Abstract
Aims: To determine possible changes in gingival crevicular fluid (GCF) antioxidant defence in chronic adult periodontal disease and to investigate the nature of the local radical scavenging mechanisms, with particular reference to glutathione.
Methods: GCF and plasma were collected from patients with chronic periodontitis and age and sex matched control subjects (n = 10). Polymorphonuclear leucocytes (PMNLs) were prepared and gingival epithelial cells (GECs) were collected by conventional methods from periodontally healthy subjects. PMNL were stimulated with F-Met-Leu-Phe after cytochalasin B treatment. Enhanced chemiluminescence was used to determine the total antioxidant capacity and to investigate the activity of cell fractions and reducing agents. GCF concentrations of reduced (GSH) and oxidised (GSSG) glutathione were determined by high performance liquid chromatography.
Results: Plasma and GCF from patients contained lower mean (SD) total antioxidant capacity (501.8 (123) μM Teq/litre and 658.3 (392) μM Teq/litre, respectively) compared with controls (577.9 (99.8) and 1351.5 (861) μM Teq/litre, respectively). Antioxidant light recovery profiles for GCF demonstrated a stepped response, not seen in plasma, which was inhibited by N-ethylmaleimide. This response was also detected in the cytosolic fraction of GEC and anaerobically stimulated PMNL. Similar antioxidant profiles, inhibitable by N-ethylmaleimide, were obtained with cysteamine, cysteine, and GSH. Control GCF contained high mean (SD) concentrations of glutathione (GSH, 1899.8 (494.4)μM; GSSG, 256.8 (152.4)μM). GCF from patients with periodontitis contained significantly lower amounts of GSH (mean, 1183.1; SD, 580.3μM) and GSSG (mean, 150.1; SD, 44.9μM).
Conclusions: GSH values and total antioxidant capacity are reduced in chronic periodontal disease. The high concentrations of GSH present in GCF in health are similar to those found extracellularly in the lung and may represent an important antioxidant and anti-inflammatory defence strategy common to exposed epithelial surfaces.
Destructive periodontal diseases affect 10–15% of the world population,1 and are a major cause of tooth loss. There is increasing evidence that the disease occurs in a predisposed group of the population that has an aberrant inflammatory/immune response to the microbial plaque that accumulates around the gingival margin.2 This exaggerated response is known to result in inadvertent or collateral host tissue damage.
The predominant inflammatory cell (96%) within the healthy connective tissues and epithelium of the gingiva is the polymorphonuclear leucocyte (PMNL).3 In aggressive and chronic forms of periodontitis, PMNLs appear to be functionally activated and exhibit increased production of reactive oxygen species (ROS).2–4 These molecules are reported to be capable of inducing periodontal tissue destruction5 and are associated with osteoclastic bone resorption.6 The degree to which ROS influence the progression of periodontal diseases is as yet unclear, but their role cannot be considered in isolation, given the range of antioxidant species that protects against excess ROS activity and maintains a delicate equilibrium within host tissues.7
The ability of the host to scavenge ROS produced by leucocytes or other cells (for example, fibroblasts) is regarded as a key protective mechanism against inadvertent ROS mediated host tissue damage. This mechanism appears to be crucial in both acute and chronic inflammatory diseases of the lung8 where, similar to periodontal diseases, exposed epithelial surfaces are associated with neutrophilic infiltration within the underlying connective tissues. Interestingly, antioxidant defence in the lungs appears to be unusual in that it is associated with high concentrations (in the 200–400μM range) of extracellular reduced glutathione (GSH). Alterations in antioxidant defence have also been detected in the cervix, with reduced tissue levels being detected in cases of intraepithelial neoplasia.9
“In aggressive and chronic forms of periodontitis, polymorphonuclear leucocytes appear to be functionally activated and exhibit increased production of reactive oxygen species”
The epithelial surfaces most involved in the periodontal diseases are the junctional and crevicular epithelia, which are bathed in a plasma derived fluid, called gingival crevicular fluid (GCF). Although the crevicular epithelium is stratified squamous epithelium, the junctional epithelium has a uniquely differentiated structure,10 which, although separating the microbial plaque biofilm on the tooth surface from the underlying gingival connective tissues, is relatively permeable. GCF continuously flows through this epithelium and enters the gingival crevice at a very slow rate (0.24–1.56 μl/minute at non-inflamed sites).11 Similarly, neutrophils migrate into the crevice by the same route. Therefore, GCF, which can be conveniently collected by non-invasive methods on paper strips, provides a sample whose contents reflect the status of the underlying periodontal tissues.
To date, there has been little published data on the role of free radicals or antioxidants in the pathogenesis of the periodontal diseases. The purpose of our study was to determine possible changes in GCF antioxidant defence in adult periodontal disease using an enhanced chemiluminescent (ECL) assay for total antioxidant capacity,12 and to investigate the nature of the local radical scavenging mechanisms, with particular reference to the presence and role of reduced glutathione.
MATERIALS AND METHODS
Chemicals
Chemicals were obtained from Sigma Aldrich Chemical Co, (Poole, Dorset, UK) unless otherwise stated.
Patients and periodontally healthy controls
Ten patients with clinically advanced stages of periodontal disease (mean age, 46.1 years) were age and sex matched (five men and five women) with periodontally healthy control subjects (mean age, 46.9 years). All volunteers were non-smokers and otherwise in good health, as confirmed by a detailed medical history. Inclusion criteria were the complete absence of vitamin supplements and no use of anti-inflammatory or antibiotic medication in the previous three months. Patients were categorised on the basis of accepted clinical criteria,13 and had mean pocket depths of 2.9 mm (SD, 0.59; range, 1–9) with a mean 21.6% (SD, 8.2%) of sites bleeding on probing. All volunteers gave written informed consent and participants were starved overnight before clinical sampling. Ethical approval for our study was obtained from the south Birmingham local research ethical committee (number LREC 0405).
ECL assay for total antioxidants
Determination of the total antioxidant capacity of biological samples was performed as described previously,12 using a BioOrbit 1250 luminometer (Labtech International, Uckfield, Sussex, UK), interfaced with a PC running software that allows simultaneous recording/display of up to 5000 data points over a period of up to one hour. Antioxidant capacities of test samples were derived from standard curves produced using the water soluble vitamin E analogue, 6-hydroxy-2,5,7,8, tetramethylchroman-2-carboxylic acid (Trolox™; assay range, 0.0625–1.6 nmol). Standard curves were run both before and after test samples. Results were expressed as a concentration (μM Trolox equivalents/litre; Teq/litre) and, for GCF samples, the amount of antioxidant activity for each 30 second sample (nmol/30 second sample).
Biological sample collection and preparation for ECL antioxidant assay
GCF samples were collected on paper strips over 30 seconds (Periopaper™ strips; Oraflow, Plainview, New York, USA), and volumes were measured using a precalibrated Periotron 8000 (Oraflow), as reported previously.14 Samples were then eluted for 30 minutes before strip removal and storage under liquid nitrogen. Strips were eluted into assay buffer (phosphate buffered saline (PBS), pH 7.6, containing 50 mg/litre bovine serum albumin; one strip for each 100 μl) for subsequent antioxidant assay or into 3.5% (vol/vol) perchloric acid (Fischer Scientific, Loughborough, UK; six strips for each 300 μl) transport medium for high performance liquid chromatography (HPLC) analysis.
For the cross sectional study, a total of 12 GCF samples for each subject were collected, with pooled eluates from six strips being used for antioxidant and six strips for HPLC analyses. Filtration experiments to investigate the molecular weight of specific GCF antioxidant species were performed on 12 GCF samples collected from a periodontally healthy volunteer and eluted into a total volume of 1200 μl assay buffer. The eluate was assayed with and without filtration through Microcon 30 kDa (10 000 ×g; 12 minutes), 10 kDa (10 000 ×g; 30 minutes), and 3 kDa (10 000 ×g; 60 minutes) microconcentrators (Amicon Inc, Beverley, Massachusetts, USA) with the unfiltered, control aliquots being centrifuged in Eppendorf tubes at the same time as the filtered samples.
To examine the contribution of thiol groups to the antioxidant activity of GCF, pooled samples from healthy controls (n = 10) were assayed with and without preincubation with N-ethylmaleimide (20nM; five minutes). All assays of GCF eluates were performed on 100 μl sample volumes.
Venous blood was collected from the antecubital fossa into lithium/heparin or plain Vacutainers™. Blood was allowed to stand at room temperature for 30 minutes then either centrifuged immediately (1000 ×g for 30 minutes at 4°C) for separation of plasma or incubated at 4°C for a further 30 minutes before centrifugation for serum (3500 ×g for five minutes at room temperature). Plasma and serum were collected and stored under liquid nitrogen. Antioxidant assays were performed on 20 μl of 1/10 diluted serum or plasma.
Artificial (substitute) GCF was created by diluting serum 1/5 with 0.9% saline to provide a solution with a protein concentration approximating that of GCF (16–20 mg/ml).15,16
GCF collection and preparation for HPLC analysis
GCF samples from six sites in patients and age/sex matched periodontally healthy controls were collected, volumes measured, eluted into 3.5% (vol/vol) perchloric acid transport medium, and stored under liquid nitrogen as described previously. GSH and oxidised glutathione (GSSG) were measured by HPLC using a fluorimetric detector17 after derivatisation with dansyl chloride. The dansylated samples were applied to a Waters HPLC system with a LiChrospher™ 100NH2 (5 μm) column, connected to a compatible pre-column (Merck, Lutterworth, Leicestershire, UK) and fluorescence detector (Jasco FP-920). Each sample contained an internal standard (γ-glu-glu) to verify sample delivery on to the column and derivatisation. Data were collected and processed by Turbochrom 4 (Perkin Elmer, Beaconsfield, Buckinghamshire, UK); concentrations were then determined according to standard curves acquired from a parallel measurement of external standards.
PMNL preparation and stimulation with FMLP
Neutrophils were prepared by density gradient centrifugation18 of peripheral blood samples (50 ml; lithium/heparin) from five healthy volunteers. Briefly, 5 ml aliquots of blood were layered over 1 ml histopaque 1.077 and 3 ml mono-poly resolving medium (ICN Biochemicals, Thame, Oxfordshire, UK) and centrifuged at 500 ×g for 30 minutes. Cell viability (> 99%) was confirmed by trypan blue dye exclusion. Cells were resuspended in RPMI 1640 (8 × 106 cells/ml) and incubated with cytochalasin B (50 μg/ml; 22 μl added to 200 μl PMNL preparation) anaerobically under 90% N2, 10% CO2 for 10 minutes at 37°C. The cells were then stimulated aerobically and anaerobically using F-Met-Leu-Phe (2.2 μl 10μM FMLP in IMS 99 carrier; final concentration 100nM)19 for 15 minutes at 37°C. Activated PMNLs were ultrasonicated and centrifuged at 8000 ×g. The supernatant was removed and the pellet resuspended in 2 ml of RPMI 1640. All samples (5–20 μl) were assayed for ECL antioxidant activity in the presence and absence of 20% serum (5 μl).
Collection of oral epithelial cells
Crevicular epithelial cells were collected from periodontally healthy controls by simple curettage. A Gracey 1/2 “mini five” curette (LM Dental, supplied by JS Davis, Potters Bar, Hertfordshire, UK) was used to curette the crevice epithelium from 25 sites/volunteer. Samples were initially pooled into 500 μl of PBS buffer. Cells were then washed three times with PBS, resuspended in 0.5 ml of assay buffer, and ultrasonicated. After centrifugation at 8000 ×g, the supernatant was removed and the pellet resuspended in 0.5 ml of assay buffer. All samples were stored at 4°C before being assayed for ECL antioxidant activity (5–20 μl aliquots assayed after the addition of 5 μl 20% serum).
ECL assay of PMNL reducing agents and thiol compounds
Titration of various PMNL reducing agents was performed to determine the minimum concentration that eliminated light emission from the assay by > 80%. Taurine (1–30nM), hypotaurine (0.24nM), cysteamine (0.1nM to 1.0μM), L-cysteine (1–5nM), oxidised and reduced glutathione (1nM), β NADPH (0.33nM), and β NADP (0.66nM) were then assayed alone and (with the exception of taurine and hypotaurine) after the addition to 5 μl of 1/5 diluted serum. Assays of cysteine and GSH were performed with and without preincubation with 20nM N-ethylmaleimide (five minutes).
Analysis of data
Data were analysed using Minitab (version 9.0). Significant differences between medians were determined by the Mann Whitney U test.
RESULTS
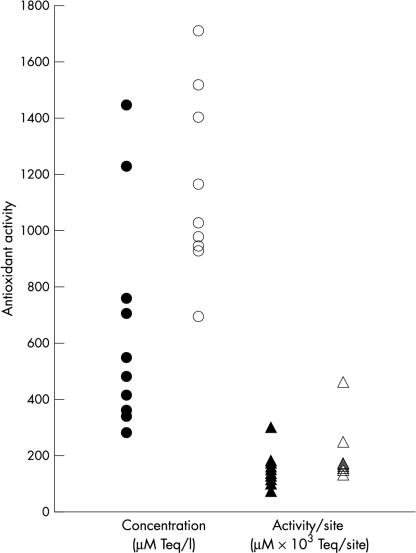
Results from antioxidant assays of the samples from the cross sectional study showed that although mean (SD) plasma concentrations of antioxidant capacity were higher in controls (577.9 (99.8) μM Teq/litre) than patients (501.8 (123) μM Teq/litre), the difference was not significant (p > 0.1). In contrast, GCF samples from controls (mean, 1352.5; SD, 861 μM Teq/litre) contained significantly higher amounts of antioxidant activity (p < 0.015) than those from patients (mean, 658.3; SD, 392 μM Teq/litre; fig 1 ▶). However, when the antioxidant capacity of GCF was calculated for each 30 second sample, the difference between control (mean, 0.2; SD, 0.1 nM/sample) and patient groups (mean, 0.15; SD, 0.06 nM/sample) was not significant (p > 0.15; fig 1 ▶). This finding reflected the higher GCF volumes obtained from patients with advanced periodontal disease (mean, 0.27; SD, 0.11 μl) compared with periodontally healthy controls (mean, 0.16; SD, 0.03 μl; p < 0.012).
Figure 1.
Total antioxidant capacity of gingival crevicular fluid from patients with periodontitis (closed symbols) and healthy controls (open symbols).
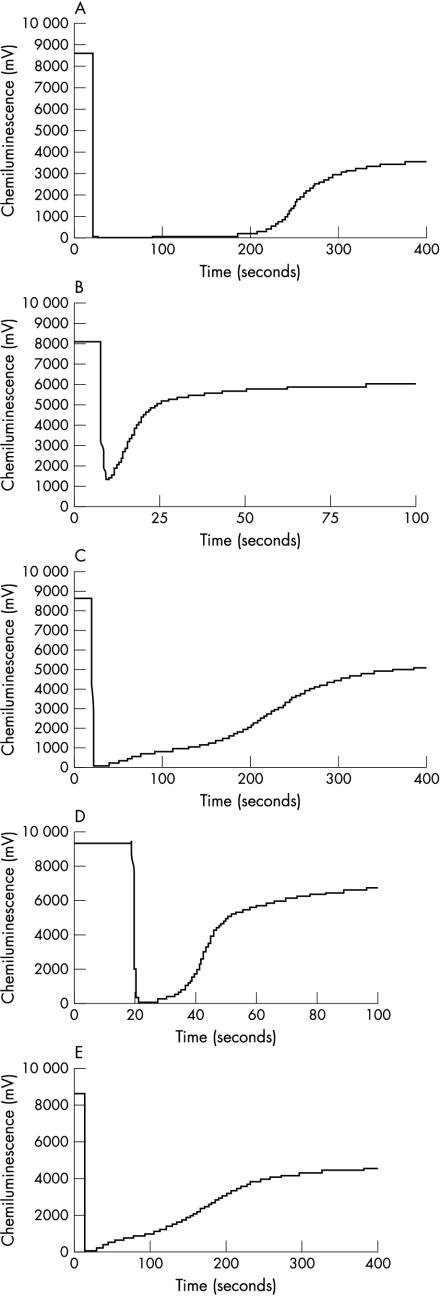
In addition to these quantitative differences in antioxidant capacity between control and patient GCF samples, qualitative differences in the chemiluminescent light recovery curve were seen between GCF and plasma samples (fig 2 ▶). Thus, plasma samples invariably gave a smooth recovery profile when all antioxidant activity had been exhausted (fig 2A ▶). In contrast, GCF samples, particularly those from control subjects, were often characterised by a more complex, stepped, recovery curve (fig 2C ▶). This response could not be reproduced by running highly diluted plasma samples (fig 2B ▶) or Trolox standards (data not shown), suggesting that it was not caused by small sample sizes or low absolute values of antioxidant activity.
Figure 2.
Antioxidant profiles for (A) plasma (1/5 dilution), (B) plasma (1/500 dilution), (C) gingival crevicular fluid (GCF) eluate, (D) GCF eluate treated with N-ethylmaleimide, (E) 3 kDa filtrate of GCF eluate.
These findings indicated that GCF contains a component, or components, with antioxidant properties not detectable in plasma. The most likely sources for this local component would be neutrophils and/or epithelial cells. Therefore, a series of experiments was performed to investigate the effects of neutrophil products and intracellular components on the assay (table 1 ▶). Initial experiments on stimulated PMNLs revealed that measurable antioxidant activity and a stepped recovery curve were only detectable in the cytosolic fraction of anaerobically stimulated PMNLs. Furthermore, the stepped recovery was only detectable when 20% serum was added to the sample, mimicking the situation in GCF. A similar response was seen with the cytosolic fraction of GECs when assayed under the same conditions.
Table 1.
Summary results of AO testing of neutrophil and epithelial cell components in the enhanced chemiluminescence AO assay
| Component tested | Quantity, dilution, and/or volume | Measurable AO activity (% loss caused by NEM) | Recovery curve profile |
| FMLP stimulated PMNL* | |||
| Aerobic | |||
| Supernatant | 5–20 μl | – | – |
| Pellet | 5–20 μl | – | – |
| Anaerobic | |||
| Supernatant | 5–20 μl | + | Stepped |
| Pellet | 5–20 μl | – | – |
| Intracellular reducing agents | |||
| Taurine | 1–30 nM | –† | None |
| Hypotaurine | 0.24 nM | –† | None |
| β NADP* | 0.66 nM | – | – |
| β NADPH* | 0.66 nM | + | Stepped |
| Cysteamine* | |||
| –NEM | 2 nM | + | Stepped |
| +NEM | 2 nM | + (80%) | Smooth |
| L cysteine* | |||
| –NEM | 1 nM | + | Stepped |
| +NEM | 1 nM | + (76%) | Smooth |
| Oxidised glutathione* | 1 nM | – | – |
| Reduced glutathione* | |||
| –NEM | 1 nM | + | Stepped |
| +NEM | 1 nM | + (80%) | Smooth |
| Gingival epithelial cells* | |||
| Supernatant | |||
| –NEM | 5–20μl | + | Stepped |
| +NEM | 5–20 μl | + (78–87%) | Smooth |
| Pellet | 5–20 μl | – | – |
| GCF | |||
| –NEM | 100 μl eluate | + | Stepped |
| +NEM | 100 μl eluate | + (75–80%) | Smooth |
| GCF filtrate | |||
| <30 kDa | 100 μl | + | Stepped |
| <10 kDa | 100 μl | + | Stepped |
| <3 kDa | 100 μl | + | Stepped |
| Plasma | 1/5–1/500 (20 μl) | + | Smooth |
| Serum | 1/5–1/500 (20 μl) | + | Smooth |
Quantity refers to nM added to assay; for FMLP stimulated PMNL and gingival epithelial cells see materials and methods for details of original cell numbers, etc; 100 μl of GCF eluate is equivalent to an entire single GCF sample; GCF filtrates were produced by passage through 30, 10, or 3 kDa Microcon concentrating filters. *Assayed with the addition of 5 μl of 20% serum; †complete light suppression without recovery.
AO, antioxidant; GCF, gingival crevicular fluid; FMLP, F-Met-Leu-Phe; NEM, N-ethylmaleimide; PMNL, polymorphonuclear leucocytes.
In an attempt to identify the nature of the component responsible for the stepped recovery, several intracellular PMNL reducing agents were individually tested for their ability to produce an ECL curve similar to that found in GCF (table 1 ▶). On their own, none of the reducing agents produced an antioxidant response showing a stepped recovery. Both taurine and hypotaurine caused irreversible light suppression in a concentration dependent manner. However, β NADPH, cysteamine, cysteine, and GSH, when assayed in the presence of 20% serum produced antioxidant effects similar to those seen in clinical GCF samples. Except for β NADPH, preincubation of these compounds with N-ethylmaleimide caused a large reduction in their antioxidant activity and complete removal of the stepped recovery curve (table 1 ▶).
To demonstrate biological relevance, N-ethylmaleimide blocking studies were performed on pooled GCF samples (fig 2C,D). These demonstrated that preincubation with N-ethylmaleimide completely removed the stepped recovery profile, indicating that thiol groups were involved in this effect. The effect on T10% values (and therefore antioxidant capacity) was a reduction of between 75% and 80% (T10% is the time taken for the light signal to recover to 10% of its initial peak value (once all antioxidants have been exhausted)). Filtration studies using Microcon microconcentrators showed that the thiol component within GCF was of low molecular weight (< 3 kDa; table 1 ▶; fig 2E ▶) and, of the molecules tested, could be cysteamine, cysteine, or GSH.
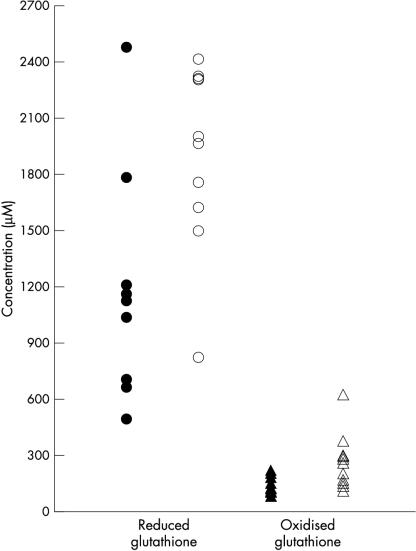
HPLC analysis of GCF samples from patients with periodontitis and controls demonstrated mean GSH concentrations in the millimolar range (fig 3 ▶). GSH concentrations were significantly lower in patients with periodontal disease (mean, 1183.1; SD, 580.3μM) relative to control subjects (mean, 1899.8; SD, 494.4μM; p < 0.022). The concentrations of GSSG in the GCF of both patients and controls were significantly lower than those of GSH (p = 0.0002). Furthermore, GCF concentrations of both GSSG and total glutathione were significantly lower in patients (mean, 150.1; SD, 44.9μM GSSG; mean, 1483; SD, 650μM total) compared with control subjects (mean, 256.8; SD, 152.4μM GSSG; p < 0.026; mean, 2431; SD, 627μM total; p < 0.015).
Figure 3.
Concentrations of reduced (open and closed circles) and oxidised (open and closed triangles) glutathione in gingival crevicular fluid from patients with periodontitis (closed symbols) and healthy controls (open symbols).
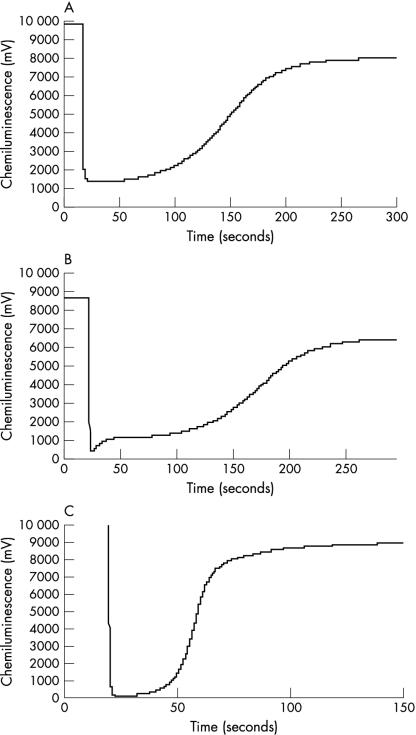
To confirm that the concentrations of GSH detected in GCF would produce changes in the antioxidant profile seen, GSH was added to 20% human plasma in saline to give a final concentration equivalent to the mean values detected by HPLC in GCF from healthy subjects. The results (fig 4A–C ▶) demonstrated antioxidant profiles that were both qualitatively and quantitatively similar to clinical GCF samples. As with GCF samples, prior blocking with N-ethylmaleimide eliminated the stepped recovery profile.
Figure 4.
Antioxidant profiles for reduced glutathione (1nM) in (A) saline, (B) 20% serum, and (C) 20% serum containing N-ethylmaleimide.
DISCUSSION
This is the first reported investigation of possible differences in GCF antioxidant capacity between periodontal health and disease. The results from this cross sectional study show that local, GCF total antioxidant capacity is significantly decreased in patients with periodontal disease compared with periodontally healthy controls. Furthermore, this local decrease was reflected systemically by lower mean antioxidant capacity in plasma from patients with periodontitis. That alterations in antioxidant capacity might be detectable both locally and systemically is supported by recent studies indicating that chronic periodontal disease is associated with peripheral neutrophils that are hyper-reactive with respect to the production of ROS in response to Fcγ stimulation.2,4,20 Although these data suggest that lowered antioxidant defence is associated with periodontal disease, they do not indicate whether this compromised antioxidant capacity is important in pathogenesis, or is essentially a consequence of hyper-reactive neutrophils and/or ROS mediated tissue damage within the periodontal tissues.
These studies have confirmed previous observations12 that the antioxidant light recovery profile obtained with GCF, irrespective of patient group, was qualitatively different from that seen with plasma, and was often characterised by a stepped response. This suggested that GCF contained a locally derived antioxidant not present in plasma. The three most likely local sources of such a component were plaque bacteria, neutrophils, and junctional/crevicular epithelium. Preliminary studies of the antioxidant profiles of washed plaque samples and periodontal bacteria (Fusobacterium nucleatum, Veillonella parvula, and Streptococcus sanguis) indicated that the flora within the gingival crevice were not the source of this novel component (data not shown). By contrast, cytosolic extracts of crevicular epithelial cells and anaerobically stimulated neutrophils elicited the stepped recovery curve characteristic of GCF. This response was also mimicked by cysteamine, cysteine, and GSH, and has also been reported in epithelial curettage samples from the uterine cervix.9
Data from the HPLC analyses indicate that GSH is the local component within GCF responsible for the stepped antioxidant response. Thus, GSH was detected in GCF at concentrations (range, 0.5–2.5mM) that were 1000 fold higher than that normally found within extracellular tissue compartments,21 and that could qualitatively and quantitatively reproduce the stepped recovery in in vitro experiments. Importantly, GCF concentrations of GSH were lower in subjects with periodontitis than in healthy controls, reflecting differences in total antioxidant values between the two groups. These findings are similar to those obtained in studies of GSH in inflammatory diseases of the lung. Alveolar epithelial lining fluid (ELF) contains high concentrations of GSH (0.4mM),22 with values being raised in smokers and deficient in patients suffering from pulmonary fibrosis23 and acute respiratory distress syndrome.24
The junctional epithelium of the gingival crevice is similar in its relative location and function to the alveolar epithelium in the lungs, where ELF rather than GCF bathes and protects the lung epithelium. The implications of the reported findings relate to the potential role of GSH within secretions/exudates that bathe epithelial lined surfaces exposed to the external environment. Both the lung and periodontium are subject to neutrophilic inflammation in response to microbial insult, within an environment high in activating cytokines produced by the lining epithelium and underlying fibroblasts and inflammatory cells. Normally, intracellular concentrations of GSH are high (0.5–10mM) but extracellular fluid values are low (0.5–5μM in human plasma). The high concentrations of GSH found in GCF and ELF are likely to influence the regulation of proinflammatory cytokines, involved in the processes that lead to host tissue damage.7 This is of potential importance in the pathobiology of such inflammatory disease processes. There is evidence that increasing cytosolic cysteine (and thus GSH) concentrations within monocytes and macrophages (using the synthetic form of cysteine, N-acetylcysteine) block ROS mediated activation of the transcription factor nuclear factor κB) NF-κB and the subsequent upregulation of proinflammatory cytokine production.25
“Data from the high performance liquid chromatography analyses indicate that reduced glutathione is the local component within gingival crevicular fluid responsible for the stepped antioxidant response”
Although the source of the high concentrations of GSH (and GSSG) in GCF remains to be elucidated, it is likely that neutrophils and/or junctional/crevicular epithelium are major contributors. It is interesting to speculate that GSH can be added to the list of defence factors in the gingival crevice and lung known to be epithelial products (for example, β defensins26 and migration inhibitory factor 827). Whether glutathione release is secondary to cell death or the result of some active secretory mechanism must also form the focus of future work. Indeed, the mechanisms underlying reduced concentrations of GSH in disease states relative to health may involve an inborn defect in the γ-glutamyl pathway of GSH synthesis (normally controlled by a complex of cellular factors),28 or other endogenous or exogenous influences such as the local microbiota. It has been shown that certain putative periodontal pathogens (Peptostreptococcus micros, Fusobacterium nucleatum fusiforme, F nucleatum polymorphum, F nucleatum vincentii, and F nucleatum nucleatum) readily degrade GSH to form hydrogen sulphide.29 The formation of hydrogen sulphide within the periodontal pocket is toxic to mammalian cells by inactivating cytochrome oxidase30 and is also reported to inhibit catalase.31 The latter activity could positively feed back into NF-κB mediated transcription of proinflammatory cytokines. The degradation of cysteine or GSH by oral microbiota may also prevent the inhibition of NF-κB mediated transcription of proinflammatory cytokines in the periodontal environment, thereby increasing the risk of cytokine related tissue damage.
Take home messages.
Plasma and gingival crevicular fluid (GCF) from patients contained lower total antioxidant capacity than controls
GCF from patients with periodontitis contained significantly lower amounts of oxidised and reduced glutathione (GSH)
The high concentrations of GSH present in GCF in health are similar to those found extracellularly in the lung and may represent an important antioxidant and anti-inflammatory defence strategy common to exposed epithelial surfaces
More work to investigate the source, function, and regulation of lining fluid GSH may help determine new therapeutic strategies based upon these apparent local disease associated reductions in concentrations
These results indicate that the gingival crevice is similar to the lung in that it is normally bathed in fluid containing high concentrations of GSH, which is known to have powerful anti-inflammatory (anti-proinflammatory cytokine) and antioxidant activity. Furthermore, at both sites, GSH concentrations are reduced in patients who suffer from chronic inflammatory diseases, where normal tissue homeostatic mechanisms fail to protect against host mediated tissue damage. The lower concentrations of glutathione (reduced, oxidised, and total) in the GCF of patients with periodontitis could be the result of a variety of factors leading to decreased synthesis and/or enhanced local degradation. Whatever the reason, these observations have important implications for the pathogenesis and treatment of periodontal disease. Additional work aimed at investigating the source, function, and regulation of lining fluid GSH may help determine new therapeutic strategies based upon these apparent local disease associated reductions in concentrations.
Acknowledgments
This work was supported in part by a project grant from Unilever Dental Research. We would also like to acknowledge the generous support of Professor S Socransky of the Forsyth Research Centre, Boston, USA, where the initial studies were performed, and the stimulating discussions with Professor S Socransky, Dr A Haffajee, and Dr S Dibart.
Abbreviations
ELF, alveolar epithelial lining fluid
ECL, enhanced chemiluminescent
FMLP, F-Met-Leu-Phe
GCF, gingival crevicular fluid
GEC, gingival epithelial cell
GSH, reduced glutathione
GSSG, oxidised glutathione
HPLC, high performance liquid chromatography
NF-κB, nuclear factor κB
PBS, phosphate buffered saline
PMNL, polymorphonuclear leucocyte
ROS, reactive oxygen species
REFERENCES
- 1.Brown LJ, Löe H. Prevalence, extent, severity and progression of periodontal disease. Periodontol 2000 1993;2:57–71. [DOI] [PubMed] [Google Scholar]
- 2.Fredriksson M, Gustafsson A, Åsman B, et al. Hyper-reactive peripheral neutrophils in adult periodontitis: generation of chemiluminescence and intracellular hydrogen peroxide after in-vitro priming and FcγR stimulation. J Clin Periodontol 1998;25:394–8. [DOI] [PubMed] [Google Scholar]
- 3.Kowashi Y, Jaccard F, Cimasoni G. Sulcular polymorphonuclear leucocytes and gingival exudate during experimental gingivitis in man. J Periodontol Res 1980;15:151–8. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson A, Åsman B. Increased release of free oxygen radicals from peripheral neutrophils in adult periodontitis after Fcγ-receptor stimulation. J Clin Periodontol 1996;23:38–44. [DOI] [PubMed] [Google Scholar]
- 5.Bartold PM, Wiebkin OW, Thonard JC. The effect of oxygen-derived free radicals on gingival proteoglycans and hyaluronic acid. J Periodontol Res 1984;19:390–400. [DOI] [PubMed] [Google Scholar]
- 6.Key LL, Wolfe WC, Gundberg CM, et al. Superoxide and bone resorption. Bone 1994;15:431–6. [DOI] [PubMed] [Google Scholar]
- 7.Chapple ILC. The role of free radicals and antioxidants in the pathogenesis of the inflammatory periodontal diseases. Mol Pathol 1996;49:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris PE, Bernard GR. Significance of glutathione in lung disease and implications for therapy. Am J Med Sci 1994;307:119–27. [DOI] [PubMed] [Google Scholar]
- 9.Cope G, Thorpe G, Holder R, et al. Serum and tissue antioxidant capacity in cervical intraepithelial neoplasia investigated using an enhanced chemiluminescent reaction. Ann Clin Biochem 1999;36:86–93. [DOI] [PubMed] [Google Scholar]
- 10.Gao Z, Mackenzie IC. Patterns of phenotypic expression of human junctional, gingival and reduced enamel epithelia in vivo and in vitro. Epithelial Cell Biol 1992;1:156–67. [PubMed] [Google Scholar]
- 11.Challacombe SJ. Passage of serum immunoglobulins into the oral cavity. In: Lehner, ed. Borderland between caries and periodontal disease, Vol. 11. London: Academic Press, 1980:51–67.
- 12.Chapple ILC, Mason GI, Matthews JB, et al. Enhanced chemiluminescent assay for measuring the total antioxidant capacity of serum, saliva and crevicular fluid. Ann Clin Biochem 1997;34:412–21. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson A, Åsman B, Bergström K. Altered relation between granulocyte elastase and α-2-macroglobulin in gingival crevicular fluid from sites with periodontal destruction. J Clin Periodontol 1994;21:17–21. [DOI] [PubMed] [Google Scholar]
- 14.Chapple ILC, Landini G, Griffiths GS et al. Calibration of the Periotron 8000™ and 6000™ by polynomial regression. J Periodontol Res 1999;34:79–86. [DOI] [PubMed] [Google Scholar]
- 15.Curtis MA, Sterne JAC, Price SJ, et al. The total protein composition of GCF sampled from male adolescents with no destructive periodontitis: baseline data of a longitudinal study. J Periodontol Res 1990;25:6–16. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson A, Asman B, Bergstrom K. Lower protein concentration in GCF from patients with periodontitis: an indicator of host-specific inflammatory reaction. J Clin Periodontol 1995;22:255–8. [DOI] [PubMed] [Google Scholar]
- 17.Martin J, White IN. Fluorimetric determination of oxidised and reduced glutathione in cells and tissues by high-performance liquid chromatography following derivatisation with dansyl chloride. J Chromatogr 1991;568:219–25. [DOI] [PubMed] [Google Scholar]
- 18.Kalmar JR, Arnold RR, Warbington ML, et al. Superior leukocyte separation with a discontinuous one-step Ficoll-Hypaque gradient for the isolation of human neutrophils. J Immunol Methods 1988;110:275–81. [DOI] [PubMed] [Google Scholar]
- 19.Borregaard N, Miller LJ, Springer TA. Chemoattractant-regulated mobilisation of a novel intracellular compartment in human neutrophils. Science 1987;237:1206–8. [DOI] [PubMed] [Google Scholar]
- 20.Fredriksson M, Gustafsson A, Åsman B, et al. Periodontitis increases chemiluminescence of the peripheral neutrophils independently of priming by the preparation method. Oral Dis 1999;5:229–33. [DOI] [PubMed] [Google Scholar]
- 21.Kelly F. Glutathione: in defence of the lung. Food Chem Toxicol 1999;37:963–6. [DOI] [PubMed] [Google Scholar]
- 22.Bernard GR. N-acetylcysteine in experimental and clinical acute lung injury. Am J Med 1991;S3C:54S–9S. [DOI] [PubMed] [Google Scholar]
- 23.Cantin A, North SL, Hubbard RC, et al. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol 1987;63:152–7. [DOI] [PubMed] [Google Scholar]
- 24.Pacht ER, Timerman AP, Lykens MG, et al. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome. Chest 1991;100:1397–403. [DOI] [PubMed] [Google Scholar]
- 25.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 1991;10:2247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dale BA, Krisanaprakornkit S. Defensin antimicrobial peptides in the oral cavity. J Oral Pathol Med 2001;30:321–7. [DOI] [PubMed] [Google Scholar]
- 27.Ross KF, Herzberg MC. Calprotectin expression by gingival epithelial cells. Infect Immun 2001;69:3248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 1999;27:922–35. [DOI] [PubMed] [Google Scholar]
- 29.Carlsson J, Larsen JT, Edlund E-D. Utilization of glutathione (L-γ-glutamyl-L-cysteinylglycine) by Fusobacterium nucleatum subspecies nucleatum. Oral Microbiol Immunol 1993;9:297–300. [DOI] [PubMed] [Google Scholar]
- 30.Nicholls P, Kim KJ. Sulfide as an inhibitor and electron donor for the cytochrome c oxidase system. Can J Biochem 1982;60:613–23. [DOI] [PubMed] [Google Scholar]
- 31.Claesson R, Granlund-Edstedt M, Persson S, et al. Activity of polymorphonuclear leukocytes in the presence of sulfide. Infect Immun 1989;57:2776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]