Abstract
Aims: The expression of proteinases and their inhibitors determines the extracellular matrix (ECM) turnover in normal and pathological processes. In cancer, proteolysis is abnormally regulated, favouring ECM degradation, which aids tumour invasion and metastasis. Previous studies have determined the expression of proteinases and inhibitors in breast cancer using a variety of techniques, including immunohistochemistry; however, most have looked at the expression of individual proteinases and/or inhibitors. Therefore, the aim of the current study was to determine the simultaneous cellular expression of matrix metalloproteinases (MMPs), plasminogen activators (PAs), and tissue inhibitors of metalloproteinases (TIMPs) in patients with breast cancer and correlate this with clinical pathological staging and survival.
Methods: Immunohistochemistry was used to determine the expression of proteinases (MMP-1, MMP-2, MMP-3, MMP-9, urokinase-type PA, and tissue-type PA) and inhibitors (TIMP-1 and TIMP-2) in 44 patients with breast cancer.
Results: The expression of all the factors studied was stronger or equivalent in tumour cells than in fibroblasts or inflammatory cells within the tumour section. Both positive and negative trends have emerged in the correlation between the cellular expression of proteinases and inhibitors and breast tumour pathology (tumour grade, lymphovascular invasion, and Nottingham prognostic index).
Conclusions: The interactions between proteinases and their inhibitors in breast cancer progression are complex. Although there are differences in the expression of these factors that relate to differences in breast cancer pathology, there are no outstanding individual factors that consistently correlate with prognosis. Therefore, different factors are probably important at different stages of the process, and the balance in the relative concentrations of proteinases and inhibitors probably determines ECM degradation in breast tumour invasion and metastasis in vivo.
Keywords: breast cancer, matrix metalloproteinases, tissue inhibitors of metalloproteinases, plasminogen activators
The major threat to patients with breast cancer is tumour invasion and metastasis. The metastatic process involves a complex cascade of events including angiogenesis, local invasion, and intravasation. Several stages within the metastatic cascade involve the organised breakdown of extracellular matrix (ECM) components by proteinases. The proteinases primarily involved in matrix degradation are the matrix metalloproteinases (MMPs) and the serine proteinases, plasminogen activators (PAs).
The MMP system consists of at least 20 human proteins, each sharing amino acid sequences and homologies (reviewed previously).1–6 MMPs are further divided into five subclasses based on their substrate specificity, namely: collagenases, gelatinases, stromelysins, membrane-type MMPs (MT-MMPs), and other MMPs. These MMPs have the combined ability to break down all ECM components and as a result are normally tightly regulated at several levels including activation and transcription; in addition, the presence of specific tissue inhibitors of metalloproteinases (TIMPs) provides an extra level of regulation.7,8
The PAs activate plasminogen to the active enzyme, plasmin, which degrades matrix components directly (for example, proteoglycans) or indirectly by activating other proteinases, such as MMPs.9,10 The PA system consists of the urokinase-type and tissue-type PAs (uPA and tPA, respectively), a receptor for uPA, which focuses proteolysis, and the plasminogen activator inhibitors PAI-1 and PAI-2. The role and regulation of the PA system has been reviewed previously.11–14
Components of both the MMP and the PA proteinase systems have been implicated in breast cancer prognosis and survival. For example uPA and PAI-1 have been associated with poor prognosis, shorter disease free time, and decreased overall survival.15–17
Individual components of both the MMP18–25 and PA systems26–31 have been analysed previously by immunohistochemistry in human breast cancer. However, most of these studies have determined the expression of individual proteinases and/or inhibitors.
“Components of both the matrix metalloproteinase and the plasminogen activator systems have been implicated in breast cancer prognosis and survival”
Therefore, the aim of our study was to determine the simultaneous cellular expression of different proteinases (MMP-1, MMP-2, MMP-3, MMP-9, uPA, and tPA) and inhibitors (TIMP-1 and TIMP-2) in 44 patients with breast cancer using serial sections. We sought to identify the cellular distribution of staining intensity within each tumour section (tumour cells, fibroblasts, or inflammatory cells) and to correlate this expression with both clinical pathological staging and outcome.
MATERIALS AND METHODS
Materials
Monoclonal antibodies against the pro forms of MMP-1, MMP-2, MMP-3, MMP-9, uPA, and tPA were purchased from the Binding Site, Birmingham, UK and against total TIMP-1 and TIMP-2 from Oncogene Research, CN Biosciences, Nottingham, UK. The Elite vector stain peroxidase kit and the 3,3′ diaminobenzidine (DAB) substrate kit were purchased from Vector Laboratories, Peterborough, UK.
Patient demographics
Immunohistochemistry was performed on paraffin wax embedded tumour specimens from 44 patients with breast cancer (June 1994 to August 1996); paired tumour and normal tissue samples had previously been analysed for proteinases and inhibitors by other laboratory techniques.25 These patients had undergone surgery at the Royal Hallamshire Hospital between January 1994 and August 1996. All patients were female with a mean age at surgery of 63 years (range, 33–83) and median follow up of 55 months (range, 12–79).
Immunohistochemistry
Paraffin wax embedded breast tumour sections were obtained from the department of histopathology, University of Sheffield. Serial 4 μm sections were cut from each embedded breast tumour and stained with monoclonal antibodies against the MMP-1, MMP-2, MMP-3, MMP-9, uPA, tPA, TIMP-1, and TIMP-2 antigens using the avidin–biotin staining technique (ABC Elite).32
After the sections were dewaxed and rehydrated, endogenous peroxidase activity was blocked by incubating the slides in 0.3% H2O2 in absolute methanol. The antigen was unmasked by incubating the sections in 0.1% trypsin for 10 minutes at 37°C. For the reduction of non-specific background staining, sections were then incubated with diluted normal blocking serum for 20 minutes at room temperature. The primary antibodies were diluted in phosphate buffered saline (PBS; 1/10, TIMP-2; 1/15 TIMP-1; 1/500, MMP-2, and the others 1/1000) and without washing, the tissue sections were incubated with the primary antibody for one hour in a humidity chamber at room temperature. For negative controls antibodies were omitted and replaced by PBS alone. Tissue sections were then incubated with biotinylated secondary antibodies (rabbit antisheep for proteinases and horse antimouse for TIMPs). Sections were then incubated with the ABC solution for 45 minutes and the reaction was revealed by incubation with 0.5 mg/ml DAB solution. Sections were counterstained with Gill’s haematoxylin, and the sections dehydrated and mounted. Between steps, the slides were washed three times with PBS.
Slides were then observed by conventional light microscopy. Staining intensities were determined by a consultant histopathologist (TJS) and each section was staged according to the proportion of cells in a section staining strongly for the antigen and were given a staining score, namely: 1, 0–25%; 2, 25–50%; 3, 50–75%; and 4, 75–100% for tumour cells, fibroblasts, and inflammatory cells.
Statistics
The Wilcoxon signed rank test was used for comparisons between the expression of the proteinases and inhibitors in the different cell types within the tumour section. Spearman’s correlation coefficient was used to determine whether a correlation existed between the expression of the proteinases and inhibitors and clinical pathological staging and outcome. Differences were considered significant at a p value of < 0.05.
RESULTS
Patients, histology, and outcome
Most invasive breast tumours were ductal carcinomas (38 of 44), three of 44 were lobular, one was ductal and lobular, one was papillary, and one was tubular. The histological grades33 of these tumours were seven grade 1, 22 grade 2, 14 grade 3, and the remaining tumour was ductal carcinoma in situ (DCIS). Fifteen tumours had undergone lymphatic or vascular invasion at the time of resection and of these, six were grade 3 tumours, eight grade 2 tumours, and one was a grade 1 tumour. The clinical prognosis as determined by the Nottingham prognostic index (NPI)33 was described as good for 17 patients, moderate for 20, and poor for seven.
Nine of 44 patients had died since the date of surgery, seven from metastases (four with grade 3 tumours and three had grade 2) and the deaths of the other two patients were unrelated to cancer (both grade 2). One patient was alive but had recurrence (grade 1 tumour) and the remaining 34 patients were alive and well at the last follow up. There was a significant correlation between expected outcome as determined by the NPI and the real outcome (p < 0.05, Spearman’s correlation).
Immunohistochemical staining
Cell type
There were wide variations in the staining scores between the different proteinases and inhibitors, and between their expression in the different cell types within breast tumour samples. The staining score for MMP-1, MMP-2, MMP-3, tPA, TIMP-1, and TIMP-2 was significantly greater in tumour cells than was seen for neighbouring inflammatory cells or fibroblasts (p < 0.05; Wilcoxon; table 1 ▶). In general, the staining score was greater for the inhibitors, TIMP-1 and TIMP-2, than for any of the proteinases (table 1 ▶).
Table 1.
Differences in the staining scores of proteinases and inhibitors in each cell type of 44 breast tumours as assessed by immunohistochemistry
| Tumour cells | Fibroblasts | Inflammatory cells | |
| MMP-1 | 1.8 (1–4)* | 1.3 (1–3) | 1.1 (1–3) |
| MMP-2 | 2.3 (1–4)* | 1.5 (1–2) | 1.3 (1–2) |
| MMP-3 | 1.6 (1–4)* | 1.2 (1–3) | 1.1 (1–3) |
| MMP-9 | 1.6 (1–3) | 1.6 (1–4) | 1.3 (1–4) |
| uPA | 2 (1–4) | 1.7 (1–4) | 1.2 (1–2) |
| tPA | 1.7 (1–4)* | 1.5 (1–3) | 1.2 (1–3) |
| TIMP-1 | 2.9 (1–4)* | 1.9 (1–4) | 1.5 (1–4) |
| TIMP-2 | 3.2 (1–4)* | 2.1 (1–4) | 2.1 (1–4) |
Values are mean (range). *p<0.05; Wilcoxon signed rank test for related samples.
MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinases; tPA, tissue-type plasminogen activator; uPA, urokinase-type plasminogen activator.
Breast tumour grade
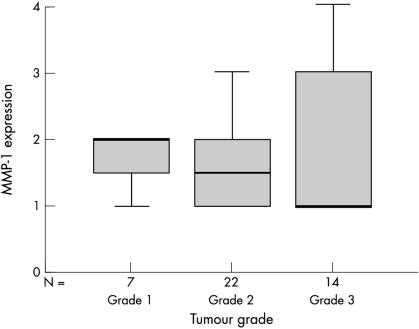
Although there were differences seen in the staining scores of the proteinases and inhibitors in each breast tumour grade, there was no significant correlation between them. The staining scores in tumour cells for all proteinases and inhibitors studied were greater in grade 1 than grade 3 breast tumours, as is illustrated in fig 1 ▶. There were no significant differences in the staining scores of proteinases or inhibitors for either inflammatory cells or fibroblasts across the breast tumour grades.
Figure 1.
Box plot showing the negative trend between matrix metalloproteinase 1 (MMP-1) expression (as determined by the staining score) and the grade of breast tumour. The line within the box plot corresponds to the median value, the box length to the interquartile range, and the lines emanating from the box (whiskers) extend to the smallest and largest observations.
Nottingham prognostic index
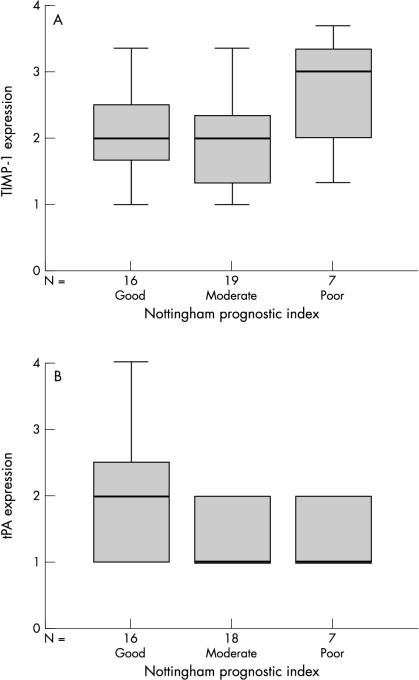
In keeping with the results for tumour grade, differences in staining scores between patients in different prognostic groups (as determined by NPI) were also seen. There were more pronounced differences between the expression of the proteinases and inhibitors in relation to the NPI in the tumour cells than in the inflammatory cells or fibroblasts. A positive but not significant correlation was seen between tumour cell staining intensity for MMP-1, MMP-2, and TIMP-1 (fig 2A ▶) and poor prognosis, as determined by NPI. However, a significant negative correlation was observed for the MMP-9 and tPA (fig 2B ▶) tumour cell staining scores, with those patients with a good clinical prognosis based on NPI demonstrating an increased staining intensity when compared with those who had a moderate or poor prognosis (p < 0.05, Spearman’s correlation). There was no difference in staining scores for MMP-3, uPA, or TIMP-2 in relation to NPI. Figure 2 ▶ shows an example of a positive and a negative correlation with NPI.
Figure 2.
Box plots demonstrating (A) the positive trend between the expression of tissue inhibitor of metalloproteinases 1 (TIMP-1) and the determined Nottingham prognostic index of the breast tumours; and (B) the significant negative trend between the expression of tissue-type plasminogen activator (tPA; p < 0.05 Spearman’s correlation) and the Nottingham prognostic index of the breast tumours. The line within the box plot corresponds to the median value, the box length to the interquartile range, and the lines emanating from the box (whiskers) extend to the smallest and largest observations.
Lymphovascular invasion
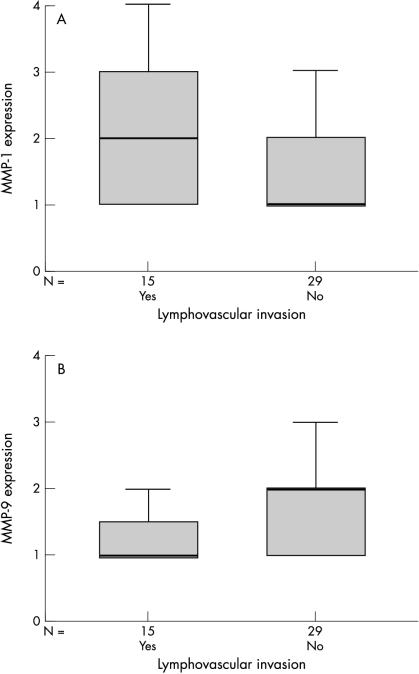
The only significant correlation between the staining score and whether the breast tumour had undergone lymphatic and/or vascular invasion at the time of resection was for the expression of MMP-1 in tumour cells (p < 0.05, Spearman’s correlation; fig 3A ▶). A positive trend was also seen for MMP-2, TIMP-1, and TIMP-2, with greater tumour cell expression seen in invasive breast tumours, whereas a negative trend was observed for both MMP-9 (fig 3B ▶) and tPA.
Figure 3.
Box plots demonstrating (A) the significant positive trend between the expression of matrix metalloproteinase 1 (MMP-1; p < 0.05, Spearman’s correlation) and whether the tumours exhibited lymph or vascular invasion at the time of resection; and (B) the negative trend between the expression of MMP-9 and whether the tumours exhibited lymph or vascular invasion at the time of resection. The line within the box plot corresponds to the median value, the box length to the interquartile range, and the lines emanating from the box (whiskers) extend to the smallest and largest observations.
Status
Most patients (34 of 44) remain alive and well at a median 57 months of follow up, so that statistical comparisons between proteinase/inhibitor expression and status cannot be determined because of the small sample numbers in the other groups; that is, one alive with metastases, seven cancer related deaths, and two unrelated deaths. However, none of the proteinases or inhibitors showed obvious differences in staining intensity between these different patient groups.
DISCUSSION
We have previously determined the protein concentrations of proteinases (MMPs and PAs) and inhibitors (TIMPs) in paired breast tumour and normal tissue using the techniques of substrate gel zymography and western blotting.25 To our knowledge, our present study is the first immunohistochemical study using tissue from patients with breast cancer to determine the cellular expression of several proteinases (MMP-1, MMP-2, MMP-3, MMP-9, uPA, and tPA) and inhibitors (TIMP-1 and TIMP-2) identified as being important in tumour invasion and metastasis. The staining intensity of these factors was correlated with breast tumour pathology, prognosis, and survival.
ECM degradation by proteinases is normally tightly regulated at several levels, including the activation of latent proteinases. MMPs are secreted from cells in a latent form and require activation extracellularly for proteolytic activity. The second level of control is the presence of specific proteinase inhibitors— TIMPs for MMPs and PAIs for the PAs—and finally both proteinases and inhibitors are regulated at the level of gene transcription. For proteolysis to occur, active proteinase concentrations must exceed those of their inhibitors. Therefore, it is probably the overall balance between the concentrations of each form of proteinase and inhibitor that will determine whether matrix degradation occurs at each stage of tumour invasion and metastasis in vivo.
Most previously published breast cancer studies have used either enzyme linked immunosorbent assays17,34–37 or in situ hybridisation20,38 to determine the expression of the MMP and PA system components. Of the few studies that have used immunohistochemistry, most have analysed a smaller number of samples26–28 or have determined the expression and localisation of individual proteinases/inhibitors.22,29,31
In our current study, both the expression and localisation of several proteinases and inhibitors were determined in 44 breast tumours. Wide variations in staining scores were seen between cell types within breast tumours, with tumour cells generally demonstrating greater staining scores than either fibroblasts or inflammatory cells. In addition, the staining scores for the inhibitors TIMP-1 and TIMP-2 were greater than those obtained for the proteinases.
Both positive and negative trends have been observed when correlating the immunohistochemical expression of proteinases and inhibitors with clinical parameters. Enhanced expression of several individual factors correlated significantly with individual clinical parameters—for example, MMP-1 expression and lymphovascular invasion. However for most, the number of samples in each subgroup for each parameter was relatively small.
Our present study has confirmed data from previous immunohistochemical studies demonstrating variations in staining scores for proteinases and inhibitors both between cell types and between breast tumour samples. In agreement with our present study, tumour cells had an increased staining score for the expression of MMP-1, MMP-2, MMP-3, and MMP-919,23 when compared with the other cell types. Similarly, uPA expression was found in both tumour and stromal cells,26,29 whereas TIMP-1 expression was predominantly localised to endothelial cells, with some expression in tumour cells and fibroblasts.1,9
“It is probably the overall balance between the concentrations of each form of proteinase and inhibitor that will determine whether matrix degradation occurs at each stage of tumour invasion and metastasis in vivo”
The differences in immunohistochemical staining of proteinases and inhibitors between studies can be explained in part by the use of different antibodies. Antibodies may be specific for different forms of the protein—for example, for MMPs the antibody may be specific for latent, active, or total MMPs, or all three; in addition, some antibodies may recognise only uncomplexed MMPs whereas others may only recognise MMPs complexed with TIMPs. Often this is not made clear, which makes comparisons between studies difficult.
There have been conflicting reports on whether the identification of individual proteinases or inhibitors correlates with or predicts surgical outcome in patients with breast cancer. In previous immunohistochemical studies, Dublin and colleagues demonstrated that strong uPA expression correlated with high tumour grade30 and Jahkola found a correlation between high uPA expression and increased risk of local recurrence.29 No other studies have shown a correlation between proteinases or inhibitors and either tumour pathology or outcome. However, in a few studies using other techniques to identify either proteinases or inhibitors, there has been a correlation between the expression of certain factors and pathology. For example, using in situ hybridisation there was a positive correlation between TIMP-1 and TIMP-2 expression and tumour grade38 and MMP-1 mRNA expression significantly correlated with the stage and invasion of breast tumours.24 However, most studies have been unable to demonstrate such a correlation.
When investigating any relation between the expression of proteinases and inhibitors and outcome/survival, only uPA has consistently been reported to be predictive of disease free and overall survival.16,29,39,40 The only other component of the MMP and PA systems that has been implicated as a strong prognostic factor for both disease free and overall survival is PAI-1.17,29,34,40 Our current study found no correlation between the cellular expression of the studied proteinases and inhibitors and either disease free or overall survival; however, the sample size was small.
Although our current study has determined the immunohistochemical expression of several proteinases and inhibitors, there are other members of these families that require consideration for the overall profile of proteolysis in vivo. These include other MMPs, such as MT-MMPs and inhibitors, such as TIMP-3 and TIMP-4, which had not been fully described at the time of our study. MT-MMPs are positively expressed in the stromal cells of invasive breast cancers.21 Although the plasminogen activator inhibitors PAI-1 and PAI-2 have been fully described, antibodies against these inhibitors were not commercially available when our study began.
Take home messages.
The expression of all the proteinases and inhibitors studied was stronger or equivalent in tumour cells than in fibroblasts or inflammatory cells within the tumour section
Both positive and negative trends were found between the expression of proteinases and inhibitors and breast tumour pathology (tumour grade, lymphovascular invasion, and Nottingham prognostic index), indicating that the interactions between these factors in breast cancer progression are complex
However, there were no outstanding individual factors that consistently correlated with prognosis so that in vivo the balance between the relative concentrations of proteinases and inhibitors probably determines extracellular matrix degradation in breast tumour invasion and metastasis
In summary, our present study determined the differential expression of proteinases and inhibitors and demonstrated trends in relation to breast tumour pathology and prognosis. With increased sample numbers the clinical relevance of identifying proteinase and inhibitor expression may be revealed. Breast tumour invasion and metastasis involve complex interactions between proteinases and inhibitors. The differential proteinase expression identified in our study suggests that distinct proteinase profiles may be involved at different stages of the metastatic cascade, depending on the surrounding components of the ECM. However, overall, it is probably the balance in the concentrations of activated proteinases and their inhibitors at each stage of the metastatic cascade that will determine whether ECM degradation and consequently tumour invasion and metastasis occur in vivo. Therefore, it is important to identify more than a single factor in any study investigating the role of proteinases and inhibitors in breast cancer progression in vivo.
Acknowledgments
The authors thank R Stewart, K Corke, and O Gallagher from the Academic Unit of Pathology, University of Sheffield for their support and advice.
Abbreviations
ABC, avidin–biotin complex
DAB, 3,3′ diaminobenzidine
ECM, extracellular matrix
MMP, matrix metalloproteinase
MT-MMP, membrane-type matrix metalloproteinase
NPI, Nottingham prognostic index
PA, plasminogen activator
PAI, plasminogen activator inhibitor
PBS, phosphate buffered saline
TIMP, tissue inhibitor of metalloproteinases
tPA, tissue-type plasminogen activator
uPA, urokinase-type plasminogen activator
REFERENCES
- 1.Matrisian LM. The matrix degrading metalloproteinases. Bioassays 1992;14:455–63. [DOI] [PubMed] [Google Scholar]
- 2.Murphy G, Docherty AJP. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol 1992;7:120–5. [DOI] [PubMed] [Google Scholar]
- 3.Raza SL, Cornelius LA. Matrix metalloproteinases: pro and anti-angiogenic activities. J Investig Dermatol Symp Proc 2000;5:47–54. [DOI] [PubMed] [Google Scholar]
- 4.Ennis BW, Matrisian LM. Matrix degrading metalloproteinases. J Neurooncol 1994;18:105–98. [DOI] [PubMed] [Google Scholar]
- 5.Ray JM, Stetler-Stevenson WG. The role of matrix metalloproteases and their inhibitors in tumour invasion, metastasis and angiogenesis. Eur Respir J 1995;7:2062–72. [PubMed] [Google Scholar]
- 6.Birkedal-Hansen H, Moore WGI, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993;4:197–250. [DOI] [PubMed] [Google Scholar]
- 7.Matrisian LM. Metalloproteinases and their inhibitors in tissue remodelling. Trends Genet 1990;6:121–5. [DOI] [PubMed] [Google Scholar]
- 8.Cottam DW, Rees RC. Regulation of matrix metalloproteinases: their role in tumour invasion and metastasis. Int J Oncol 1993;2:861–72. [DOI] [PubMed] [Google Scholar]
- 9.Saksela O, Rifkin DB. Cell associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol 1998;4:93–126. [DOI] [PubMed] [Google Scholar]
- 10.Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumour invasion. Physiol Rev 1993;73:161–95. [DOI] [PubMed] [Google Scholar]
- 11.Vassalli JD, Sappino AP, Belin D. The plasminogen activator/plasmin system. J Clin Invest 1991;88:1067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dano K, Andreasen PA, Grondahl-Hansen J, et al. Plasminogen activators, tissue degradation and cancer. Adv Cancer Res 1985;44:139–266. [DOI] [PubMed] [Google Scholar]
- 13.Kluft C, Dooijewaard G, Emeis JJ. Role of the contact system in fibrinolysis. Semin Thromb Hemost 1987;13:50–68. [DOI] [PubMed] [Google Scholar]
- 14.Gandolfo GM, Conti L, Vercillo M. Fibrinolysis components in breast cancer and colorectal carcinoma. Anticancer Res 1996;16:2155–60. [PubMed] [Google Scholar]
- 15.Duffy MJ, Maguire TM, McDermott EW, et al. Urokinase plasminogen activator: a prognostic marker in multiple types of cancer. J Surg Oncol 1999;71:130–5. [DOI] [PubMed] [Google Scholar]
- 16.Janicke F, Schmitt M, Pache L, et al. Urokinase (uPA) and its inhibitor PAI-1 are strong and independent prognostic factors in node negative breast cancer. Breast Cancer Res Treat 1993;24:195–208. [DOI] [PubMed] [Google Scholar]
- 17.Bouchet C, Hacene K, Martin PM, et al. Dissemination risk index based on plasminogen activator system components in primary breast cancer. J Clin Oncol 1999;17:3048–57. [DOI] [PubMed] [Google Scholar]
- 18.Monteagudo C, Merino MJ, San-Luan J, et al. Immunohistochemical distribution of type IV collagenase in normal, benign and malignant breast tissue. Am J Pathol 1990;136:585–92. [PMC free article] [PubMed] [Google Scholar]
- 19.Clavel C, Polette M, Doco M, et al. Immunolocalisation of matrix metallo-proteinases and their tissue inhibitors in human mammary pathology. Bull Cancer 1992;79:261–70. [PubMed] [Google Scholar]
- 20.Polette M, Clavel C, Cockett M, et al. Detection and localisation of mRNAs encoding matrix metalloproteinases and their tissue inhibitors in breast pathology. Invasion Metastasis 1993;13:31–7. [PubMed] [Google Scholar]
- 21.Chenard MP, Lutz Y, Mechine-Neuville A, et al. Presence of high levels of MT1-MMP protein in fibroblastic cells of human invasive carcinomas. Int J Cancer 1999;82:208–12. [DOI] [PubMed] [Google Scholar]
- 22.Ishigaki S, Toi M, Ueno T, et al. Significance of membrane type matrix metalloproteinase expression in breast cancer. Jpn J Cancer Res 1999;90:516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebeau A, Nerlich AG, Sauer U, et al. Tissue distribution of major matrix metalloproteinases and their transcripts in human carcinomas. Anticancer Res 1999;19:4257–64. [PubMed] [Google Scholar]
- 24.Nakapoulou L, Giannopoulou I, Gakiopoulou H, et al. Matrix metalloproteinase-1 and -3 in breast cancer: correlation with progesterone receptors and other clinicopathologic features. Hum Pathol 1999;30:436–42. [DOI] [PubMed] [Google Scholar]
- 25.Garbett EA, Reed MWR, Stephenson TJ, et al. Proteolysis in human breast cancer. Mol Pathol 2000;53:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy S, Duffy MJ, Duggan C, et al. Semi-quantitation of urokinase plasminogen activator and its receptor in breast carcinomas by immunocytochemistry. Br J Cancer 1998;77:1638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrier CM, de Witte HH, van Tienoven DH, et al. Comparison of immunohistochemistry with immunoassay (ELISA) for the detection of components of the plasminogen activation system in human tumour tissue, Br J Cancer 1999;79:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildenbrand R, Wolf G, Bohme B, et al. Urokinase plasminogen activator receptor (CD87) expression of tumour associated macrophages in ductal carcinoma in situ, breast cancer and resident macrophages of normal breast tissue. J Leukoc Biol 1999;66:40–9. [DOI] [PubMed] [Google Scholar]
- 29.Jahkola T, Toivonen T, von Smitten K, et al. Cathepsin D, urokinase plasminogen activator and type-1 plasminogen activator inhibitor in early breast cancer: an immunohistochemical study of prognostic value and relations to tenascin-C and other factors. Br J Cancer 1999;80:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dublin E, Hanby A, Patel NK, et al. Immunohistochemical expression of uPA, uPAR and PAI-1 in breast carcinoma. Am J Pathol 2000;157:1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hildenbrand R, Leitz M, Magdolen V, et al. Validation of immunolocalization of the urokinase receptor expression in ductal carcinoma in situ of the breast: comparison with detection by non-isotopic in-situ hybridisation. Histopathology 2000;36:499–504. [DOI] [PubMed] [Google Scholar]
- 32.Hsu SM, Raine L, Fanger H. The use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase technique: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem 1981;29:577–80. [DOI] [PubMed] [Google Scholar]
- 33.Miller WR, Ellis IO, Sainsbury JRC, et al. ABC of breast diseases: prognostic factors. BMJ 1995;309:1573–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harbeck N, Thomssen C, Berger U, et al. Invasion marker PAI-1 remains a strong prognostic factor after long-term follow-up both for primary breast cancer and following first relapse. Breast Cancer Res Treat 1999;54:147–57. [DOI] [PubMed] [Google Scholar]
- 35.Rha SY, Yang WI, Gong SJ, et al. Correlation of tissue and blood plasminogen activation system in breast cancer. Cancer Lett 2000;150:137–45. [DOI] [PubMed] [Google Scholar]
- 36.Foekens JA, Peters HA, Look MP, et al. The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients. Cancer Res 2000;60:636–43. [PubMed] [Google Scholar]
- 37.Duffy MJ, Blaser J, Duggan C, et al. Assay of matrix metalloproteases types 8 and 9 by ELISA in human breast cancer. Br J Cancer 1995;71:1025–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brummer O, Athar S, Riethdorf L, et al. Matrix metalloproteinases 1, 2 and 3 and their tissue inhibitors 1 and 2 in benign and malignant breast lesions: an in situ hybridisation study. Virchows Arch 1999;435:566–73. [DOI] [PubMed] [Google Scholar]
- 39.Fisher JL, Field CL, Zhou H, et al. Urokinase plasminogen activator system gene expression is increased in human breast carcinoma and its bone metastases—a comparison of normal breast tissue, non-invasive and invasive carcinoma and osseous metastases. Breast Cancer Res Treat 2000;61:1–12. [DOI] [PubMed] [Google Scholar]
- 40.Meijer-van Gelder ME, Look MP, Bolt-de Vries J, et al. Breast-conserving therapy: proteases as risk factors in relation to survival after local relapse. J Clin Oncol 1999;17:1449–57. [DOI] [PubMed] [Google Scholar]