Abstract
Aims: To ascertain whether the expression and enzyme activity of thymidylate synthase (TS) are related to the rapidity of cell proliferation in human cancer cell lines.
Methods: Ten asynchronously growing human cancer cell lines of different origin were used, characterised by various doubling times. TS expression was evaluated by western blot analysis using the TS 106 monoclonal antibody. TS activity was determined by the enzyme catalytic assay. The quantitative variation of TS in different phases of the cell cycle was investigated using two parameter flow cytometry for the TS protein and DNA analysis. The number of proliferating cells was evaluated by Ki67 immunostaining.
Results: TS expression and activity were significantly related to each other (r = 0.765; p = 0.01) and to the cell doubling time (r = −0.899; p < 0.001 and r = −0.919; p < 0.001, respectively). Ki67 immunolabelling showed no association between the number of cycling cells and TS protein expression and activity. Two parameter flow cytometry indicated that differences of TS expression in the cell lines were not related to the cell cycle phases or to the proportion of S phase cells.
Conclusions: These results show that the expression and activity of the TS protein in asynchronously growing cancer cells are significantly related to the cell doubling time; the faster the cell proliferation, the greater the expression and activity of TS. These findings could explain why TS values are of prognostic value per se and why tumours with high TS expression benefit more from chemotherapy.
Keywords: cancer cell lines, cell doubling time, thymidylate synthase, 5-FU chemotherapy
Thymidylate synthase (TS) is necessary for the de novo synthesis of DNA in proliferating cells because it catalyses the methylation of deoxyuridine monophosphate to thymidine monophosphate.1 TS is the target for fluoropyrimidine drugs such as 5-fluorouracil (5-FU),2 which are currently used in the treatment of several solid tumours. 5-FU is the drug of choice for systemic treatment in colorectal cancer.3 Intrinsic tumour TS values are thought to be of importance in predicting the response to chemotherapy with 5-FU and survival in patients with colorectal cancer. Patients with low tumour TS expression have a significantly better outcome than those with high TS expression.4 In the past, this was thought to be because high intrinsic TS tumour values would confer forms of resistance to treatment with 5-FU.5 However, there is also evidence that TS protein expression is a prognostic factor per se, irrespective of 5-FU sensitivity.6–8 Moreover, adjuvant 5-FU based or 5-FU containing treatments have been shown significantly to improve disease free and overall survival only for patients with high levels of TS expression.9,10 Therefore, the relation between TS expression and tumour prognosis should be explained on a different basis to that of tumour 5-FU resistance. Precise identification of the cause of the association between TS expression and tumour clinical outcome and chemotherapy sensitivity is the prerequisite for studies carried out to define the efficacy of TS inhibitors and to develop more appropriate cancer treatment strategies. The relation between TS expression and the cell cycle has been investigated extensively both in normal and cancer cells, but there have been contradictory results with regard to the relation between TS expression and the parameters of cell proliferation.11–13 Furthermore, independently of the relation with proliferation, TS expression varies greatly among different cell lines, suggesting that individual cell lines are characterised by different constitutive levels of expression.14
“Adjuvant 5-fluorouracil (5-FU) based or 5-FU containing treatments have been shown significantly to improve disease free and overall survival only for patients with high levels of TS expression”
The aim of our present study was to ascertain whether TS expression and enzyme activity are related to the rate of cell proliferation. For this purpose, we used a series of human cancer cell lines of different origin, characterised by various doubling times. TS expression was measured by densitometric evaluation of autoradiographic signals obtained after western blot analysis performed using the TS 106 monoclonal antibody on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) separated cell proteins. TS activity was measured by determining the catalytic activity of TS. The cell growth fraction of each cell line was measured by evaluating the Ki67 labelling index. The quantitative variation of TS in different phases of the cell cycle was investigated using two parameter flow cytometry for TS protein and DNA analysis.
METHODS
Cell lines and population doubling time
Ten established cell lines were used that were derived from human tumours of different origin: the CHP212, SJNKP, IMR32, and SY5Y cell lines from neuroblastomas; the LoVo, COLO205, SW48, SW480, and SW620 cell lines from colonic adenocarcinomas; and the TG cell line from a tubal carcinoma. All cell lines were maintained in asynchronous growth as monolayer cultures, as described previously.15 The population doubling times were determined by counting the cells in triplicate samples at 24, 48, and 72 hourly intervals, according to the method described by Patterson.16
Preparation of protein extracts, SDS-PAGE, and immunoblotting
Proteins were extracted from whole cells. Protein samples (10 μg) were loaded in each lane. Proteins samples were electrophoresed in 10% SDS-PAGE gels. Size standards from 200 to 205 kDa, purchased from Sigma Chemical Company (St Louis, Missouri, USA), were included. Polypeptides were electrotransferred to reinforced cellulose nitrate membranes (Hybond C Extra; Amersham, Little Chalfont, Buckinghamshire, UK). After electroblotting, filters were stained with Ponceau solution (Sigma Chemical Company) and the immunoblotting was carried out as described previously.15 The following primary antibodies were used: the TS 106 monoclonal antibody (Chemicon International, Temecula, California, USA) diluted 1/200, and anti-β actin (Santa Cruz Biotechnology, Santa Cruz, California, USA) diluted 1/100 in 5% non-fat dry milk in 1.5% bovine serum albumin (BSA)-TBS-T (3% BSA, 0.2% Tween 20 in phosphate buffered saline (PBS)). Autoradiographs obtained using the enhanced chemoluminescence kit (Amersham) were acquired with a scanner (DUOSCAN; Agfa) and signals were quantified using specific densitometric software (GelPro analyser 3.0; Media Cybernetics, Silver Spring, Maryland, USA).
Thymidylate synthase enzyme activity
The assay determines the catalytic activity of TS by means of tritiated water released during the TS catalysed conversion of [5-3H] dUMP to dTMP. The enzyme activity was measured in the range of linearity with respect to time and enzyme concentration, essentially as described previously.17 Briefly, cell pellets were suspended at a concentration of 4 × 106 cells/ml in 50mM Tris/HCl buffer, pH 7.5, containing 2mM dithiothreitol. Cell suspensions were sonicated on ice for 15 seconds at 20 kHz, centrifuged at 10 0000 ×g for 30 minutes, and the protein concentration of the supernatants was determined by measuring the optical density at 280 nm. The assay mixture contained, in a final volume of 55 μl: 50mM Tris/HCl buffer, pH 7.5; 2mM dithiothreitol; 0.1μM [3H] dUMP (3.7 × 103 Bq, specific activity 599 GBq/mmol); 0.63mM 5,6,7,8-tetrahydrofolate; and 5–25 μl cell supernatants. Samples were incubated at 37°C for 15 minutes and then 300 μl of a suspension containing 15% activated charcoal in 4% trichloroacetic acid was added to each sample. After centrifugation at 5000 ×g for 10 minutes the radioactivity present in 150 μl of the supernatants was determined by liquid scintillation counting.
Ki67 immunostaining
Cytological preparations were obtained from each cell line by cytocentrifugation on precleaned slides; these were then air dried and permeabilised with ice cold acetone (−20°C) for 10 minutes. Ki67 immunostaining was performed as described previously.18 A negative control was performed by omitting the anti-Ki67 monoclonal antibody. The Ki67 labelling index (Ki67 LI), expressed as per cent labelled cells, was evaluated by counting at least 1000 nuclei on each slide.
Two parameter flow cytometry
DNA content and TS protein expression were simultaneously evaluated by flow cytometry as described previously.13 Cells were fixed in 70% ethanol in PBS containing 0.05% Tween 20 at 4°C, washed in PBS, and placed in blocking buffer BSA-T at 4°C for 30 minutes. Samples were pelleted and incubated with 200 μl of the primary antibody (TS 106 monoclonal antibody diluted 1/20 in PBS) for one hour at 4°C. After one wash, samples were stained with 200 μl of a goat antimouse IgG–fluorescein isothiocyanate conjugate (Coulter Immunology, Miami, Florida, USA) diluted 1/50 in PBS containing 0.1% goat serum and 0.5% Tween 20. After washing twice, cells were stained with DNA Prep kit (Coulter Immunology), according to the manufacturer’s instructions. Two parameter analysis was performed by an EPICS XL flow cytometer (Coulter Immunology) with dedicated software to restrict the analysis to singlets for propidium iodide. TS 106 analysis was performed by evaluating the mean fluorescence intensity from each phase of the cell cycle. Ratios of TS staining intensities between cell cycle phases were then evaluated, after subtracting non-specific staining.
Statistical analysis
Correlations were evaluated by simple linear regression analysis. A p value < 0.05% was considered to be significant.
RESULTS
TS protein expression and activity and cell doubling time
Table 1 ▶ shows the doubling times of the 10 human cancer cell lines studied.
Table 1.
Doubling times (DT), Ki67 labelling index (LI), and thymidylate synthase (TS) expression and activity in cancer cell lines
| Cell line | DT (hours) | TS expression (TS/βactin) | TS activity | Ki67 LI (%) |
| TG | 20 | 2.56 | 486 | 77 |
| CHP212 | 21 | 3.96 | 417 | 79 |
| SJNKP | 24 | 3.8 | 341 | 81 |
| SW480 | 24 | 3.62 | 455 | 87 |
| SW620 | 35 | 4.37 | 232 | 85 |
| LoVo | 55 | 0.37 | 207 | 80 |
| COLO | 62 | 0.33 | 55 | 82 |
| SW48 | 66 | 0.34 | 41 | 78 |
| IMR32 | 70 | 0.34 | 166 | 78 |
| SY5Y | 77 | 0.22 | 69 | 86 |
TS activity was measured in pmoles of tritiated water released/mg protein/hour.
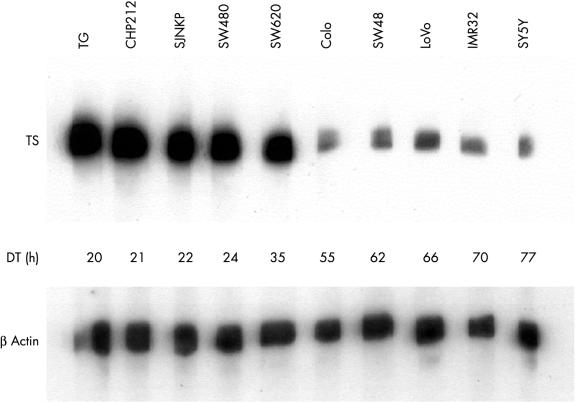
The expression of TS by the cancer cell lines was determined by immunoblotting of the whole cell protein content using the TS 106 monoclonal antibody (fig 1 ▶). The immunostaining resulted in specific bands corresponding to the expected molecular weight of 36 kDa. The integrated optical density values were standardised with those of β actin. The TS to β actin ratio values ranged from 0.22 (SY5Y cell line) to 4.37 (SW620 cell line) (table 1 ▶). The five rapidly proliferating cell lines with doubling times ranging from 20 to 35 hours were characterised by much higher TS protein values (mean, 3.66; SD, 0.67) than the five slowly proliferating cell lines with doubling times ranging from 55 to 77 hours (mean, 0.32; SD, 0.06). When TS expression and doubling time values were analysed using the linear regression model, a highly significant inverse correlation was found (r = −0.899; p < 0.001).
Figure 1.
Western blots of cell extracts from the 10 cell lines using the TS 106 monoclonal antibody and an anti-β actin antibody. DT, doubling time.
In the same series of cell lines TS activity was evaluated by the enzyme catalytic assay. TS activity values ranged from 509 pmoles of tritiated water released/mg protein/hour (SW48 cell line) to 6267 pmoles of tritiated water (CHP212 cell line), and were found to be significantly related both to TS expression (r = 0.765; p = 0.01) and to cell doubling time (r = −0.919; p < 0.001).
TS expression and activity in relation to cell growth fraction
To evaluate the cell growth fraction, Ki67 immunostaining was performed on cytological preparations obtained from the first series of cell lines. The Ki67 antigen is expressed in the G1, S, and G2/M cell cycle phases and is absent in resting (G0) cells. The Ki67 LI ranged from 77% in TG cells to 87% in SW480 cells. No significant correlation was found between Ki67 LIs and TS expression (r = 0.240; p = 0.505), TS activity (r = −0.053; p = 0.885), or doubling time values (r = 0.014; p = 0.969).
TS expression in relation to cell cycle phases
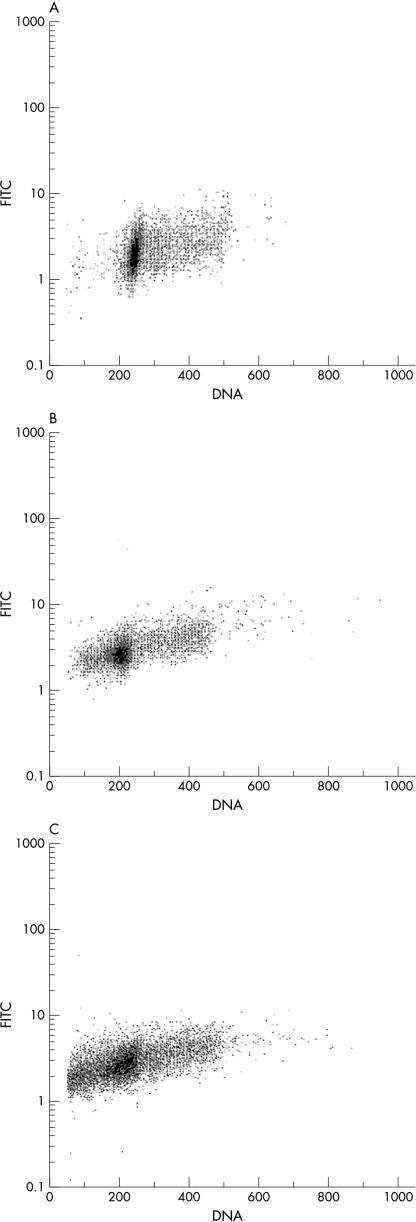
To evaluate TS expression with respect to the cell cycle phase, three cell lines (CHP212, SW620, and LoVo) with different doubling times were analysed by two parameter flow cytometry for TS protein and DNA content. Figure 2 ▶ shows the analysis of TS staining intensity by cell cycle phase. In the CHP212, SW620, and LoVo cell lines, respectively, TS values were 1.45, 1.53, and 1.59 times higher in the S–G2/M phases than in the G0/G1 phases. The expression of TS was not related to the proportion of S phase cells, which was similar in the three cell lines (28.3% in CHP212, 22.8% in SW620, and 22.7% in LoVo).
Figure 2.
Two parameter flow cytometry of the thymidylate synthase (TS) protein (y axis) and DNA (x axis) on the (A) CHP212, (B) SW620, and (C) LoVo cell lines. TS protein staining was performed with TS 106 as the primary antibody and a goat antimouse fluorescein isothiocyanate (FITC) conjugate as the secondary antibody. DNA staining was performed with propidium iodide.
DISCUSSION
Our study shows that there is a significant correlation between TS protein expression and activity and cell doubling time in human cancer cell lines. We used cancer cell lines of different origin to evaluate the expression of the TS protein. SDS-PAGE separated proteins were immunoblotted with the specific TS 106 monoclonal antibody and revealed by a chemoluminescent reaction. The integrated optical density values of the autoradiographic signals, standardised with β actin values, correlated inversely with the cell population doubling time: the greater the TS protein value, the shorter the doubling time. In the same series of human cancer cell lines we measured the catalytic activity of TS using total cell protein extracts. The TS activity values were inversely related to the cell doubling time. As expected,19 a strict correlation was found between the TS protein expression values and TS activity.
Large quantitative changes of TS expression and activity have been reported to occur when resting cells are stimulated to proliferate, or when proliferating cells are induced to pass from the exponential to the confluent growth phase.12 Therefore, we investigated whether the TS variations recorded by us might have been related to differences in the numbers of cells in the G0 phase. We excluded this possibility by measuring the proportion of cycling cells in each of the 10 cancer cell lines by means of the Ki67 antibody, which reveals the nuclear antigen expressed in proliferating cells in the G1, S, and G2/M phases.20 The Ki67 LI ranged only from 77 to 87, independently of doubling time and TS expression and activity.
“In asynchronously proliferating cells, the expression and activity of thymidylate synthase are exclusively related to the doubling time and not to the number of cycling cells or to the number of cells in S phase”
We also considered the possible influence of variations in TS expression during the various phases of the cell cycle. Studies have shown that, in cycling cells, the largest variations in TS values occur during the transition from the G1 to the S phase.11,12 Therefore, different percentages of S phase cells in asynchronously growing cell lines might conceivably influence the amount of TS protein. For this reason, we measured the quantitative variation in the cell cycle phases in the CHP212, SW620, and LoVo cell lines using two parameter flow cytometry for TS protein and DNA content. In these three cell lines, characterised by different doubling times, the ratios between the TS values of cells in the S–G2/M phase and those in the G0/G1 phase were 1.45, 1.53, and 1.59, respectively, and the percentages of cells in the S phase were 28.3%, 22.8%, and 22.7%, respectively. These data excluded the possibility that the different TS values observed among the 10 cancer cell lines might have been related to different numbers of cells in S–G2/M phase. Our data are consistent with those reported by Pestalozzi and colleagues13 on quantitative TS changes in the cell cycle phases, indicating that no more than onefold variations in TS protein values can be found between the G0/G1 and the S–G2/M phases.
Take home messages.
Both the expression and activity of the thymidylate synthase (TS) protein in asynchronously growing cancer cells are significantly related to the cell doubling time—the faster the cell proliferates the greater the expression and activity of TS
Thus, TS expression and activity can be regarded as a parameter of the cell proliferation rate
This might explain why TS values are of prognostic value—tumours expressing high amounts of TS protein, being very rapidly growing, will be more aggressive than those with low TS protein values, which are characterised by a low proliferation rate
It might also explain why tumours with high TS values benefit more from chemotherapy, which is more effective in rapidly growing tumours than in tumours with a low growth rate
Taken together, our results clearly indicate that in asynchronously proliferating cells, the expression and activity of TS are exclusively related to the doubling time and not to the number of cycling cells or to the number of cells in S phase. This association might be the result of differences in the duration of S phase among the cell lines examined. In the CHP212, SW620, and LoVo cell lines, the proportions of cells in the S phase were practically the same, thus indicating an S phase duration proportional to the doubling time. The faster the proliferation, the shorter the S phase. Therefore, rapidly proliferating cells have to synthesise a greater amount of DNA (and consequently a greater quantity of TS must be present) per time unit than slowly proliferating ones. The TS protein expression and/or activity value can therefore be regarded as a parameter of the cell proliferation rate.
These data can explain the contradictory observations that, on the one hand, cancers with high TS protein expression are associated with a worse clinical outcome,4 yet on the other hand benefit more from 5-FU based chemotherapy.9,10 The tumour growth rate is an established prognostic parameter of cancer lesions: the faster the growth of the tumour mass, the worse the disease prognosis. If we consider TS protein expression to be a parameter of the cell proliferation rate, as our results indicate, it is obvious that tumours expressing high amounts of TS protein, being very rapidly growing, will be more aggressive than those with low TS protein values, which are characterised by a low proliferation rate. This is also the case regardless of the adjuvant treatment. However, in contrast, there is evidence that chemotherapy is greatly influenced by cancer cell kinetics: it is more effective in rapidly growing tumours than in tumours with a low growth rate. Once again, considering that tumours with high TS values are more rapidly proliferating than those with low TS values, it is not surprising that adjuvant 5-FU based chemotherapy resulted in a significant improvement in disease prognosis for patients with high TS values but not for those with low TS values.
Acknowledgments
This work was supported by Grants from the Ministero della Ricerca Scientifica e Tecnologica (MURST) 40 and 60%, Pallotti’s Legacy for Cancer Research, and the University of Bologna (funds for selected research topics). The authors are grateful to Mr RMT Cooke for editing.
Abbreviations
BSA, bovine serum albumin
5-FU, 5-fluorouracil
LI, labelling index
PBS, phosphate buffered saline
SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis
TS, thymidylate synthase
REFERENCES
- 1.Danenberg PV. Thymidylate synthetase—a target enzyme in cancer chemotherapy. Biochim Biophys Acta 1977;473:73–92. [DOI] [PubMed] [Google Scholar]
- 2.Danenberg PV, Langenbach RJ, Heidelberger C. Structures of reversible and irreversible complexes of thymidylate synthetase and fluorinated pyrimidine nucleotides. Biochemistry 1974;13:926–33. [DOI] [PubMed] [Google Scholar]
- 3.Moertel CG. Chemotherapy of gastrointestinal cancer. N Engl J Med 1978;299:1049–52. [DOI] [PubMed] [Google Scholar]
- 4.van Triest B, Peters GJ. Thymidylate synthase: a target for combination therapy and determinant of chemotherapeutic response in colorectal cancer. Oncology 1999;57:179–94. [DOI] [PubMed] [Google Scholar]
- 5.Peters GJ, van der Wilt CL, van Triest B, et al. Thymidylate synthase and drug resistance. Eur J Cancer 1995;31A:1299–305. [DOI] [PubMed] [Google Scholar]
- 6.Yamachika T, Nakanishi H, Inada K, et al. A new prognostic factor for colorectal carcinoma, thymidylate synthase, and its therapeutic significance. Cancer 1998;82:70–7. [PubMed] [Google Scholar]
- 7.Edler D, Kressner U, Ragnhammar P, et al. Immunohistochemically detected thymidylate synthase in colorectal cancer: an independent prognostic factor of survival. Clin Cancer Res 2000;6:488–92. [PubMed] [Google Scholar]
- 8.Edler D, Hallstrom M, Johnston PG, et al. Thymidylate synthase expression: an independent prognostic factor for local recurrence, distant metastasis, disease-free and overall survival in rectal cancer. Clin Cancer Res 2000;6:1378–84. [PubMed] [Google Scholar]
- 9.Johnston PG, Fisher ER, Rockette HE, et al. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol 1994;12:2640–7. [DOI] [PubMed] [Google Scholar]
- 10.Takenoue T, Nagawa H, Matsuda K, et al. Relation between thymidylate synthase expression and survival in colon carcinoma, and determination of appropriate application of 5-fluorouracil by immunohistochemical method. Ann Surg Oncol 2000;7:193–8. [DOI] [PubMed] [Google Scholar]
- 11.Haurani FI, Kardinal CG, Biermann WA. Thymidylate synthetase and dihydrofolic acid reductase in the stimulated human lymphocyte. J Cell Physiol 1978;95:49–55. [DOI] [PubMed] [Google Scholar]
- 12.Navalgund LG, Rossana C, Muench AJ, et al. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem 1980;255:7386–90. [PubMed] [Google Scholar]
- 13.Pestalozzi BC, McGinn CJ, Kinsella TJ, et al. Increased thymidylate synthase protein levels are principally associated with proliferation but not cell cycle phase in asynchronous human cancer cells. Br J Cancer 1995;71:1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Triest B, Pinedo HM, van Hensbergen Y, et al. Thymidylate synthase level as the main predictive parameter for sensitivity to 5-fluorouracil, but not for folate-based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell lines. Clin Cancer Res 1999;5:643–54. [PubMed] [Google Scholar]
- 15.Derenzini M, Sirri V, Treré D, et al. The quantity of nucleolar proteins nucleolin and protein B23 is related to cell doubling time in human cancer cells. Lab Invest 1995;73:497–502. [PubMed] [Google Scholar]
- 16.Patterson MK. Measurement of growth and viability of cells in culture. Methods Enzymol 1979;58:141–52. [DOI] [PubMed] [Google Scholar]
- 17.Mirjolet JF, Barberi-Heyob M, Merlin JL, et al. Thymidylate synthase expression and activity: relation to S-phase parameters and 5-fluorouracil sensitivity. Br J Cancer 1998;78:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derenzini M, Treré D, Pession A, et al. Nucleolar size indicates the rapidity of cell proliferation in cancer tissues. J Pathol 2000;191:181–6. [DOI] [PubMed] [Google Scholar]
- 19.Johnston PG, Drake JC, Trepel J, et al. Immunological quantitation of thymidylate synthase using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive and -resistant human cancer cell lines. Cancer Res 1992;52:4306–12. [PubMed] [Google Scholar]
- 20.Gerdes J, Lemke H, Baisch H, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984;133:1710–15. [PubMed] [Google Scholar]