogenic factors that affect mangrove growth. We used multi-spectral satellite images from moderate resolution imaging spectroradiometer (MODIS) Aqua, Landsat-5 Thematic Mapper (TM), Landsat-8 operational land imager (OLI), and modern-era retrospective analysis for research and applications, version 2 (MERRA-2) captured during 1990–2022. The data were processed and analysed using the optimised soil-adjusted vegetation index, classification method, overlay, and change detection to develop a digital map of the spatio-temporal changes in the mangroves from 1990 to 2022. The results indicated a decrease in mangrove density in the study area. Furthermore, the mangroves did not experience any significant increase in coverage from 1990 to 2022, with the highest rate of increase being 0.27 %. The highest coverage rate (0.29 %) was recorded in 1990, and the lowest (0.24 %) was recorded in 2013. This study concludes that optimal temperature, rain, wind, waves, salinity, nutrient concentration, and bay characteristics are the important favourable factors for mangrove growth. In contrast, anthropogenic activities (particularly those that lead to pollutant release), overgrazing, and diseases are harmful factors that lead to mangrove deterioration. Our study highlights the need to support and develop mangrove rehabilitation projects while minimising the influence of anthropogenic activities on the ecosystem.
Keywords: Multi-spectral satellite images, Landsat, Mangroves, Optimised soil-adjusted vegetation index, Red Sea coast
Graphical abstract
Icon attributions:- Mangrove plants icons created by Freepik: https://www.flaticon.com/free-icon/mangrove- Density of mangroves icons created by Freepik: https://www.flaticon.com/free-icon/mangrove- Environmental factors icons created by Freepik: https://www.flaticon.com/free-icon/disaster- Anthropogenic factors icons created by Freepik: https://www.flaticon.com/free-icon/waste-water- Satellites icons created by Freepik: https://www.flaticon.com/free-icon/satellite- Date icons created by Freepik: https://www.flaticon.com/free-icon/calendar- Images overlay icons created by Freepik: https://www.flaticon.com/free-icon/gallery.
Highlights
-
•
Spatial and temporal changes in the mangroves of the Red Sea coast were studied.
-
•
Multi-spectral satellite images (1990–2022) revealed declining mangrove density.
-
•
Digitised map depicted 4 positive and 3 negative change periods in mangrove density.
-
•
Factors such as rain, temperature, and salinity improved mangrove coverage.
-
•
Anthropogenic activities, overgrazing, and diseases cause mangrove degradation.
1. Introduction
Mangroves provide environmental, social, and economic benefits that increase their value to local communities [1,2]. Mangroves are breeding grounds for fish and home for mammals, crustaceans, birds, and other animals, thus possessing a high commercial value [[3], [4], [5], [6]]. Furthermore, mangroves protect beaches from waves, floods, strong winds, hurricanes, and other natural disasters [7,8]. They maintain the quality of coastal waters [9], reduce wave-induced coastal erosion [10,11], and protect seagrass and neighbouring coral-reef ecosystems [12,13]. They also contribute to nutrient retention, sediment and pollutant filtration [14], carbon sequestration and storage [15], and the storage of blue carbon in sediments [[16], [17], [18]]. Thus, mangroves play an essential role in preserving the natural environment [1].
However, mangroves are fragile ecosystems [19]; they are highly vulnerable to climate change, sea-level rise, high salinity, low oxygen, and variations in temperature, precipitation, wind intensity, wave strength, and nutrient input, leading to their degradation or even decimation [[20], [21], [22], [23], [24]]. Anthropogenic activities practised in coastal areas, e.g., aquaculture, logging, commercial fish and shrimp farming, and tourism, can destroy a large proportion of mangroves worldwide [14,17,[25], [26], [27], [28], [29], [30]]. An estimated 20–35 % of the total mangrove vegetation area in the world has already been lost over the past two decades [31,32]. Therefore, to promote the conservation, growth, and rehabilitation of mangroves, it is important to monitor the changes in mangrove vegetation [[33], [34], [35]].
Remote sensing techniques, geographic information systems, drones, and machine learning techniques can provide data regarding the Earth's surface that can be utilised to map, monitor, and detect the changes in mangroves [30,[36], [37], [38], [39], [40], [41], [42]]. Among these, satellite data are the most widely used information tools. However, the spatial resolutions of these data may differ, affecting their ability to distinguish mangrove vegetation from other plant species or terrestrial targets [14,29,43]. The majority of existing studies use medium-resolution satellite data, particularly from the Landsat and Sentinel satellites [5,8,12,17,28,32,42,[44], [45], [46], [47]], because of the availability of the data for different dates, easy access to the data, and favourable spectral resolutions of the images [18].
The current literature covers the mangroves in several regions, including Brazil, Indonesia, Malaysia, Japan, Hong Kong, Vietnam, Bangladesh, Ghana, and the United Arab Emirates [5,11,12,16,18,44,46,[48], [49], [50], [51], [52]]. However, only a few previous studies used satellite images to monitor or detect the changes in the mangroves in the Kingdom of Saudi Arabia, even though it contains a vast coastline that overlooks the Red Sea and Arabian Gulf [[53], [54], [55], [56], [57]]. Notably, most of these studies did not investigate the changes in mangrove vegetation in recent years (2017–2022); this may be because of the expanse of the Red Sea coast and the challenges that arise from the extensive scale of the region. The coastal areas are also exposed to urbanisation and tourism development, which may affect mangrove quality and lead to their deterioration/decimation; thus, it is important to monitor these areas for detecting changes.
Concerning literature reviews, Saifullah [53] only reviewed the existing information about the mangrove ecosystem of Saudi Arabia, without focussing on the changes in these regions. El-Juhany [54] randomly sampled the mangrove plants along the Red Sea coast, reporting results that were not detailed. The study did not portray the actual extent of change in the mangrove vegetation along the coastline; however, it addressed the effects of camel grazing, garbage disposal, and salinity fluctuations on the plants. Almahasheer et al. [55] used the Landsat-4/5/7 images of 1972–2013 and focussed on the southern part of the coast in the Jizan region. Al-Guwaiz et al. [57] did not study the change in mangrove plants but focused on the percentage of carbon in the plants in an area outside the boundaries of the study area (Yanbu City). Almahasheer et al. [56] studied mangrove vegetation on Tarut Bay in the Arabian Gulf. Taken together, none of these studies monitored the changes in the mangrove vegetation of the Red Sea coast. Notably, to confront climate change, the current economic and development policies of Saudi Arabia highlight the necessity of preserving mangroves as a natural resource and important carbon sink and encourage their monitoring. This study is the first to combine the monitoring of the spatio-temporal changes in mangrove vegetation and determine the factors responsible for these changes. The aim of this study was to preserve and monitor the environment, improve the current understanding of mangroves, and identify the harmful practices that affect the biological environment, based on multi-temporal satellite images captured during 1990–2022. We used multi-temporal satellite images to detect the changes in the mangrove vegetation between Allith and Alqahma on the Red Sea coast. The primary objectives were to 1) detect the changes in the density of mangrove vegetation in the region, 2) analyse mangrove coverage, 3) create a digital map of the spatio-temporal changes in mangrove vegetation, and 4) identify the environmental factors responsible for the spatio-temporal changes.
2. Materials and methods
2.1. Study area
The study area is located in the western region of the Kingdom of Saudi Arabia, in the southwestern part of the coastal plain of the Red Sea, located between 17°55′–20°09′ N and 40°13′–41°42′ E (Fig. 1). The geomorphological forms in the study area vary significantly, from swamps [such as the Allith marsh [58] to short valleys that flow into vast marshes or the Red Sea and deep sandy beds near the coast [59]. Lava fields are present throughout this region. Furthermore, the study area is characterised by the presence of bays and islands [e.g., Thara Island [60]. Udipsamment soil (which remains wet due to tides) covers most of the area. Notably, the soil has a high salinity, which is highest near the surface (extending to the depth of a few centimetres). These natural conditions affect mangrove growth [61].
Fig. 1.
(A) Location of the study area in the Kingdom of Saudi Arabia, modified from the General Authority for Survey and Geospatial Information [80]. (B) Satellite image of the study area (between Allith and Alqahma, on the Red Sea coast), sourced from the United States Geological Survey (USGS, 2020).
2.2. Mangrove characteristics
Mangroves are distributed in scattered patches along the Red Sea coast from Allith in the north to Alqahma in the south. The vegetation portrays an increase in density and height towards the south; the plants serve as a habitat for several species of birds, such as gulls birds (Fig. 2A), insects such as mosquitoes (Fig. 2B), snails, fish, and shrimp (Fig. 2C).
Fig. 2.
Animals that inhabit the mangroves in the study area: (A) gulls birds forage and roost along the mangroves; (B) flies and mosquitoes breed in the mangroves; (C) periwinkles (marine snails) feed on the mangrove plants.
The mangrove plants found in this area include Avicennia marina and Rhizophora mucronata. These plants are evergreen, and their heights vary from 50 cm to 3 m (Table 1), with leaf sizes of 3–4 cm. The mangrove plants in the study area are characterized by different heights, as there are tall plants (Fig. 3A), medium-height plants (Fig. 3B), short plants (Fig. 3C), and very short plants (Fig. 3D). They bear orange-yellow flowers (Fig. 4A) with a distinctive scent; notably, they grow to heights of 0.5–1 cm and are 0.4–0.8 cm in width. The fruits are green, conical, and 1–2 cm in length (Fig. 4B). The mangroves also exhibit root growth above the water surface (Fig. 5A–D, E) or above the soil surfaces (Fig. 5B, C, F, G) (aerial roots, which help them breathe), which vary from 3 cm to 30 cm in length; the root colours range from brownish-grey to dark-green.
Table 1.
Morphological characters of the mangroves in the study area.
| Family | Genus | Species | Plant length | Leaf length | Flower colour | Flower length | Fruits | Roots |
|---|---|---|---|---|---|---|---|---|
| Acanthaceae | Avicennia | Avicennia marina | 50 cm–3 m | 3–4 cm | Orange-Yellow | 0.5–1 cm | Green Conical | 3–30 cm |
| Rhizophoraceae | Rhizophora | Rhizophora mucronata |
Fig. 3.
Photos of the mangroves portraying the (A) dense and tall trees (>2 m in height); (B) plants that are 1–1.50 m in height; (C) plants that are 40–70 cm in height; (D) plants that are <20 cm in height (at the initial stage of growth).
Fig. 4.
Photos of mangrove plant (A) inflorescence and (B) fruits.
Fig. 5.
Aerial roots of different lengths rising above the (A, D, E) water and (B, C, F, G) soil surfaces.
2.3. Data collection
2.3.1. Satellite data
This study used the Landsat-5 thematic mapper (TM) Landsat-8 operational land imager (OLI) data captured by a multi-spectral level 2A product. These data have undergone geometric and atmospheric corrections; thus, users do not need to carry out engineering or meteorological corrections [62]. To cover the study area, we required four images for each year of study (8 years were chosen); thus, we considered a total of 32 images for the period 1990–2022. As mangroves are evergreen throughout the year, seasonal changes do not affect their growth. For this study, we selected the months for cloud-free images (Table 2). The images were obtained from the USGS website [83].
Table 2.
Details of the satellite data used in this study.
| Area | Satellite | Spatial resolution (m) | Spectral resolution | Path/Row | Daily/Monthly | Capture date |
|---|---|---|---|---|---|---|
| Allith-Alqahmah | Landsat-5 TM and Landsat-8 OLI | 30 | 11 bands | 168/047 168/048 169/046 169/047 |
Daily | December 18, 1990, December 27, 1999, June 4, 2000, July 23, 2002, July 10, 2013, November 28, 2015, January 21, 2021, July 3, 2022 |
| MODIS Aqua | 500 | 250 m (bands 1–2) 500 m (bands 3–7) 1000 m (bands 8–36) |
17°44′–20.16′ N; 39°49′–41°0842′ E | Monthly | July 2013, November 2015, January 2021, July 2022 | |
| MERRA-2 | 0.625° × 0.5° | – |
Abbreviations.
Thermal mapper (TM).
operational land imager (OLI).
Moderate-resolution imaging spectroradiometer (MODIS).
Modern-era retrospective analysis for research and applications, version 2 (MERRA-2).
We used the moderate resolution imaging spectroradiometer (MODIS) Aqua satellite data to calculate the sea surface temperature. All the data for this satellite were level-2A data (geometrically, atmospherically, and radiometrically corrected) for 2002–2022, obtained from the National Aeronautics and Space Administration (NASA) Ocean Colour website (https://oceancolor.gsfc.nasa.gov/(accessed on September 25, 2022) [82]. The study period began in 2002, as seawater temperature data from previous years was unavailable. We also relied on the monthly modern-era retrospective analysis for research and applications, version 2 (MERRA-2) model data to calculate the rainfall, wind speed, and salinity in the region, obtained from the Giovanni website (https://giovanni.gsfc.nasa.gov/giovanni/, accessed on November 10, 2022).
2.3.2. Field data
The field data for observing the growth of mangroves along the Red Sea coast, beginning from Allith southward to Alqahma, were collected during 26–30 July 2022. During the field visit, the results of the satellite data analysis were confirmed, mangrove classification was validated, and pixels that represented a mixture of plants and other structures were identified, which were accessed using a Global Positioning System (GPS) device (Fig. 6A). The morphological characteristics of the mangroves were also identified, as well as field measurements (Fig. 6B). The images that depicted the state of the mangroves in the study area, particularly those that portrayed the impact of human activities, were selected.
Fig. 6.
(A) Field investigation of mangrove pixels using a global positioning system (GPS) device to access the sites. (B) Field measurements to analyse vegetation characteristics.
2.4. Data processing
Fig. 7 portrays the steps used to process the data.
Fig. 7.
Schematic diagram of the data-processing steps carried out in this study. Abbreviations: modern-era retrospective analysis for research and applications, version 2 (MERRA-2), moderate resolution imaging spectroradiometer (MODIS), optimised soil-adjusted vegetation index (OSAVI), sea surface temperature (SST). SeaDAS is a comprehensive software package used for data processing.
2.4.1. Pre-processing
The spectral bands of the Landsat satellite images were collected using the “layer stack” tool in the Earth Resource Data Analysis system (ERDAS) Imagine software (version 2014). The images were grouped using the “mosaic image” function to obtain one image for each selected study year. The study area was excised from the Landsat data using the “clip” tool in ArcGIS (version 10.3). To obtain accurate results, all other areas were excluded from the images.
2.4.2. Optimised soil-adjusted vegetation index (OSAVI)
Intervening factors affect the ability of spectral vegetation indices (VIs) to distinguish between plants and other land components. However, three main factors affect these indices: soil reflectance, canopy architecture, and vegetation cover [63]. Although mangroves are located on the coastline, they can have various types of soils. In our study area, the soil was sand, and the area was dry; therefore, the optimised soil-adjusted vegetation index (OSAVI) was the most appropriate VI. Notably, OSAVI is an effective method for monitoring the vegetation changes in arid and semi-arid areas while excluding any effects resulting from the fluctuations in soil background [64,65]. The OSAVI model was proposed by Rondeaux et al. [63]. Its value ranges from +1 (for dense vegetation) to −1 (for exposed bare soil). An ideal correction factor, x (with a value of 0.16), was added to the OSAVI equation as follows:
| (1) |
where NIR is the value of the near-infrared band, and Red is the value of the red band. The equation was created using the ERDAS Imagine software (version 2014). Subsequently, the time series of the changes in the mangroves was constructed in MS Excel (version 2016); furthermore, we calculated the regression and coefficient of determination of the time trend line of changes.
2.4.3. Threshold
OSAVI was applied to all the images of the area using the ERDAS Imagine software (version 2014), and we also calculated the threshold value used for separating the pixels containing the mangrove plants from the rest of the pixels (see Table 3).
Table 3.
Threshold values used for distinguishing the pixels containing mangrove plants.
| Year | Threshold |
|---|---|
| 1990 | 0.070 |
| 1999 | 0.090 |
| 2000 | 0.056 |
| 2002 | 0.070 |
| 2013 | 0.110 |
| 2015 | 0.080 |
| 2021 | 0.150 |
| 2022 | 0.150 |
2.4.4. Classification
The mangroves were classified using the iterative self-organising data analysis technique algorithm (ISODATA) to perform unsupervised classification for all years of the study, from 1990 to 2022, using the ERDAS Imagine software (version 2014). This algorithm iteratively changes the number of tuples from one iteration to the next by merging, splitting, deleting, and recalculating the statistics [66] to reach a maximum ratio between successive pixel iterations. The ISODATA algorithm randomly selects each input data's cluster centre. The repeated pixels are grouped into a cluster based on a minimum spectral distance between the cluster centre and the pixel to form the clusters, which is calculated based on the Euclidean distance calculation. The cluster averages are updated after each iteration, and then the clusters can be merged or split according to the spread of the classified data [67]. The images were classified according to the stages of the study. In the first stage, the land use of the area was classified. The mangrove areas were isolated from the remaining land use in the second stage. In the third stage, the area covered by mangrove plants was calculated (for all the years). Finally, eight images were overlaid to produce a map depicting the changes in mangrove vegetation in the region.
In addition, the wind speed and wave height were classified based on the Beaufort scale, which divides wind speeds into 10 categories (levels), starting from calm winds that lead to waves with no height (0 m) to hurricanes with wave heights that exceed 16 m [68].
2.4.5. Classification accuracy
The classification accuracy was calculated by constructing a confusion matrix, extracting the values of product accuracy, user accuracy, and total accuracy for all the study years, using ERDAS by matching the satellite and field data to verify the accuracy of the spectral index classification and image analysis results. Twenty regions were identified along the coast of the Red Sea, each containing both mangrove and non-mangrove pixels. The locations were surveyed over three days using a global positioning system (GPS; Garmin GPSMAP, USA). The classification accuracy was calculated using the kappa coefficient (K); this method allowed for the analysis of the actual agreement between the classified image and field data. The equation for K is shown below [69]:
| (2) |
where Po and Pe denote the actual and expected proportions of correctly classified classes, respectively.
These results were interpreted using the principles developed by Landis and Koch [70]. The values of the Kappa coefficient ranged between 0 and 1 and were categorised into five groups: 0.01–0.20 (slight classification), 0.21–0.40 (fair classification), 0.41–0.60 (moderate classification); 0.61–0.80 (substantial classification); and 0.81–1.00 (portraying an almost perfect rating) [70]. After classifying the data and verifying their accuracy, the statistical data were processed in MS Excel to determine the impact of the mangrove plants on natural and human factors; furthermore, we calculated the correlation between them, determined the statistical significance value for all the relationships, and generated detailed graphs.
2.4.6. Percentage of change
We calculated the percentage change for all the study years to measure the extent of changes in the mangrove plants. The following equation was used to calculate the percentage change in the mangroves:
| (3) |
where RD is the value of change for the current year, and ED is the change value for the previous year [81].
3. Results and discussion
3.1. Monitoring the changes in the density of mangroves
Table 4 and Fig. 8 portray a decline in the general average OSAVI. The OSAVI value across the study period amounted to −0.007, representing the decrease in the density, greenery, and growth of the mangroves in the study area. Sari and Rosalina [8] reported a decrease in mangrove density, whereas Wiguna et al. [71] indicated that the density of mangrove vegetation was medium, according to the normalised difference vegetation index (NDVI). However, some areas along the Red Sea coastline and nearby islands are rich in mangroves, forming an evergreen and growing plant community, portraying an average OSAVI value of 0.61. The lowest OSAVI value was −0.44, representing areas devoid of mangroves. However, when each year was examined separately, the average OSAVI values for all the years were generally similar and negative, reaching a minimum of −0.020 in July 2002. The highest OSAVI value was recorded in July 2022 (0.002), indicating the scarcity of mangroves in the region. The range between the highest and lowest OSAVI values was high for November 2015, indicating that the largest amount of green plant leaves occurred that year. The majority of negative changes (50 %) in mangrove vegetation density occurred between 2002 and 2013, whereas the majority of positive changes (150 %) occurred between 2015 and 2021; this indicates an increased interest in mangrove plants in recent years.
Table 4.
Optimised soil-adjusted vegetation index (OSAVI) values for 1990–2022.
| Year | High | Low | Mean | Increase (%) | Decrease (%) |
|---|---|---|---|---|---|
| 1990 | 0.567 | −0.584 | −0.0171 | 0 | 0 |
| 1999 | 0.408 | −0.341 | −0.018 | 0 | 5.26 |
| 2000 | 0.554 | −0.554 | −0.02 | 0 | 11.11 |
| 2002 | 0.374 | −0.312 | −0.002 | 90 | 0 |
| 2013 | 0.573 | −0.436 | −0.003 | 0 | 50 |
| 2015 | 0.998 | −0.585 | −0.002 | 33.33 | 0 |
| 2021 | 0.911 | −0.159 | 0.001 | 150 | 0 |
| 2022 | 0.411 | −0.411 | 0.002 | 100 | 0 |
| Average | 0.619 | −0.445 | −0.0076 |
Fig. 8.
Changes in mangrove vegetation density according to the optimised soil-adjusted vegetation index (OSAVI) values for 1990–2022 (Top panels: December 1990, December 1999, June 2000; Middle panels: July 2002, July 2013, November 2015; Bottom panels: January 2021, July 2022, while the panel at the bottom right shows the time series of OSAVI values).
3.2. Monitoring the changes in mangrove coverage
The proposed method could effectively extract the coverage of mangrove plants and isolate their areas from those of other land uses despite the similarity of the spectral fingerprints of mangrove plants to other coastal plants. Table 5 presents the variation in product, user, and overall accuracy values across all years. The value of the K for 1990–2021 (which ranged from 0.70 to 0.80); according to the Landis and Koch [70] scale, this range provides substantial classification. The K value increased in 2022, reaching 0.81, indicating an almost perfect rating, therefore portraying an increase in the reliability of the study results.
Table 5.
Assessment accuracy of mangrove classification.
| Year | Producer's accuracy (%) |
User's accuracy (%) |
Overall accuracy (%) | Kappa coefficient (K) value | ||||
|---|---|---|---|---|---|---|---|---|
| Mangrove | Non-mangrove |
Mangrove | Non-mangrove |
|||||
| Soil | Water | Soil | Water | |||||
| 1990 | 28.57 | 46.87 | 96.58 | 8.00 | 7.00 | 87.22 | 83.50 | 0.76 |
| 1999 | 27.27 | 35.00 | 96.69 | 6.00 | 5.00 | 86.86 | 82 | 0.74 |
| 2000 | 22.58 | 38.88 | 94.68 | 7.00 | 46.66 | 83.37 | 79 | 0.70 |
| 2002 | 17.85 | 32.35 | 94.81 | 5.00 | 36.66 | 85.89 | 79 | 0.70 |
| 2013 | 22.22 | 33.33 | 94.93 | 6.00 | 35.71 | 87.28 | 81 | 0.73 |
| 2015 | 35.00 | 39.47 | 95.85 | 7.00 | 53.57 | 87.76 | 83.50 | 0.76 |
| 2021 | 31.58 | 5.00 | 96.83 | 6.00 | 60.71 | 90.67 | 86.50 | 0.80 |
| 2022 | 44.44 | 48.64 | 97.24 | 8.00 | 64.28 | 90.21 | 87 | 0.81 |
The coverage of mangroves in the study area indicates a state of change in the plants in the region. From 1990 to 2022, mangrove vegetation did not experience any significant increase in its coverage rate of 0.27 % because of its concentration in a narrow strip that extended over some locations along the Red Sea coast and nearby islands (the Muqaymar, Al-Rubais, Al-Oud, Sirin, Thara, and Marka islands). The extent of the dunes on the coast also prevented the growth of mangrove plants in some locations, e.g., the area between Qunfudhah and Al Quoz in the study area. This result is consistent with the findings of El-Juhany [54], although the author employed random sampling and not a comprehensive survey of all sites. Our observations were similar to the changes observed by Singh et al. [72]. Moreover, upon moving eastward from the coastline towards the land, most of the study area (99.73 %) was devoid of mangroves (Table 6 and Fig. 9).
Table 6.
Monitored changes in mangrove coverage area from 1990 to 2022.
| Year | Mangrove |
Non-mangrove |
Total |
Change rate (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Area (ha) | Percentage (%) | Area (ha) | Percentage (%) | Area (ha) | Percentage (%) | Increase | Decrease | |
| 1990 | 3103.48 | 0.29 | 1084359.05 | 99.71 | 1087462.53 | 100 | 0 | 0 |
| 1999 | 2999.86 | 0.28 | 1084462.67 | 99.72 | 0 | 3.34 | ||
| 2000 | 2903.72 | 0.27 | 1084558.81 | 99.73 | 0 | 3.20 | ||
| 2002 | 2968.19 | 0.27 | 1084494.34 | 99.73 | 2.22 | 0 | ||
| 2013 | 2592.37 | 0.24 | 1084870.16 | 99.76 | 0 | 12.66 | ||
| 2015 | 2615.53 | 0.25 | 1084847.00 | 99.75 | 0.89 | 0 | ||
| 2021 | 2899.69 | 0.27 | 1084562.84 | 99.73 | 10.86 | 0 | ||
| 2022 | 3009.31 | 0.28 | 1084530.22 | 99.73 | 3.78 | 0 | ||
| Average | 2886.5187 | 0.27 | 1, 084,576.011 | 99.73 | 2.4 | 2.2 | ||
Fig. 9.
Changes in mangroves coverage (area) from 1990 to 2022; the sub-boxes at the top of the image display samples of some sites covered by mangroves2022 (Top panels: December 1990, December 1999, June 2000; Middle panels: July 2002, July 2013, November 2015; Bottom panels: January 2021, July 2022, while the panel at the bottom right shows the time series of mangrove area change).
By observing the annual changes in the mangroves, we determined the fluctuations in plant coverage from one year to another. The highest mangrove coverage was recorded in December 1990 (0.29 % of the total land area); however, the proportion of the mangrove plant coverage declined gradually, reaching 0.27 % by December 2002. The lowest mangrove coverage (0.24 %) was recorded in December 2013. McGowan et al. [9] reported a significant decline in the mangrove area. Approximately five years later, the mangrove coverage area increased slightly, reaching the same level as before (0.27 %) in January 2021. In July 2022, the mangrove area increased slightly compared to the previous year, reaching approximately 0.28 % of the total land area. Although the values fluctuated, this slight increase indicates the recent interest in the biological environment and increased awareness of the importance of preserving the mangroves and implementing mangrove planting projects. Almahasheer et al. [56] indicated a slight increase in mangrove vegetation along the Red Sea coast, similar to the results of our study. When considering the positive and negative changes experienced by the mangroves (Table 6), we observed that the region experienced four periods during which the mangroves were subjected to a positive change. The first occurred during 2000–2002 when the number of plants increased by 2.22 %. The second occurred during 2013–2015, with a rate of change of 0.89 %. The most significant increase in the mangroves (10.86 %) occurred during 2015–2021 because of enhanced regional mangrove cultivation and an increase in the population's awareness of the region's importance [56]. This slight increase (3.78 %) continued until 2022. Notably, encouraging mangrove cultivation resulted in a similar increase in the mangrove area when employed in Vietnam [11]. Concerning negative changes, the mangroves were subjected to three such periods: 1990–1999 (when the number of plants declined at a rate of 3.34.21 %), 1999–2000 (at a rate of 3.20 %), and 2002–2013 (at a rate of 12.66 %). These periods reflected the state of mangrove deterioration in the study area. Notably, the mangroves were subjected to various changes over the 32 years considered for this study, with the plant coverage decreasing by approximately 2.2 % and increasing by 2.4 %. These changes are shown in the digital map in Fig. 10.
Fig. 10.
Map portraying the spatio-temporal changes in mangrove vegetation during 1990–2022. The sub-boxes are for selected sites that have been zoomed in to show the spatiotemporal variation in mangrove vegetation.
3.3. Environmental factors that influence mangrove ecosystems
This study analysed the effects of environmental factors, such as temperature, rain, wind, wave action, bay characteristics, and nutrient inputs, on mangrove growth and coverage [31].
3.3.1. Temperature
Temperature is one of the most important factors that influence mangrove plant growth. The study area was characterised by a moderate temperature (average of 30 °C) across the 32 years, with the highest temperature average being 32.14 °C (Table 7 and Fig. 11). The lowest temperature average recorded during the study period was 27.83 °C, with the difference between the highest and lowest temperatures being 4.31 °C. The correlation between temperature and mangrove growth in the study area was weak (−0.33), owing to the lack of temperature variation in the region. The statistical significance value was 0.58, confirming no relationship between the region's temperature and mangrove plant growth. These results agree with the findings of Zhang et al. [13], who demonstrated that temperature did not affect mangrove inflorescence/deterioration.
Table 7.
Variations in the water temperature of the Red Sea during 2002–2022.
| Year | High (°C) | Low (°C) | Mean (°C) | Mangrove coverage (%) |
|---|---|---|---|---|
| July 2002 | 32.58 | 27.46 | 30.11 | 0.27 |
| July 2013 | 32.49 | 29.33 | 30.50 | 0.24 |
| November 2015 | 32.75 | 28.84 | 31.04 | 0.25 |
| January 2021 | 29.62 | 26.28 | 27.70 | 0.27 |
| July 2022 | 33.25 | 27.23 | 30.67 | 0.28 |
| Average | 32.14 | 27.83 | 30.00 | 0.26 |
| Correlation | −0.33 | |||
| ap-value | 0.58 | |||
Statistical significance (p-value <0.05).
Fig. 11.
Variations in the water temperature of the Red Sea (°C) during 2002–2022 (Top panels: July 2002, July 2013, November 2015; Bottom panels: January 2021, July 2022).
The results of the time-series analysis shown in Fig. 12 portray that the highest temperature was 33.25–27.23 °C, with an average of 30.67 °C (in July 2022, summer), whereas the lowest temperature was 29.62–26.28 °C, with an average of 27.70 °C (in January 2021, winter). Thus, there was no significant difference between the temperatures in summer and winter; both periods were suitable for mangrove plant growth.
Fig. 12.
Time series of the variations in the water temperature (°C) of the Red Sea during 2002–2022.
3.3.2. Rainfall
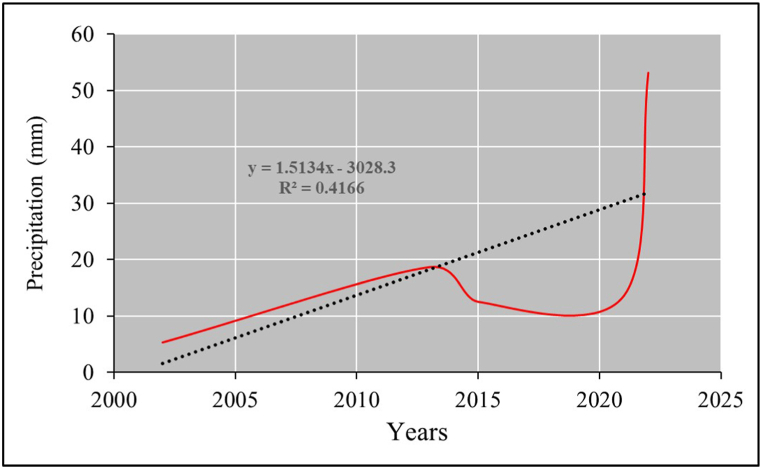
Mangroves require rainwater to reduce salinity, obtain nutrients, and stimulate optimal growth [73]. The study area is located in an arid–semi-arid region. Table 8 and Fig. 13 showed that the area's average rainfall during the study period was 20.63 mm. Notably, the highest rainfall during the study period was recorded in July 2022 (53.16 mm), and the lowest was recorded in July 2002 (5.26 mm), indicating annual rainfall fluctuations in the region. The time-series analysis results shown in Fig. 14 portray the changes in the annual rainfall amounts across the study period, except for 2022, during which the amount of rain increased significantly compared to other years due to climate change. The correlation between rainfall and mangrove plant growth was 0.44, indicating a moderate correlation. The statistical significance value of 0.45 indicated the rejection of the hypothesis of a relationship between rainfall and mangrove plant growth; because the plants grow in seawater near the shores of the study area, their growth is unrelated to rainfall.
Table 8.
Variations in the mangrove area in the study region during 2002–2022 concerning precipitation (mm).
| Year | Precipitation (mm) | Mangroves area (%) |
|---|---|---|
| July 2002 | 5.26 | 0.27 |
| July 2013 | 18.64 | 0.24 |
| November 2015 | 12.49 | 0.25 |
| January 2021 | 13.59 | 0.27 |
| July 2022 | 53.16 | 0.28 |
| Average | 20.63 | 0.26 |
| Correlation | 0.44 | |
| ap-value | 0.45 | |
Statistical significance (p-value <0.05).
Fig. 13.
Variations in precipitation (mm) in the study area during 2002–2022 (Top panels: July 2002, July 2013, November 2015; Bottom panels: January 2021, July 2022).
Fig. 14.
Time series of precipitation (mm) in the study area during 2002–2022.
3.3.3. Wind speed and wave height
Mangroves protect life and property from storms by mitigating the effects of storm surges [74]. However, the study area was characterised by the absence of strong winds and hurricanes (Table 9 and Fig. 15), as the general wind speed across the study period did not exceed 5.09 m s−1, reflecting the predominance of a gentle breeze, with the wave height ranging between 0.5 m and 1 m (towards the shores of the Red Sea). However, the highest wind speed in some parts of the coast was approximately 6.96 m s−1, indicating that the region was dominated by a moderate breeze, contributing to waves 1–2 m in height. The wind speed decreased in other parts of the coast, with the lowest speed being 3.89 m s−1, leading to a gentle breeze contributing to waves of 0.5–1 m in height.
Table 9.
Variations in wind speed (m s−1) in the study area during 2002–2022.
| Year | Wind speed (m s−1) |
Description | Wave height (m) | Mangroves (%) | ||
|---|---|---|---|---|---|---|
| High | Low | Mean | ||||
| July 2002 | 6.635 | 3.901 | 4.800 | Gentle breeze | 0.5–1 | 0.27 |
| July 2013 | 6.848 | 3.783 | 5.085 | Gentle breeze | 0.5–1 | 0.24 |
| November 2015 | 6.437 | 3.944 | 5.023 | Gentle breeze | 0.5–1 | 0.25 |
| January 2021 | 6.627 | 3.627 | 4. 80 | Gentle breeze | 0.5–1 | 0.27 |
| July 2022 | 8.275 | 4.211 | 5.774 | Moderate breeze | 1–2 | 0.28 |
| Average | 6.964 | 3.893 | 5.096 | Gentle breeze | 0.5–1 | 0.26 |
| Correlation | 0.32 | |||||
| ap-value | 0.59 | |||||
Statistical significance (p-value <0.05).
Fig. 15.
Changes in the wind speed (m s−1) in the study area during 2002–2022 (Top panels: July 2002, July 2013, November 2015; Bottom panels: January 2021, July 2022).
As indicated by the wind-speed time series (Fig. 16), the prevailing wind speeds on the Red Sea shores for all the study years were similar, with the only exception noted for July 2022. Thus, the area is an environment suitable for mangrove growth. In July 2022, the wind speed reached 5.77 m s−1 (moderate breeze), leading to a 1–2 m wave height. Some parts of the coast in the same year (July 2022) recorded a high wind speed of 8.27 m s−1, the highest among all the study years, which resulted in the dominance of fresh breeze and increased the wave height to 2–3 m. The wind speed decreased gradually, especially in the winter, reaching the lowest speed of 3.62 m s−1 in January 2021. Thus, at present, a gentle breeze prevails over the region, leading to waves with heights of 0.5–1 m. Therefore, the type of prevailing winds in the region and wind speed may not impact mangrove plant growth, thus portraying a weak relationship (0.32) with a statistical significance value of 0.59.
Fig. 16.
Time series of wind speed (m s−1) during 2002–2022.
3.3.4. Salinity
The Red Sea waters generally have a high salinity (Fig. 17) of 36–40 ppt [75]. However, the study area has a high salinity (∼38), which is suitable for mangrove growth and influences the area's leaf size, thickness, and plant height [31].
Fig. 17.
Salinity in the (A) Red Sea water and (B) study area.
As for the salinity of the air surrounding the waters (airborne salinity) of the Red Sea in the study area, the average salinity across the study period was 50.80 kg/m3, ranging from 103.20 kg/m3 (highest air salinity) to 9 kg/m3 (lowest air salinity), as shown in Table 10 and Fig. 18, Fig. 19. Air salinity varies from year to year. Notably, for our study area, this value increased with the distance from the coastline, reaching >145 kg/m3 in the middle of the sea in July 2022; for the rest of the year, the salinity ranged from 59 kg/m3 to 133 kg/m3. On the coastline, the airborne salinity increased from 5 kg/m3 in 2021 to 14 kg/m3 in 2022. The correlation between mangroves and sea airborne salinity was weak (−0.14), indicating that mangrove growth was not affected by airborne salinity; this result was supported by the P-value, which did not exceed 0.81; this was also proven by Zhang et al. [13].
Table 10.
Changes in airborne salinity in the study area during 2002–2022.
| Year | Sea salt surface |
Mangroves (%) | ||
|---|---|---|---|---|
| High | Low | Mean | ||
| July 2002 | 82 | 6 | 38 | 0.27 |
| July 2013 | 133 | 12 | 66 | 0.24 |
| November 2015 | 97 | 8 | 49 | 0.25 |
| January 2021 | 59 | 5 | 27 | 0.27 |
| July 2022 | 145 | 14 | 74 | 0.28 |
| Average | 103.20 | 9 | 50.80 | 0.26 |
| Correlation | −0.14 | |||
| p-value | 0.81 | |||
∗ Statistical significance (p-value <0.05).
Fig. 18.
Changes in the airborne salinity in the study area during 2002–2022 (Top panels: July 2002, July 2013, November 2015; Bottom panels: January 2021, July 2022).
Fig. 19.
Time series of airborne salinity of the Red Sea (kg/m3) during 2022–2022.
3.3.5. Nutrient inputs
The study area is characterised by the presence of large valleys, such as Allith, Alshaqa, Alduqa, Alasbah, Qununa, Yabih, Aldiqah, Hali, Shafaqa, Amq, and Dhaban, all of which dip from the east (Sarawat Mountains) to the west, toward the Red Sea or close to the coast (Fig. 20). The rivers of these valleys are distinguished by their rapid flow and high loads of sand, mud, and silt, particularly when heavy rains occur in the Sarawat Mountains [61]. Thus, these valleys act as nutrient sources for mangroves and are the most critical factors affecting mangrove growth in the region. Therefore, the area of mangrove vegetation at the mouth of the valleys is increasing [12]. However, the impact of this increase along the coastline may vary from one region to another because of the annual rainfall fluctuations in the region.
Fig. 20.
Map portraying runoff from different valleys; their rivers serve as nutrient sources for the Red Sea coast.
3.3.6. Bays
As shown in Fig. 21, the study area contains several bays suitable for mangrove growth because of the region's lack of tidal movement and minimal wind movement. The bays are distributed from the north to the south, although their frequency increases in the south towards Alqahma (panels 1–3 show less dense mangroves and 4–6 show dense mangroves). Therefore, these bays are heavily vegetated; this was also observed in a field study in the area.
Fig. 21.
Mangroves concentrated in bays along the Red Sea coast.
3.4. Effects of anthropogenic factors on mangrove growth
Anthropogenic activities in the coastal areas bordering the Red Sea have led to the deterioration of mangrove vegetation [54]. The vegetation area decreased from approximately 0.56 % in July 1990 to 0.22 % in November 2015 (Table 6). Fig. 22A and B show the change in vegetation cover in Alqahma Bay in 1990 compared to 2022. Fig. 22G and H also show a decrease in vegetation cover in 2022 compared to 1990 in Albirk Beach. Urban growth, road construction along the shores of the Red Sea, and the conversion of coastlines into residential areas and paved roads that extend along the coast (Fig. 22C, D, E, F), particularly in Alqahma, Albirk, and Allith, have led to the burial of mangrove vegetation. Oyebade et al. [76] also reported similar effects of urban growth on mangrove forests.
Fig. 22.
Effects of anthropogenic activity on Mangrove vegetation between 1990 and 2022. (A, B) Alqahma Bay. (G.H) Albirk Beach. (C, D) Extension of the buildings towards the mangroves. (E, F) Partial filling of the mangrove areas and extension of paved roads.
The construction of dams and bridges has also prevented the access of nutrients to mangrove areas, as observed in Albirk Beach (Fig. 23A) and Khor Nahud (Fig. 23F), leading to their deterioration and conversion into dry land (Fig. 23C–E). Car tracks were observed at some locations, which may have prevented the growth of new mangroves in the area (Fig. 23B–D).
Fig. 23.
(A, F) Sites affected by vehicles and the lack of nutrients. (B, D) Impact of car tracks on mangroves leading to decreased coverage. (C, E) Mangrove dehydration and deterioration due to reduced nutrient supply.
Notably, some mangroves from the Alqahma Bay area (Fig. 24A) have been reduced while others have been buried, currently used fishing harbours and marinas (Fig. 24B and C). Additionally, fish and shrimp farming projects and the establishment of a water desalination plant on the coast of Allith contribute to the decline in mangrove density in the region (Fig. 24D and E), as reported in other studies [77,78].
Fig. 24.
(A) Mangroves in the Alqahma Bay area (B, C) Conversion of part of Alqohma Bay, the largest mangrove agglomeration in the study area, into a port. (D, E) Continuation of land reclamation work at Alqohma Bay.
Tourism development in the region has also transformed parts of the coast covered by mangroves into recreational beaches and coastal parks, where residents practice fishing and swimming (Fig. 25B and C), e.g. shores of the Albirk, Hali, and Aljumayyat beaches in Alquoz (Fig. 25A).
Fig. 25.
(A) Mangroves in the Albirk Beach area. (B, C) Parts of mangrove areas on the beaches of Albirk have been converted into recreational areas.
Mangrove plants are similar to other plants: animals feed on them, as in Aljumayyat Beach (Fig. 26A). Some residents practice camel overgrazing in the region. overgrazing would be a major threat to the mangroves along the coast (Fig. 26B and C). El-Juhany [54] demonstrated that camel herding had been practised by shepherds in the region since ancient times, contributing to the deterioration of the mangroves in this region; therefore, local governments must prevent overgrazing and limit the movement of herders.
Fig. 26.
(A) Mangroves of Aljumayyat Beach in the Alquoz area. (B, C) Effect of camel overgrazing on the mangroves of Aljumayyat Beach in Alquoz.
Furthermore, the mangroves along the Red Sea coast suffer, as observed at Alqahma Beach and Almuzaylif Beach. (Fig. 27ِA, G), from waste and sewage pollution (Fig. 27ِB, C), similar to those in the Indus Delta [43]. Pollution is a major contributor to mangrove deterioration. Waste piles that accumulate between the branches and roots of mangroves hinder their growth and cause rotting, attracting mosquitoes and other harmful insects (Fig. 27ِD, E, F).
Fig. 27.
(A) Mangroves at Alqahma Beach. (G) Mangroves at Almuzaylif Beach. (B, C) Effect of waste and sewage pollution on mangroves at Alqahma Beach. (D, E, F) Waste accumulation at Almuzaylif Beach.
Like other plants, mangroves are exposed to several diseases that may lead to deterioration, as observed in several locations (Fig. 28D, E, F). Previous studies have shown that mangrove plants are vulnerable to leaf spots, leaf loss, stem death, and rot, often caused by fungi [79]. In the study area, mangrove plants exhibited leaf erosion, white spots (Fig. 28B), and tiny black spots (Fig. 28C) on the leaves. These spots remained dormant for some time and then enlarged to 3–5 mm in size; this may be attributed to Curvularia aff. tsudae and Pestalotiopsis sp. [11]; this may lead to plant leaf loss and the ecological degradation of mangrove vegetation (Fig. 28A).
Fig. 28.
(A) Leaf damage by insects in Almuzaylif Beach. (B) White spots on mangrove leaves at Amaq Beach. (C) Black spots on plant leaves in Alqohma Bay. (D, E, F) Sites where mangroves have been exposed to diseases.
4. Conclusions
The coastal environment of the Red Sea is rich in flora and fauna, including mangroves, which are sporadically distributed along the coast. Like other plants, mangrove plants are affected by environmental and anthropogenic factors. The vast expansion of mangroves along the Red Sea coast has limited the number of studies on this area. Therefore, we used multi-temporal satellite data to detect the changes in the mangroves between Allith and Alqahma on the Red Sea coast. A digital map of the temporal and spatial changes was produced, and the positive and negative factors that affected mangrove growth and density were identified. These observations were based on satellite images (MODIS Aqua, Landsat-5 TM, Landsat-8 OLI, and MERRA-2) captured from 1990 to 2022. The results portrayed a decrease in the average OSAVI (−0.007) value, indicating a reduction in the mangrove vegetation density in the study area. However, some sites rich in mangrove vegetation portrayed OSAVI values of 0.61.
The index also revealed that the most positive changes in mangrove vegetation density occurred during 2002–2013, and the most negative changes occurred during 2015–2021. We conclude that the mangrove coverage area in the region did not increase significantly because the average coverage rate did not exceed 0.27 %. The highest mangrove coverage percentage was recorded in December 1990 (0.29 %), and the lowest was recorded in July 2013 (0.24 %). The study area experienced four periods wherein the plants were subjected to a positive change, and the area of plant coverage increased, along with three periods when the coverage area decreased. Furthermore, mangrove vegetation was concentrated at high densities in narrow bays and islands near the coast, forming an evergreen plant community that makes these areas suitable for plant growth. Moderate temperature, wind, seawater salinity, and nutrient availability are among the factors that favour mangrove growth in this region. Anthropogenic activity, waste pollution, wastewater, and plant diseases are among the most critical factors that deteriorate the mangrove vegetation in the region. Therefore, we recommend further monitoring and controlling the positive and negative changes in the mangroves on the Red Sea coast using multi-spectral satellite data. Additionally, we recommend curbing anthropogenic activities in the areas where mangroves are widespread while preserving them as natural reserves. The recultivation of mangroves in the areas that have experienced deterioration, particularly in the Alqahma and Albirk regions, is necessary.
This study highlights that relying on satellite data effectively detects changes in vegetation cover; however, there are some spatial and temporal accuracy limitations. During the field visit, we noted areas where mangroves were densely distributed; however, because of their small size and location, they were not detected in the Landsat satellite images. Similarly, the abundance of aerosols and clouds in the study area and the occasional lack of a sequence in the dates of the images for the same year, particularly from 2003 to 2012, allowed the use of only a limited number of images. In addition, the abundance of silt and moisture in the soil surrounding the mangroves limits access to some sites. It is recommended that these limitations be overcome with drones, high-resolution satellites, or machine learning to classify mangrove vegetation, its structure, and its biomass. This study is expected to benefit those interested in monitoring the biological environment, as it reveals the challenges faced by mangrove plants in dry areas and proposes methods to preserve them in order to tackle the global climate change and its impact on the environment.
Data availability statement
Data will be made available on request. For requesting data, please write to the corresponding author.
Funding
This study received no specific grants from the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
I thank my husband who accompanied me during the fieldwork and helped me collect mangrove plant samples and measure their parameters.
References
- 1.Kamal M., Phinn S. Hyperspectral data for mangrove species mapping: a comparison of pixel-based and object-based approach. Remote Sens. 2011;3:2222–2242. doi: 10.3390/rs3102222. [DOI] [Google Scholar]
- 2.Setiawan Y., Effendi H., Prasetyo L.B., Siregar I.Z. Editorial. Procedia Environ. Sci. 2016;33:1–2. doi: 10.1016/j.proenv.2016.03.049. [DOI] [Google Scholar]
- 3.Nagelkerken I.S.J.M., Blaber S.J.M., Bouillon S., Green P., Haywood M., Kirton L.G., Meynecke J.-O., Pawlik J., Penrose H.M., Sasekumar A., Somerfield P.J. The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat. Bot. 2008;89:155–185. doi: 10.1016/j.aquabot.2007.12.007. [DOI] [Google Scholar]
- 4.Sitoe A.A., Mandlate L.J.C., Guedes B.S. Biomass and carbon stocks of Sofala Bay mangrove forests. Forests. 2014;5:1967–1981. doi: 10.3390/f5081967. [DOI] [Google Scholar]
- 5.Lima M.C.F., de Souza F.E.S., Lima J.C.F., de Oliveira J.B. Monitoring two mangrove forests of Rio Grande do Norte, Brazil, using multi-temporal satellite data. Intell. Environ. 2017;22:98–107. doi: 10.3233/978-1-61499-796-2-98. IOS Press. [DOI] [Google Scholar]
- 6.Pradit S., Noppradit P., Loh P.S., Nitiratsuwan T., Le T.P.Q., Oeurng C., Mohamed C.A.R., Lee C.W., Lu X., Anshari G.Z., Kandasamy S., Wang J. The occurrence of microplastics in sediment cores from two mangrove areas in southern Thailand. J. Mar. Sci. Eng. 2022;10:418. doi: 10.3390/jmse10030418. [DOI] [Google Scholar]
- 7.Weber S.J., Keddell L., Kemal M. Myanmar Ecological Forecasting: utilizing NASA Earth Observations to Monitor, map, and analyze mangrove forests in Myanmar for enhanced conservation (No. NASA/CR-2014-218274) NTRS - NASA Technical Reports Server, 20140006912. 2014 [Google Scholar]
- 8.Sari S.P., Rosalina D. Mapping and monitoring of mangrove density changes on tin mining area. Procedia Environ. Sci. 2016;33:436–442. doi: 10.1016/j.proenv.2016.03.094. [DOI] [Google Scholar]
- 9.McGowan T., Cunningham S.L., Guzmán H.M., Mair J.M., Guevara J.M., Betts T. Mangrove forest composition and structure in las Perlas Archipelago, Pacific Panama. Rev. Biol. Trop. 2010;58:857–869. doi: 10.15517/rbt.v58i2.5251. [DOI] [PubMed] [Google Scholar]
- 10.Sweetman A.K., Middelburg J.J., Berle A.M., Bernardino A.F., Schander C., Demopoulos A.W.J., Smith C.R. Impacts of exotic mangrove forests and mangrove deforestation on carbon remineralization and ecosystem functioning in marine sediments. Biogeosciences. 2010;7:2129–2145. doi: 10.5194/bg-7-2129-2010. [DOI] [Google Scholar]
- 11.Nguyen H.H., Tran L.T.N., Le A.T., Nghia N.H., Duong L.V.K., Nguyen H.T.T., Bohm S., Premnath C.F.S. Monitoring changes in coastal mangrove extents using multi-temporal satellite data in selected communes, Hai Phong City, Vietnam, for. Soc., Hai Phong City, Vietnam. 2020;4:256–270. doi: 10.24259/fs.v4i1.8486. [DOI] [Google Scholar]
- 12.Wang H., Peng Y., Wang C., Wen Q., Xu J., Hu Z., Jia X., Zhao X., Lian W., Temmerman S., Wolf J., Bouma T. Mangrove loss and gain in a densely populated urban estuary: lessons from the Guangdong-Hong Kong-Macao Greater Bay Area. Front. Mar. Sci. 2021;8 doi: 10.3389/fmars.2021.693450. [DOI] [Google Scholar]
- 13.Zhang Y., Meng X., Xia P., Li Z. Response of mangrove development to air temperature variation over the past 3000 years in Qinzhou Bay, Tropical China. Front. Earth Sci. 2021;9 doi: 10.3389/feart.2021.678189. [DOI] [Google Scholar]
- 14.Alexandris N., Chatenoux B., Lopez Torres L., Peduzzi P. Monitoring the restoration of mangrove ecosystems from Space. UN Environ. Program/GRID. 2014 doi: 10.13140/RG.2.2.27685.81126. Geneva. [DOI] [Google Scholar]
- 15.Broadhead J.S., Bukoski J.J., Beresnev N.N. Mangrove carbon estimator and monitoring guide. Tech. Rep. Bangkok, Thailand, Un Food Agri. Organ. 2016 https://www.fao.org/3/i6500en/i6500en.pdf [Google Scholar]
- 16.Mensah J.C. Vol. 24. Graduate School of Oceanography, University of Rhode Island; 2013. (Remote Sensing Application for Mangrove Mapping in the Ellembelle District in Ghana, USAID Integrated Coastal and Fisheries Governance Program for the Western Region of Ghana, Narragansett, Rhode Island, Coastal Resources Center). [Google Scholar]
- 17.Omar H., Misman M.A., Musa S. In: Geographic Information Systems and Science. Rocha J., Abrantes P., editors. John Wiley & Sons; 2019. GIS and remote sensing for mangroves mapping and monitoring; pp. 101–115. [DOI] [Google Scholar]
- 18.Elmahdy S.I., Ali T.A., Mohamed M.M., Howari F.M., Abouleish M., Simonet D. Spatiotemporal mapping and monitoring of mangrove forests changes from 1990 to 2019 in the Northern Emirates, UAE using random forest, Kernel logistic regression and Naive Bayes tree models. Front. Environ. Sci. 2020;8:102. doi: 10.3389/fenvs.2020.00102. [DOI] [Google Scholar]
- 19.Jhonnerie R., Siregar V.P., Nababan B., Prasetyo L.B., Wouthuyzen S. Random forest classification for mangrove land cover mapping using Landsat 5 TM and ALOS PALSAR imageries. Procedia Environ. Sci. 2015;24:215–221. doi: 10.1016/j.proenv.2015.03.028. [DOI] [Google Scholar]
- 20.Ellison J., Jungblut V., Anderson P., Slaven C. Secretariat of the Pacific Regional Environment Programme. 2012. Manual for mangrove monitoring in the pacific islands region. [Google Scholar]
- 21.Kauffman J.B., Donato D.C. Bogor; Indonesia: 2012. Protocols for the Measurement, Monitoring and Reporting of Structure, Biomass and Carbon Stocks in Mangrove Forests, Center for International Forestry Research. [Google Scholar]
- 22.Duncan C., Owen H.J.F., Thompson J.R., Koldewey H.J., Primavera J.H., Pettorelli N. Satellite remote sensing to monitor mangrove forest resilience and resistance to sea level rise. Methods Ecol. Evol. 2018;9:1837–1852. doi: 10.1111/2041-210X.12923. [DOI] [Google Scholar]
- 23.Giusto B.D., Le T.M.N., Nguyen T.T.M., Nguyen T.T.H., Vu N.U.M., Lavallee J.P. Development versus adaptation? Facing climate change in Ca Mau, Vietnam. Atmosphere. 2021;12:1160. doi: 10.3390/atmos12091160. [DOI] [Google Scholar]
- 24.Moxon S.D.G. (Doctoral dissertation, Palacký University Olomouc, Czechia); 2021. Mapping Mangrove Forests: Processing and Visualization of Multi-Sensor Earth Observation Data for the Colombian Pacific Coast. [Google Scholar]
- 25.Zuhair M.M., Hussin Y.A., Weir M.J.C. Workshop Proceedings “The Balance between Biodiversity Conservation and Sustainable Use of Tropical Rain forests.”. The Tropenbos Foundation; Wageningen, the Netherlands: 2001. Monitoring mangrove forests using remote sensing and GIS; pp. 251–257. [Google Scholar]
- 26.Vaiphasa C. Wageningen University and Research; the Netherlands: 2006. Remote Sensing Techniques for Mangrove Mapping (Doctoral Thesis. [Google Scholar]
- 27.Dat P.T., Yoshino K. In: 32nd Asian Conference on Remote Sensing, ACRS 2011. Hook R., editor. Asian Association on Remote Sensing; New York: 2011. Monitoring mangrove forest using multi-temporal satellite data in the northern coast of Vietnam; pp. 169–174. [Google Scholar]
- 28.Nguyen L.D., Nguyen C.T., Le H.S., Tran B.Q. Mangrove mapping and above-ground biomass change detection using satellite images in coastal areas of Thai Binh Province, Vietnam, for. Soc. 2019;3:248–261. doi: 10.24259/fs.v3i2.7326. [DOI] [Google Scholar]
- 29.Pham T.D., Xia J., Ha N.T., Bui D.T., Le N.N., Tekeuchi W. A review of remote sensing approaches for monitoring blue carbon ecosystems: mangroves, seagrasses and salt marshes during 2010–2018. Sensors. 2019;19:1933. doi: 10.3390/s19081933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauser L.T., An Binh N., Viet Hoa P., Hong Quan N., Timmermans J. Gap-free monitoring of annual mangrove forest dynamics in Ca Mau Province, Vietnamese Mekong delta, using the Landsat-7-8 archives and post-classification temporal optimization. Remote Sens. 2020;12(22):3729. doi: 10.3390/rs12223729. [DOI] [Google Scholar]
- 31.Lugo A.E., Medina E. In: Coastal and Marine Environments. Yeqiao W., editor. CRC Press; Boca Raton, Florida: 2020. Mangrove forests; pp. 117–133. [Google Scholar]
- 32.Chowdhury M.S., Hafsa B. Multi-decadal land cover change analysis over Sundarbans Mangrove Forest of Bangladesh: a GIS and remote sensing based approach. Glob. Ecol. Conserv. 2022;37 doi: 10.1016/j.gecco.2022.e02151. [DOI] [Google Scholar]
- 33.Trisasongko B.H. Tropical mangrove mapping using fully-polarimetric radar data. ITB J. Eng. Sci. 2009;41:98–109. doi: 10.5614/itbj.sci.2009.41.2.4. [DOI] [Google Scholar]
- 34.Zhang H., Wang T., Liu M., Jia M., Lin H., Chu L.M., Devlin A.T. Potential of combining optical and dual polarimetric SAR data for improving mangrove species discrimination using rotation forest, Rem. Sens. 2018;10:467. doi: 10.3390/rs10030467. [DOI] [Google Scholar]
- 35.Susantoro T.M., Wikantika K., Yayusman L.F., Tan A., Ghozali M.F. Monitoring of mangrove growth and coastal changes on the north coast of brebes, central java, using Landsat data. IJReSES. 2019;16:197–214. doi: 10.30536/j.ijreses.2019.v16.a3221. [DOI] [Google Scholar]
- 36.Green E.P., Clark C.D., Mumby P.J., Edwards A.J., Ellis A.C. Remote sensing techniques for mangrove mapping. Int. J. Rem. Sens. 1998;19:935–956. doi: 10.1080/014311698215801. [DOI] [Google Scholar]
- 37.Heumann B.W. An object-based classification of mangroves using a hybrid decision tree—support vector machine approach. Remote Sens. 2011;3:2440–2460. doi: 10.3390/rs3112440. [DOI] [Google Scholar]
- 38.Rhyma Purnamasayangsukasih P.R., Norizah K., Ismail A.A.M., Shamsudin I. A review of uses of satellite imagery in monitoring mangrove forests. IOP Conf. Ser. Earth Environ. Sci. 2016;37 doi: 10.1088/1755-1315/37/1/012034. [DOI] [Google Scholar]
- 39.Sanyal P., Ray R., Paul M., Gupta V.K., Acharya A., Bakshi S., Jana T.K., Mukhopadhyay S.K. Assessing the dynamics of dissolved organic matter (DOM) in the coastal environments dominated by mangroves, Indian Sundarbans. Front. Earth Sci. 2020;8:218. doi: 10.3389/feart.2020.00218. [DOI] [Google Scholar]
- 40.Xia J., Yokoya N., Pham T.D. Probabilistic mangrove species mapping with multiple-source remote-sensing datasets using label distribution learning in Xuan Thuy National Park, Vietnam, Rem. Sens. 2020;12:3834. doi: 10.3390/rs12223834. [DOI] [Google Scholar]
- 41.Rumee A.R. IoT system for remote monitoring of the mangrove forests of Sundarbans. J. Comput. Syst. Sci. Int. 2021;20:254–258. doi: 10.35784/jcsi.2703. [DOI] [Google Scholar]
- 42.Moreno G.M.D.S. Dissertation for Master’s degree, University of Brasília, Brazil; 2022. Deep Semantic Segmentation of Mangrove Combining Spatial, Temporal and Polarization from Sentinel-1 Time Series in the Brazilian Territory. [Google Scholar]
- 43.Giri C., Long J., Abbas S., Murali R.M., Qamer F.M., Pengra B., Thau D. Distribution and dynamics of mangrove forests of South Asia. J. Environ. Manag. 2015;148:101–111. doi: 10.1016/j.jenvman.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 44.Alsaaideh B., Al-Hanbali A., Tateishi R., Kobayashi T., Hoan N.T. Mangrove forests mapping in the southern part of Japan using Landsat ETM+ with DEM. J. Geogr. Inf. Syst. 2013;5(4):369–377. doi: 10.4236/jgis.2013.54035. [DOI] [Google Scholar]
- 45.Dan T.T., Chen C.F., Chiang S.H., Ogawa S. Mapping and change analysis in mangrove forest by using Landsat imagery. ISPRS Ann. Photogramm. Remote Sens. Spatial Inf. Sci. 2016;III–8:109–116. doi: 10.5194/isprsannals-III-8-109-2016. [DOI] [Google Scholar]
- 46.Mukhtar E., Raynaldo A., Novarino W. Carbon stock mapping using mangrove discrimination indices in Mandeh Bay, West Sumatra. Aquac Aquar. Conserv. Legis. 2021;14:430–440. [Google Scholar]
- 47.Azeez S.A., Gnanappazham L., Muraleedharan K.R., Revichandran C., John S., Seena G., Thomas J. Multi-decadal changes of mangrove forest and its response to the tidal dynamics of Thane Creek, Mumbai. J. Sea Res. 2022;180 doi: 10.1016/j.seares.2021.102162. [DOI] [Google Scholar]
- 48.Dutta M.K., Mukherjee R., Mukhopadhyay S.K. Atmospheric ozone and its biosphere-atmosphere exchange in a mangrove forest ecosystem: a case study from Sundarbans, NE coast of India. Int. J. Sci. Technol. Res. 2015;4:196–201. [Google Scholar]
- 49.Rahman M.M., Islam S.A. Phenophases of five mangrove species of the Sundarbans of Bangladesh. Int. J. Bus. Soc. Sci. Res. 2015;4:77–82. [Google Scholar]
- 50.Waluyo Jati I.W., Pribadi R. Vol. 31. EDP Sciences; 2018. Economic valuation as an instrument to determine the management strategy of Baros mangrove forest, Bantul, Yogyakarta, Indonesia. (E3S Web Conf. E3S Web Conference, the 2nd International Conference on Energy, Environmental and Information System (ICENIS 2017)). [DOI] [Google Scholar]
- 51.Li R., Zhang L., Xue B., Wang Y. Abundance and characteristics of microplastics in the mangrove sediment of the semi-enclosed Maowei Sea of the South China Sea: new implications for location, rhizosphere, and sediment compositions. Environ. Pollut. 2019;244:685–692. doi: 10.1016/j.envpol.2018.10.089. [DOI] [PubMed] [Google Scholar]
- 52.de Lacerda L.D., Ward R.D., Godoy M.D.P., de Andrade Meireles A.J., Borges R., Ferreira A.C. 20-years cumulative impact from shrimp farming on mangroves of Northeast Brazil. Front. For. Glob. Change. 2021;4 doi: 10.3389/ffgc.2021.653096. [DOI] [Google Scholar]
- 53.Saifullah S.S. Mangrove ecosystem of Saudi arabian Red Sea coast- an overview, mar. Sci. 1996;7:263–270. doi: 10.4197/mar.7-1.23. [DOI] [Google Scholar]
- 54.El-Juhany L.I. Present status and degradation trends of mangrove forests on the southern Red Sea coast of Saudi Arabia. Am. Eur. J. Agric. Environ. Sci. 2009;6:328–340. [Google Scholar]
- 55.Almahasheer H., Al-Taisan W., Mohamed M.K. Mangrove deterioration in Tarut bay on the eastern province of the kingdom of Saudi Arabia. Pakhtunkhwa J. Life Sci. 2013;1:49–59. [Google Scholar]
- 56.Almahasheer H., Aljowair A., Duarte C.M., Irigoien X. Decadal stability of Red Sea mangroves. Estuar. Coast Shelf Sci. 2016;169:164–172. doi: 10.1016/j.ecss.2015.11.027. [DOI] [Google Scholar]
- 57.Al-Guwaiz S.M., Alatar A.A., El-Sheikh M.A., Al-Gehni G.A., Faisal M., Qahtan A.A., Abdel-Salam E.M. Role of mangrove rehabilitation and protection plans on carbon storage in Yanbu Industrial City, Saudi Arabia: a case study. Sustainability. 2021;13 doi: 10.3390/su132313149. [DOI] [Google Scholar]
- 58.Alwalayi A. second ed. Al. Mumtaz Foundation; Riyadh: 1997. Geology and Geomorphology of the Kingdom of Saudi Arabia (Types of the Earth's Surface) [Google Scholar]
- 59.Alnafie A.H. King Fahad National Library; Riyadh: 2019. Physical Geography of the Kingdom of Saudi Arabia. [Google Scholar]
- 60.Saqqa A. second ed. Kunouz al-Maarifa Publishing; Jeddah: 1998. Physical Geography of the Kingdom of Saudi Arabia. [Google Scholar]
- 61.Alnafie A.H. Knowledge Stars; Riyadh: 2004. Phytogeography of the Kingdom of Saudi Arabia. [Google Scholar]
- 62.EROS, USGS EROS archive – Landsat archives – Landsat, OLI/TIRS level-2 data products – surface reflectance. Earth Resour. Observ. Sci. (EROS) Center. 2018;8 https://www.usgs.gov/centers/eros/science/usgs-eros-archive-landsat-archives-landsat-8-olitirs-level-2-data-products?qt-science_center_objects=0#qt-science_center_objects [Google Scholar]
- 63.Rondeaux G., Steven M., Baret F. Optimization of soil-adjusted vegetation indices, Rem. Sens. Environ. 1996;55:95–107. doi: 10.1016/0034-4257(95)00186-7. [DOI] [Google Scholar]
- 64.Huete A.R. A soil-adjusted vegetation index (SAVI), Rem. Sens. Environ. 1988;25:295–309. doi: 10.1016/0034-4257(88)90106-X. [DOI] [Google Scholar]
- 65.Fern R.R., Foxley E.A., Bruno A., Morrison M.L. Suitability of NDVI and OSAVI as estimators of green biomass and coverage in a semi-arid rangeland. Ecol. Indic. 2018;94:16–21. doi: 10.1016/j.ecolind.2018.06.029. [DOI] [Google Scholar]
- 66.Shahi A.P., Rai P.K., Rabi-ul-Islam M., Mishra V.N. Chapter 5, Remote sensing data extraction and inversion techniques: a review. Atmos. Remote. Sens. 2023:85–104. doi: 10.1016/B978-0-323-99262-6.00021-3. [DOI] [Google Scholar]
- 67.Melesse A.M., Jordan J.D. A comparison of fuzzy vs. augmented-ISODATA classification algorithms for cloud-shadow discrimination from Landsat images. Photogramm. Eng. Rem. Sens. 2002;68:905–912. [Google Scholar]
- 68.Risanti A.A., Marfai M.A. The effects of hydrodynamic process and mangrove ecosystem on sedimentation rate in Kendal coastal area, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020;451 doi: 10.1088/1755-1315/451/1/012070. [DOI] [Google Scholar]
- 69.Cohen J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 70.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 71.Wiguna P.P.K., Sutari N.W.S., Febriarta E., Permatasari A.L., Suherningtyas I.A., Pulungan N.A.H.J., Sukraini T.T., Gani M. Spatial analysis of mangrove distribution using Landsat 8 oli in badung regency and Denpasar City, Bali province, Indonesia, T. And T. Forum Geogr. 2022;36:21–29. doi: 10.23917/forgeo.v36i1.14711. [DOI] [Google Scholar]
- 72.Singh A.A., Maharaj A.A., Naidu H., Raj K., Charan D., Chand R., Singh S., Joseph L., Singh P., Prasad R. Change detection of a coastal woodland mangrove forest in Fiji by integration of remote sensing with spatial mapping. Asian J. Conserv. Biol. 2021;10:222–233. doi: 10.53562/ajcb.66492. [DOI] [Google Scholar]
- 73.Krauss K.W., Lovelock C.E., McKee K.L., López-Hoffman L., Ewe S.M.L., Sousa W.P. Environmental drivers in mangrove establishment and early development: a review. Aquat. Bot. 2008;89:105–127. doi: 10.1016/j.aquabot.2007.12.014. [DOI] [Google Scholar]
- 74.Das S., Crépin A.S. Mangroves can provide protection against wind damage during storms. Estuar. Coast Shelf Sci. 2013;134:98–107. doi: 10.1016/j.ecss.2013.09.021. [DOI] [Google Scholar]
- 75.Mezger E.M., De Nooijer L.J., Boer W., Brummer G.J.A., Reichart G.J. Salinity controls on Na incorporation in Red Sea planktonic foraminifera. Paleoceanography. 2016;31:1562–1582. doi: 10.1002/2016PA003052. [DOI] [Google Scholar]
- 76.Oyebade B.A., Emerhi E.A., Ekeke B.A. Quantitative review and distribution status of mangrove forest species in West Africa. Afr. Res. Rev. 2010;4 doi: 10.4314/afrrev.v4i2.58292. [DOI] [Google Scholar]
- 77.C.-F, Chen N.-T., Son N.-B., Chang C.-R., Chen L.-Y., Chang M., Valdez G., Centeno C.A., Thompson J. L. Aceituno. Multi-decadal mangrove forest change detection and prediction in Honduras, Central America, with Landsat imagery and a Markov chain model. Remote Sens. 2013;5:6408–6426. doi: 10.3390/rs5126408. [DOI] [Google Scholar]
- 78.Islam M.M., Borgqvist H., Kumar L. Monitoring Mangrove forest landcover changes in the coastline of Bangladesh from 1976 to 2015. Geocarto Int. 2019;34:1458–1476. doi: 10.1080/10106049.2018.1489423. [DOI] [Google Scholar]
- 79.Osorio J.A., Wingfield M.J., Roux J. A review of factors associated with decline and death of mangroves, with particular reference to fungal pathogens. South Afr. J. Bot. 2016;103:295–301. doi: 10.1016/j.sajb.2014.08.010. [DOI] [Google Scholar]
Further reading
- 80.GASGI General authority for survey and geospatial information, official map of the kingdom of Saudi Arabia. 2020. https://gasgi.gov.sa/en/products/publicmaps/pages/official-map-of-the-kingdom-of-saudi-arabia.aspx
- 81.A. Haupt, T.T. Kane, Population handbook, the population reference bureau, Washington District of Columbia, 2004. Giovanni website, 2022. https://giovanni.gsfc.nasa.gov/giovanni. (accessed 10 November 2022).
- 82.National Aeronautics and Space Administration (NASA) Ocean Colour Website. https://oceancolor.gsfc.nasa.gov/. (accessed 25 September 2022).
- 83.USGS Global visualization viewer. 2022. https://glovis.usgs.gov/app 20–27 July 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request. For requesting data, please write to the corresponding author.