Abstract
Objectives
This study investigated the longitudinal progression of retinal structure and microvasculature over 3 years in patients with relapsing–remitting multiple sclerosis (RRMS) using optical coherence tomography (OCT) and OCT angiography (OCTA). It also explored the correlation between these changes and the Expanded Disability Status Scale (EDSS) scores.
Methods
In this prospective, longitudinal study, we enrolled 66 patients with RRMS without history of optic neuritis and 124 healthy controls. All participants underwent full ophthalmological examination, OCT/OCTA scans, and disability scoring (EDSS) at baseline and after 12 and 24 months. OCT data were analyzed for retinal layer thickness, while OCTA assessed microvascular perfusion in the retinal capillary plexuses and choriocapillaris. Statistical models evaluated yearly rates of change and their association with EDSS scores.
Results
The patients with RRMS exhibited 3.6 times faster thinning of the inner plexiform layer (IPL; − 0.47 µm per year, P = 0.001) compared to controls over 3 years. Additionally, superficial retinal capillary layer perfusion density decreased more rapidly at − 0.44% per year (P = 0.006) in patients with MS. A strong correlation was found between worsening EDSS scores and accelerated ONL thinning (estimated coefficient: − 1.62 µm/per unit change of EDSS score, P = 0.004).
Discussion
This study demonstrates progressive retinal neurodegeneration and microvascular dysfunction in patients with RRMS without a history of optic neuritis. The association between ONL thinning and increased disability supports the potential of OCT/OCTA as valuable tools for monitoring disease progression and severity in RRMS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-025-12930-7.
Keywords: Relapsing–remitting multiple sclerosis, Optical coherence tomography, Optical coherence tomography angiography, Neurodegeneration, Microvascular dysfunction, Expanded disability status scale
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease that primarily affects the central nervous system (CNS), with demyelination and neuroinflammation being its hallmark features [1]. Over time, neurodegeneration has emerged as a critical contributor to disease progression, often correlating with increasing disability [2]. As part of the CNS, the retina offers a unique, non-invasive window to study MS-related neurodegeneration, given its anatomical and embryological continuity with the brain [3].
Optical coherence tomography (OCT) and OCT angiography (OCTA) have become indispensable tools in MS research due to their ability to detect subtle structural and microvascular changes in the retina with high resolution, reproducibility, and accessibility [4–9]. OCT provides quantitative measurements of retinal layer thickness, particularly the retinal nerve fiber layer (RNFL) and ganglion cell-inner plexiform layer (GCIPL), which are known to reflect axonal and neuronal damage in MS. RNFL and GCIPL thinning have been linked to disease activity and progression, making OCT a valuable biomarker for MS-related neurodegeneration [3, 10–12]. The 2017 McDonald criteria for MS diagnosis do not currently include the optic nerve as a distinct CNS region [13]. However, proposed revisions, presented at the 2024 European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) meeting, suggest adding the optic nerve as a fifth topography for fulfilling dissemination in space criteria [13]. Using OCT to detect optic nerve lesions could facilitate earlier MS diagnosis and therapy initiation, potentially improving long-term outcomes.
OCTA, on the other hand, enables non-invasive imaging of the retinal microvasculature, offering unique insights into MS-related vascular changes. Studies suggest that MS may involve widespread microvascular dysfunction, potentially linked to neuroinflammation, hypoxia, or impaired perfusion [14]. OCTA allows the examination of capillary plexuses, including the superficial capillary plexus (SCP) and deep capillary plexus (DCP), and has revealed parafoveal vessel density (VD) alterations in MS. However, findings remain inconsistent, with some studies reporting increased VD [15], while others document reductions or stability over varying follow-up periods [3, 12, 16, 17].
Despite their promise, prior studies utilizing OCT and OCTA have been limited by small sample sizes and relatively short follow-up periods, often 1–2 years [12, 15–17]. Furthermore, most have focused primarily on the RNFL and GCIPL, leaving the longitudinal changes in other retinal layers and vascular structures largely unexplored. These limitations underscore the need for longer-term studies with robust methodologies to capture the full spectrum of retinal changes in MS.
To address these gaps, we conducted a 3-year longitudinal investigation of retinal structure and microvasculature in patients with relapsing–remitting MS (RRMS) without a history of optic neuritis. By analyzing all ten retinal layers using OCT and perfusion in the SCP, DCP, and choriocapillaris with OCTA, we aimed to elucidate the progressive retinal changes in RRMS and their correlation with clinical disability, as measured by the Expanded Disability Status Scale (EDSS). Additionally, we recruited healthy controls to establish a normative database for age-related retinal changes, providing a reliable baseline for comparison with our MS cohort.
Methods
This study was approved by the Emergency University Hospital Bucharest Institutional Review Board (protocol number 11285) and adhered to the 1964 Declaration of Helsinki. Written informed consent was obtained from all participants before study inclusion.
Study design and patient population
This study was designed as a prospective, longitudinal study, and it was conducted between August 2020 and May 2023. All participants were at least 18 years of age, able to understand, and willing to comply with the procedures and actions outlined in the study protocol.
The participants with MS were included in the study by the treating neurologist based on the 2017 McDonald criteria [18]. EDSS evaluations were performed by a neurologist, and the score ranged from 0 to 10 in 0.5-unit increments, with higher scores representing higher levels of disability [19]. Only participants with RRMS who were on disease-modifying therapies were included. The patients with MS with acute optic neuritis or a history of optic neuritis and/or patients with either primary or secondary progressive MS were excluded from the study. Medical records were screened to determine the disease-modifying therapies used, disease duration, number of relapsing episodes, and a history of optic neuritis.
Normal controls were examined and recruited in the same clinic and were free of neurological and/or other relevant medical conditions. MS patients and healthy controls were excluded from the study if they had a history of ocular surgery, sight-threatening ocular diseases (e.g., glaucoma and age-related macular degeneration), or other ophthalmic pathologies that could affect retinal structure or microvasculature, such as diabetic retinopathy, uveitis, or retinal vein occlusion.
All the participants received a comprehensive ophthalmological examination, including visual acuity using the ETDRS acuity charts, refractive error, intraocular pressure, axial length measurements, slit-lamp examination, and fundoscopy [5]. All participants with MS underwent assessments at baseline, after 12 and 24 months, whereas controls underwent assessments only once at baseline.
OCT/OCTA imaging
All scans were performed by a single trained technician on the same day with the other measurements, using the Cirrus AngioPlex HD-5000 Spectral-Domain OCT (Carl Zeiss Meditec, Inc, Dublin, CA, USA) [20]. Pupils were not dilated before imaging; instead, room lighting was dimmed to achieve pupil dilation. At each visit, each participant received two macula-centered scans for each eye: 512 A-scans × 128 B-scans; 6 × 6 mm OCT scan and 3 × 3 mm OCTA scan [20, 21]. The quality of scans was reviewed by a single trained grader masked to the participant’s characteristics according to Advised Protocol for OCT Study Terminology and Elements (APOSTEL) recommendations and the OSCAR-IB protocol [22, 23]. Briefly, eyes with poor-quality images (signal strength score below 6, movement artifacts causing off-centration, breakages, weak local signal caused by artifacts such as floaters, algorithm failures resulting in segmentation errors), and/or eyes with media transparency impairment and missing variables were excluded from analysis [24–26]. The same trained grader reviewed the quality of all OCTA scans. Briefly, OCTA scans were systematically assessed for alignment, segmentation errors, and artifacts [21, 27]. Eyes were excluded if the OCTA image exhibited a poor signal strength index (< 6), significant motion artifacts (e.g., irregular vessel patterns visible on the en face angiogram), local weak signal caused by artifacts such as floaters, or errors due to misalignment or incorrect segmentation.
Extraction of OCT measurements
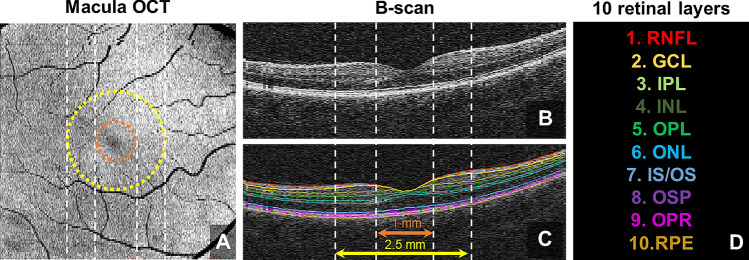
We segmented ten individual retinal layers: RNFL; ganglion cell layer (GCL); inner plexiform layer (IPL); inner nuclear layer (INL); outer plexiform layer (OPL); outer nuclear layer (ONL); photoreceptor inner/outer segments (IS/OS); outer segment of photoreceptors (OSP); outer segment photoreceptor/retinal pigment epithelium complex (OPR); retinal pigment epithelium (RPE) from the macular OCT images using the Iowa Reference Algorithm (Retinal Image Analysis Lab, Iowa Institute for Biomedical Imaging, Iowa City, IA, USA) (Fig. 1). Average retinal thickness was calculated within an annulus centered on the fovea with an inner diameter of 1.0 mm and an outer diameter of 2.5 mm.
Fig. 1.
Framework of optical coherence tomography (OCT) image processing; A annulus mask applied onto macular OCT image for analysis; B and C corresponding B-scans, showing the surface of each retinal layer; D color code for ten retinal layers: retinal nerve fiber layer (RNFL); ganglion cell layer (GCL); inner plexiform layer (IPL); inner nuclear layer (INL); outer plexiform layer (OPL); outer nuclear layer (ONL); photoreceptor inner/outer segments (IS/OS); outer segment of photoreceptors (OSP); outer segment photoreceptor/retinal pigment epithelium complex (OPR); retinal pigment epithelium (RPE)
Extraction of OCTA measurements
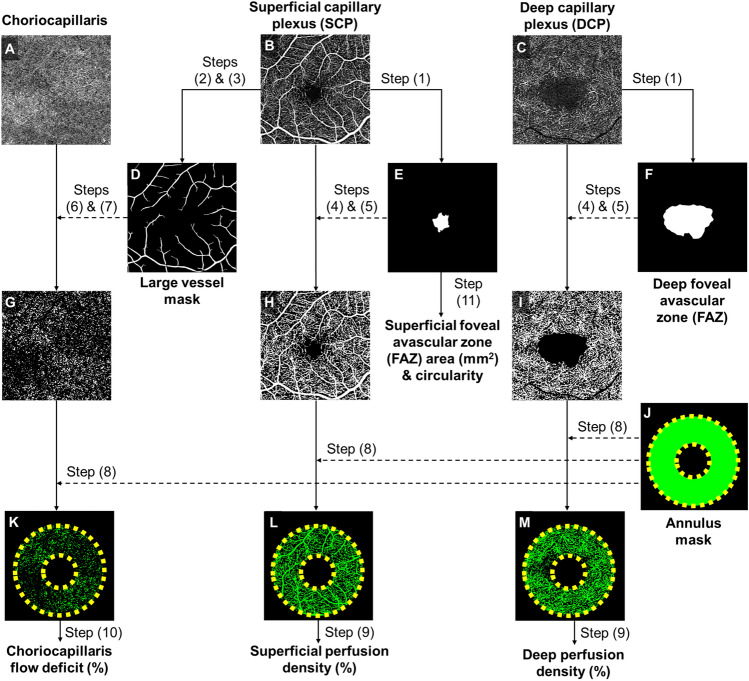
Each OCTA scan was automatically segmented into the SCP, DCP, and choriocapillaris using the Cirrus Review Software (Carl Zeiss Meditec, version 11.0.0.29946) [21, 28]. The SCP spans the inner limiting membrane (ILM) to the IPL, while the DCP spans the INL to the OPL [29]. The choriocapillaris spans from 31 µm below the RPE to 40 µm below the RPE [30]. Images were checked by a technician using the review software to ensure correct segmentation, and no manual adjustment was needed. Projection artifacts from the overlying retinal circulation were removed from the DCP using the software integrated with the instrument.
OCTA images of the three slabs were subsequently loaded into a customized MATLAB (The MathWorks Inc., Natick, MA) algorithm to extract the OCTA measurements (Fig. 2). The OCTA processing framework comprised the following steps: (1) outlining the foveal avascular zone (FAZ) border manually on the SCP and DCP slabs, (2) enhancing the contrast of the large vessels on the SCP with a combination of Hessian and Gabor filters, (3) then subsequently binarizing it to produce a large vessel mask; (4) binarization by setting a threshold at the mean intensity on the SCP and DCP slabs, (5) after which masking of FAZ regions. (6) Large vessel artifacts were removed from the choriocapillaris slab, and (7) flow deficits were then binarized by setting a threshold that was 1 standard deviation below the mean intensity of the respective OCTA images. Analysis (8) with a fovea centered annulus (inner diameter 1.0 mm, outer diameter 2.5 mm) was performed to obtain the results (Fig. 2).
Fig. 2.
Framework of optical coherence tomography angiography (OCTA) image post-processing. A–C Raw OCTA images extracted from the OCTA machine. D Large vessels segmented and binarized from the superficial capillary plexus (SCP). E–F Foveal avascular zones (FAZ) are manually delineated from SCP and deep capillary plexus (DCP). G Choriocapillaris flow deficits binarized from the OCTA image by applying a mask to remove large vessel artifacts. H, I Vessels binarized from the SCP and DCP. FAZ regions were masked from the binarized images. J Fovea-centered annulus mask has an inner diameter of 1.0 mm and an outer diameter of 2.5 mm. K Choriocapillaris flow deficit density is obtained within the annulus mask. L, M Perfusion density is obtained by binarizing vascular images with an annulus mask. Dotted lines represent the overlay of masks
The evaluation of perfusion density was computed as the percentage of perfused area per total imaged area. Area and circularity of FAZ in the SCP were computed, where the circularity of the FAZ refers to the ratio between the perimeter of the FAZ and the perimeter of an equivalent circle (i.e., circle of the same area). The circularity value ranges from 0.0 to 1.0. A value closer to 0.0 indicates an irregular shape of the FAZ, while a value closer to 1.0 indicates a more circular shape. For the choriocapillaris slab, the evaluation was based on the absence of flow signals. Flow deficit density was computed as the percentage of flow deficit area per total imaged area.
Statistical analysis
Both eyes of each participant were included in this study according to the eligibility criteria described. The primary outcome variables were the yearly rates of change in OCT and OCTA measurements, as well as their association with EDSS scores. To assess the normal distribution of the variables, the Shapiro–Wilk test was employed. Descriptive statistics were calculated as mean ± standard deviation for normally distributed data, and median [interquartile range (IQR)] for non-normally distributed data. All statistical analyses were performed using R version 4.0.4. A P value less than 0.05 was considered statistically significant.
To examine the yearly rates of change in retinal structure and microvasculature in participants with MS, a mixed-effects linear regression model was used, adjusting for baseline age, scan signal strength at each visit, and within-participant and within-eye correlations. The association between changes in retinal structure and microvasculature (independent variables) and EDSS scores (dependent variable) was evaluated using a similar mixed-effects model, adjusting for baseline age, signal strength of scans at each visit, follow-up period, within-participant, and within-eye correlations.
Retinal thinning can be influenced by age-related factors. Therefore, it is essential to compare retinal thinning in patients with MS to age-matched controls during follow-up. In our study, we estimated normal age-related retinal decline using a multivariate linear regression analysis of cross-sectional data. This approach helped to minimize the impact of non-disease factors, enhancing our ability to identify subtle MS-related changes. To establish normal aging patterns, a control group of 124 healthy individuals with ages ranging from 18 to 79 was recruited. We applied a multivariate linear regression model to evaluate the annual rate of change in OCT/OCTA measurements within the control group. This model was designed to quantify the relationship between participants’ age and retinal structural or microvascular parameters, allowing us to estimate the expected rate of decline associated with normal aging. By incorporating age as the primary independent variable, the model provided an estimate of the annual change in each retinal parameter, represented by the regression coefficient. The regression model adjusted for potential confounders, including sex, signal strength (to account for image quality), and inter-eye correlation (to account for the non-independence of measurements between eyes). A negative beta coefficient in the model was interpreted as an age-related decline in the measured parameters, providing a baseline for understanding normal aging patterns in retinal and choroidal structures.
EDSS changes were calculated as the difference between the EDSS score obtained at the third visit and the EDSS score obtained at the first visit (ΔEDSS = EDSS at Visit 3—EDSS at Visit 1). Visits were spaced approximately 1 year apart over 2 years. Pearson correlation coefficients were calculated to assess the strength and direction of the relationships between ONL thickness and each OCTA measurement.
Results
Initially, 123 participants with MS were recruited for the study (Supplementary Fig. 1). Of these, 33 were excluded, because they missed one of the follow-up visits, and 24 were excluded because of poor-quality scans in both eyes. Finally, we analyzed the data of 66 patients (101 eyes), where 35 of them had good-quality scans in both eyes, and 31 had good-quality scans in one eye.
Table 1 presents the demographic and clinical characteristics of the participants with MS, stratified by three yearly visits. The mean follow-up period was 25.21 ± 2.83 months. Patient’s mean age was 40 ± 12 years, 61% were female (n = 40), and the mean disease duration was 12 ± 7 years. At baseline (Visit 1), the median EDSS score was 2.0 (IQR: 1.5–3.0), with a mean of 2.25 ± 0.73. EDSS scores increased significantly over visits, with a mean of 2.36 ± 0.76 at Visit 2 and 2.39 ± 0.82 at Visit 3 (β = 0.077 units/year; P < 0.001). The mean change in EDSS from baseline was 0.11 ± 0.39 and 0.14 ± 0.43, respectively.
Table 1.
Comparison of demographic and clinical characteristics stratified by visits
| Demographic or clinical characteristic | Visit 1 | Visit 2 | Visit 3 | P value* |
|---|---|---|---|---|
| Number of patients, eyes | 66, 101 | 66, 101 | 66, 101 | |
| Age, years (mean ± SD) | 40 ± 12 | 41 ± 11 | 43 ± 12 | |
| Sex, n(%) | ||||
| Male | 26 (39%) | |||
| Female | 40 (61%) | |||
| Diabetes | 1 (1.5%) | |||
| Hypertension | 2 (3%) | |||
| Hyperlipidemia | 4 (6%) | |||
| Duration of MS, years (mean ± SD) | 12 ± 7 | |||
| EDSS (median (interquartile range)) | 2.0 (1.5–3.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | < 0.001 |
| EDSS (mean ± SD) | 2.25 ± 0.73 | 2.36 ± 0.76 | 2.39 ± 0.82 | < 0.001 |
| Number of attacks prior to baseline | 4.4 ± 5.8 | |||
| Attack-free during the 3-year follow-up | ||||
| Attack-free | 48 (73%) | |||
| Ever attack | 18 (27%) | |||
| Number of attacks during the three visits | 1.8 ± 1.2 | |||
| Disease-modifying therapies | ||||
| Glatiramer acetate | 25 (38%) | |||
|
Subcutaneous interferon β−1a Teriflunomide Interferon β−1b |
14 (21%) 8 (12%) 7 (11%) |
|||
| Intramuscular interferon β−1a | 5 (8%) | |||
| Natalizumab | 4 (6%) | |||
|
Fingolimod Dimethyl fumarate Ocrelizumab |
1 (1.5%) 1 (1.5%) 1 (1.5%) |
EDSS expanded disability status scale
*A mixed-effects linear regression model was used to assess whether there was a statisticallysignificant trend in EDSS across the visits, adjusting for within-participant correlation
A sample (n = 124) of healthy reference participants was also analyzed to model the rates of normal, age-related changes in the retinal structure and microvasculature. Of these normal controls, 61% (n = 76) were female and had a mean age of 42 ± 13 years, extending from 18 to 79 years. Their age composition was 29 or younger (n = 31; 25%), 30–39 (n = 18; 15%), 40–49 (n = 31; 25%), 50–59 (n = 25; 20%), 60–69 (n = 14; 11%), and 70 or older (n = 5; 4%). The median (IQR) signal strength was 9 (8–10) for OCT scans and 10 (9–10) for OCTA scans.
Table 2 details the changes in OCT/OCTA measurements across the three visits in participants with MS. Although there was a slight decline in OCT image quality over the three visits, from the median (IQR) of 10 (9–10) on the first visit to 9 (8–10) on the third visit (P = 0.013), the overall scan quality remained exceptionally high. After adjusting for baseline age, disease duration, signal strength, and inter-eye correlation, IPL (− 0.47 µm per year, P = 0.001) showed a significant thinning over time in participants with MS. The rate of thinning in the IPL was 3.6 times faster than the normal, age-related changes of the IPL (− 0.13 µm per year, P < 0.001). However, ONL (− 0.31 µm per year, P = 0.100) is not significant.
Table 2.
Changes in OCT/OCTA measurements across three yearly visits
| Visit 1 | Visit 2 | Visit 3 | MS-related change (µm per year) | P value* | Age-related change (µm per year) | P value* | |
|---|---|---|---|---|---|---|---|
| OCT measurements | |||||||
| Retinal nerve fiber layer, µm | 22.15 ± 4.01 | 21.96 ± 3.06 | 22.09 ± 3.71 | − 0.06 (− 0.28–0.16) | 0.618 | 0.01 (− 0.03–0.05) | 0.586 |
| Ganglion cell layer, µm | 45.36 ± 9.66 | 45.08 ± 10.06 | 45.47 ± 9.71 | − 0.06 (− 0.45–0.33) | 0.754 | − 0.14 (− 0.21–0.07) | < 0.001 |
| Inner plexiform layer, µm | 36.89 ± 3.77 | 36.66 ± 3.36 | 35.67 ± 3.71 | − 0.47 (− 0.71–0.22) | 0.001 | − 0.13 (− 0.16–0.10) | < 0.001 |
| Inner nuclear layer, µm | 38.41 ± 4.11 | 38.61 ± 3.82 | 38.69 ± 4.24 | 0.09 (− 0.09–0.27) | 0.347 | − 0.02 (− 0.05–0.03) | 0.457 |
| Outer plexiform layer, µm | 28.84 ± 3.64 | 28.39 ± 3.74 | 28.37 ± 3.48 | − 0.23 (− 0.49–0.03) | 0.079 | 0.02 (− 0.02–0.06) | 0.384 |
| Outer nuclear layer, µm | 96.81 ± 8.05 | 96.95 ± 8.23 | 95.77 ± 8.34 | − 0.31 (− 0.68–0.06) | 0.100 | − 0.24 (− 0.35–0.14) | < 0.001 |
| Photoreceptor inner/outer segments, µm | 10.30 ± 0.40 | 10.27 ± 0.43 | 10.25 ± 0.39 | − 0.02 (− 0.04–0.01) | 0.218 | 0.02 (0.01–0.03) | < 0.001 |
| Outer segment of photoreceptors, µm | 15.18 ± 3.16 | 14.88 ± 2.76 | 15.41 ± 3.27 | − 0.02 (− 0.30–0.26) | 0.895 | 0.11 (0.07–0.15) | < 0.001 |
| Outer segment photoreceptor/retinal pigment epithelium complex, µm | 21.85 ± 3.73 | 22.31 ± 3.54 | 22.10 ± 3.74 | 0.31 (0.01–0.61) | 0.048 | − 0.09 (− 0.13–0.05) | < 0.001 |
| Retinal pigment epithelium, µm | 14.93 ± 0.28 | 14.97 ± 0.29 | 14.95 ± 0.29 | 0.01 (− 0.01–0.02) | 0.574 | − 0.01 (− 0.01–0.01) | 0.003 |
| Signal strength of OCT scan† | 10 (9–10) | 9 (8–10) | 9 (8–10) | 0.013 | 9 (8–10) |
| OCTA measurements | MS-related change (% or mm2 per year) | P value* | Age-related change (% or mm2 per year) | P value* | |||
|---|---|---|---|---|---|---|---|
| Superficial perfusion density, % | 42.31 ± 2.46 | 41.44 ± 2.54 | 41.23 ± 2.47 | − 0.44 (− 0.75–0.13) | 0.006 | − 0.04 (− 0.07–0.01) | 0.016 |
| Deep perfusion density, % | 38.21 ± 4.10 | 38.87 ± 3.76 | 38.46 ± 3.75 | 0.09 (− 0.15–0.33) | 0.453 | − 0.13 (− 0.17–0.09) | < 0.001 |
| Choriocapillaris flow deficits density, % | 16.64 ± 1.16 | 16.69 ± 1.26 | 16.60 ± 1.08 | − 0.01 (− 0.14–0.11) | 0.870 | 0.03 (0.01–0.04) | 0.001 |
| Foveal avascular zone at superficial layer, area, mm2 | 0.29 ± 0.10 | 0.31 ± 0.10 | 0.31 ± 0.10 | 0.01 (0.00–0.02) | < 0.001 | 0.01 (− 0.01–0.01) | 0.100 |
| Foveal avascular zone at superficial layer, circularity | 1.17 ± 0.15 | 1.16 ± 0.15 | 1.16 ± 0.08 | − 0.00 (− 0.02–0.01) | 0.681 | 0.01 (− 0.01–0.01) | 0.194 |
| Signal strength of OCTA scan† | 10 (9–10) | 10 (9–10) | 10 (9–10) | 0.358 | 10 (9–10) |
MS multiple sclerosis, OCT optical coherence tomography, OCTA OCT angiography
*A mixed-effects linear regression model was used to assess the change in OCT/OCTA measurements over visits, adjusting for baseline age, disease duration, scan signal strength at each visit, and within-participant and within-eye correlations
†Values are presented as median (interquartile range) as signal strength did not meet the assumptions of normality, as assessed by the Shapiro–Wilk test
The OCTA image quality remained stable throughout the three examinations, with a consistent signal strength of 10 (9–10) (P = 0.358; Table 2). Among the participants with MS, only superficial retinal perfusion density showed a significant decline over time at − 0.44% per year (P = 0.006) after adjusting for baseline age, signal strength, and inter-eye correlation. This rate of decline was 11 times faster than the normal, age-related change (− 0.04% per year, P = 0.016).
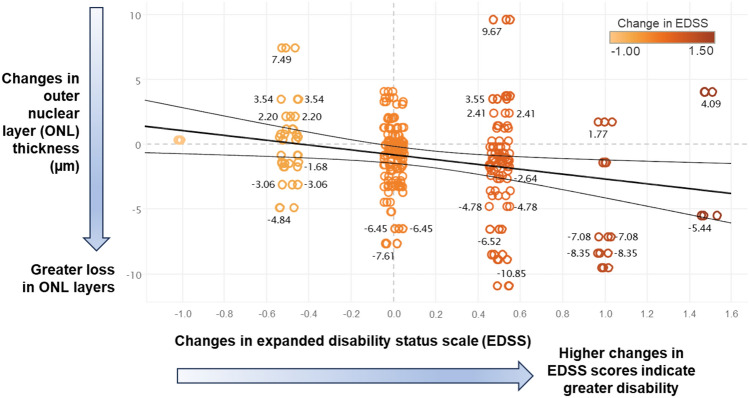
Table 3 explores the relationship between changes in EDSS scores and OCT/OCTA measurements over time. Of all the parameters, we found a statistically significant connection between higher EDSS scores and thinning of the ONL (estimated coefficient: − 1.62 µm/per unit change of EDSS score, P = 0.004) after adjusting for baseline age, signal strength, and inter-eye correlation. In other words, for every unit increase in the EDSS score (worse disability), the ONL layer thins by an average of 1.62 µm. Figure 3 further illustrates the relationship between worsening disability scores in patients with MS and ONL thinning in their retina.
Table 3.
Relationship between changes in OCT/OCTA measurements and changes in EDSS scores
| Estimated coefficients (per unit change of EDSS score; 95% confidence intervals) | P value* | |
|---|---|---|
| OCT measurements | ||
| Retinal nerve fiber layer, µm | − 0.47 (− 1.09–0.15) | 0.139 |
| Ganglion cell layer, µm | − 0.74 (− 1.94–0.43) | 0.220 |
| Inner plexiform layer, µm | − 0.33 (− 0.97–0.31) | 0.311 |
| Inner nuclear layer, µm | − 0.23 (− 0.78–0.31) | 0.412 |
| Outer plexiform layer, µm | 0.54 (− 0.14–1.22) | 0.122 |
| Outer nuclear layer, µm | − 1.62 (− 2.73–0.53) | 0.004 |
| Photoreceptor inner/outer segments, µm | − 0.06 (− 0.12–0.01) | 0.081 |
| Outer segment of photoreceptors, µm | 0.14 (− 0.45–0.74) | 0.640 |
| Outer segment photoreceptor/retinal pigment epithelium complex, µm | − 0.14 (− 0.85–0.56) | 0.695 |
| Retinal pigment epithelium, µm | 0.03 (− 0.02–0.08) | 0.202 |
| OCTA measurements | ||
| Superficial perfusion density, % | 0.21 (− 0.26–0.68) | 0.384 |
| Deep perfusion density, % | 0.20 (− 0.49–0.89) | 0.567 |
| Choriocapillaris flow deficits density, % | 0.15 (− 0.10–0.41) | 0.247 |
| Foveal avascular zone at superficial layer, area, mm2 | 0.00 (− 0.01–0.02) | 0.620 |
| Foveal avascular zone at superficial layer, Circularity | − 0.03 (− 0.06–0.00) | 0.061 |
EDSS expanded disability status scale, OCT optical coherence tomography, OCTA OCT angiography
*A mixed-effects linear regression model was used to assess the change in OCT/OCTA measurements over the change in EDSS, adjusting for baseline age, disease duration, the signal strength of scans at each visit, follow-up period, and within-participant, and within-eye correlations
Fig. 3.
A significant association can be seen between worsening disability scores [Expanded Disability Status Scale (EDSS)] and a faster rate of thinning in the outer nuclear layer (ONL) of the retina in patients with relapsing–remitting multiple sclerosis
We analyzed the relationship between global ONL thickness and OCTA parameters (Table 4). In MS individuals, significant positive correlations were found between global ONL thickness and superficial perfusion density (ρ = 0.236, P = 0.002) as well as FAZ area (ρ = 0.174, P = 0.025). However, no significant correlations were observed between ONL thickness and deep perfusion density or choriocapillaris flow deficits.
Table 4.
Correlation of OCTA measurements with global outer nuclear layer thickness
| OCTA measurements | Pearson correlation coefficients | P value |
|---|---|---|
| Superficial perfusion density, % | 0.236 | 0.002 |
| Deep perfusion density, % | − 0.115 | 0.140 |
| Choriocapillaris flow deficits density, % | − 0.004 | 0.958 |
| Foveal avascular zone at superficial layer, Area, mm2 | 0.174 | 0.025 |
| Foveal avascular zone at superficial layer, Circularity | − 0.058 | 0.460 |
Discussion
Our study provides a comprehensive analysis of structural changes in all ten retinal layers and microvascular alterations in all three capillary plexuses over 3 years in patients with MS without a history of optic neuritis. We found that retinal neurodegeneration and microvascular dysfunction are accelerated in patients with MS, even before the onset of optic neuritis. This suggests that the MS disease process, which primarily affects the CNS, also has early implications for the eyes. Additionally, we observed a correlation between the thinning of the ONL and the EDSS scores, indicating the potential of OCT as a valuable tool for assessing disease progression and monitoring treatment responses.
Retinal structural changes in patients with MS without a history of optic neuritis
Our study observed significantly accelerated rates of thinning in the IPL (3.6 times faster) of the retina in patients with MS without a history of optic neuritis, compared to age-related changes. These findings suggest that the inflammatory and demyelinating processes characteristic of MS may drive neurodegenerative changes in the retina. The IPL, rich in synapses between inner retinal neurons, appears particularly susceptible to damage in MS [31]. Interestingly, a longitudinal study [32] with over 800 participants reported that IPL thinning preceded the progression of MS, suggesting a strong link between synaptic loss and functional decline, including cognitive impairment and visual disturbances.
Interestingly, we observed a significant association between worsening EDSS and ONL thinning over 3 years, while the overall rate of ONL thinning across the cohort was not statistically significant. These findings reflect distinct aspects of retinal changes in MS. The rate of ONL thinning over time represents the average yearly change across the cohort. The non-significant result (− 0.31 µm per year, P = 0.100) suggests that the temporal change in ONL thickness was small and variable, making it challenging to detect a consistent thinning trend over 3 years. This finding aligns with the previous studies reporting variability in ONL changes in MS cohorts [33, 34]. Such variability likely reflects the heterogeneous nature of MS progression, where some participants experience substantial retinal changes, while others remain stable. In contrast, the significant association between worsening EDSS and ONL thinning (− 1.62 µm/per unit change of EDSS score, P = 0.004) highlights the relationship between disability progression and ONL changes at the individual level. This result indicates that individuals with greater disability progression (worsening EDSS) are more likely to experience pronounced ONL thinning, regardless of the overall cohort trend. Notably, ONL thinning may not occur uniformly over time but could be influenced by episodic disease activity, such as relapses or subclinical inflammation, which are better captured by measures of disability progression.
The ONL houses the photoreceptor nuclei and is likely susceptible to inflammatory insults, potentially contributing to the visual disturbances experienced by some patients with RRMS [35]. Retinal inflammation in MS, characterized by microglial activation and the release of inflammatory cytokines, may disrupt the microenvironment required for photoreceptor cell survival, ultimately leading to ONL atrophy [36, 37]. This aligns with the broader understanding of MS-related neurodegeneration in the CNS, where chronic inflammation drives progressive damage to neuronal structures [1, 2]. Based on these parallels, we speculate that the MS-related inflammatory status in the retina contributes to ONL atrophy, mirroring the process of CNS neurodegeneration observed in MS.
Our study found that the RNFL and GCL remained stable in patients with MS over 3 years. However, in healthy controls, we observed a significant age-related decline in the GCL. This aligns with the previous research that showed no significant changes in RNFL in patients with MS, but found RNFL thinning in healthy controls [38]. Other studies on MS have reported both decreases [3, 10, 39] and stability [11, 12, 40] in RNFL and GCL thickness. The lack of significant decline in RNFL and GCL in our patients with MS might be due to factors such as the duration of the disease, the specific type of MS, and the presence of other health conditions. Our findings suggest that measuring RNFL and GCL thickness alone may not be enough for accurately tracking the progression of MS.
Our study did not find significant changes in the INL of the retina in patients with RRMS without a history of optic neuritis. However, previous studies have suggested that INL changes may be more noticeable in the early stages of MS, especially in patients with higher inflammatory burden [34, 41]. Additionally, a study that included patients with both RRMS and PPMS observed more pronounced INL changes [34]. We believe that this difference might be due to the generally higher inflammatory burden in patients with progressive MS. By focusing on patients with RRMS without a history of optic neuritis, we aimed to study a group with a lower inflammatory burden.
Currently, assessing disease progression in MS primarily relies on the EDSS, which can be influenced by subjective individual and external factors. This can make it challenging to accurately track disease progression [42]. Objective retinal measurements from OCT offer a promising alternative. Previous studies have primarily focused on changes in the RNFL [43] and GCIPL [44, 45]. Our 2-year follow-up study, however, revealed a significant correlation between worsening disability in patients with RRMS and thinning of the ONL, suggesting that ONL measurements could be a valuable tool for monitoring MS progression. While previous studies did not examine the ONL due to limitations in commercial OCT devices, our findings highlight the potential of using OCT devices to measure ONL thinning as a more accurate and objective marker of disease progression.
Retinal microvascular changes in patients with MS without a history of optic neuritis
Our longitudinal study examined retinal blood flow in patients with MS over 3 years. We discovered a significant decline in superficial retinal perfusion density (− 0.44% per year) compared to the expected age-related decrease (− 0.04% per year). Deep retinal perfusion remained stable.
Previous studies with similar follow-up periods have shown similar reductions in superficial perfusion [3, 12, 16], but inconsistencies exist for the DCP, which can be decreased [12, 16] or unaffected [3] over time. These inconsistencies likely stem from methodological variations, including a lack of healthy controls, small sample sizes (patients with MS = 15–27 [12, 17]; healthy controls = 15–46 [12, 15–17]), different disease forms[12, 15], and diverse OCT devices (Optovue Angiovue [12, 15]; Heidelberg [3, 16]; Cirrus [17]).
Crucially, our study observed an increase in the superficial FAZ area, aligning with the decreased perfusion. This suggests a potential loss of blood vessels. Our findings support our previous hypothesis, based on a cross-sectional study [21], that ongoing microvascular changes occur in the retina of patients with MS.
The correlation analysis revealed a significant positive association between superficial perfusion density and ONL thickness, suggesting that superficial vascular integrity is relevant to ONL preservation or degeneration. Given that only ONL thinning was significantly linked to EDSS (a measure of disability progression in MS), the observed vascular dropout in superficial perfusion density could be interpreted as a vascular response to neuronal cell loss in the ONL, reflecting reduced metabolic demand. This hypothesis is supported by evidence in other conditions where retinal vascular reduction occurs in response to neurodegenerative structural changes.
In our earlier studies, we observed that our MS patients exhibited thinner RNFL, thinner GCL, higher superficial perfusion density, and lower deep perfusion density compared to healthy controls [5, 20, 21]. Whether baseline retinal measures influence disease progression remains an important question. Patients with thinner RNFL or GCL at baseline may experience faster disability progression (e.g., worsening EDSS). While beyond the scope of this study, we plan further analyses to explore the prognostic value of baseline retinal changes in predicting MS progression.
Strengths and limitations
The strengths of this study include the longitudinal prospective design, with a well-phenotype cohort of patients with RRMS who were diagnosed according to internationally accepted criteria [18]. We included only patients with MS without a history of optic neuritis to prevent a bias associated with optic nerve direct damage, since optic neuritis is related to retinal changes [46]. Additionally, the comprehensive retinal analysis, which included all ten retinal layers and three capillary plexuses, provides a detailed understanding of retinal changes in patients with MS.
The limitations of this study are related to the small number of participants, which restricts the data's applicability to the entire MS population. A significant number of participants were excluded, because they missed one of the follow-up visits or because of poor-quality scans in both eyes. Furthermore, the results are limited to the Cirrus device. Another limitation is the absence of MRI data to investigate the relationship between brain atrophy, EDSS, OCT, and OCTA parameters.
Conclusions
This study suggests progressive retinal neurodegeneration and microvascular dysfunction in RRMS patients without a history of optic neuritis. Additionally, faster rates of ONL thinning may correlate with increased disability. Superficial retinal perfusion density also showed a more rapid decline in patients with MS, supporting the theory that there is an adverse association between retinal capillaries and MS disability. These findings suggest that OCT/OCTA could be a valuable ocular imaging device for monitoring disease progression and severity in patients with RRMS patients without a history of optic neuritis.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CNS
Central nervous system
- DCP
Deep capillary plexus
- ECTRIMS
European committee for treatment and research in multiple sclerosis
- EDSS
Expanded disability status scale
- GCL
Ganglion cell layer
- ILM
Inner limiting membrane
- INL
Inner nuclear layer
- IPL
Inner plexiform layer
- IS/OS
Photoreceptor inner/outer segments
- MS
Multiple sclerosis
- OCT
Optical coherence tomography
- OCTA
Optical coherence tomography angiography
- ONL
Outer nuclear layer
- OPL
Outer plexiform layer
- OPR
Outer segment photoreceptor/retinal pigment epithelium complex
- OSP
Outer segment of photoreceptors
- PPMS
Primary-progressive multiple sclerosis
- RNFL
Retinal nerve fiber layer
- RPE
Retinal pigment epithelium
- RRMS
Relapsing–remitting multiple sclerosis
- SCP
Superficial capillary plexus
- SD
Standard deviation
- VD
Vessel density
Author’s contribution
Jacqueline Chua, Alina Popa-Cherecheanu, and Leopold Schmetterer had full access to all the data in the study and are responsible for the integrity of the data and the accuracy of the data analysis. Study concept and design: Jacqueline Chua, Mihai Bostan, Alina Popa-Cherecheanu, and Leopold Schmetterer. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: Mihai Bostan, Jacqueline Chua, and Leopold Schmetterer. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Jacqueline Chua. Obtained funding: Jacqueline Chua and Leopold Schmetterer. Administrative, technical, or material support: all authors. Study supervision: Jacqueline Chua, Alina Popa-Cherecheanu, and Leopold Schmetterer.
Funding
This work was funded by grants from the National Medical Research Council (OFLCG/004c/2018-00; MOH-000249-00; MOH-000647–00; MOH-001001-00; MOH-001015–00; MOH-000500-00; MOH-000707-00; MOH-001072-06; MOH-001286-00), National Research Foundation Singapore (NRF2019-THE002-0006 and NRF-CRP24-2020-0001), Agency for Science, Technology and Research (A20H4b0141), Khoo Bridge Funding Award (Duke-NUS-KBrFA/2024/088), and the Singapore Eye Research Institute and Nanyang Technological University [SERI-NTU Advanced Ocular Engineering (STANCE) Program].
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
Declarations
Conflicts of interest
The authors declare no competing interests.
Contributor Information
Alina Popa-Cherecheanu, Email: alina_cherecheanu@yahoo.com.
Jacqueline Chua, Email: jacqueline.chua.y.m@seri.com.sg.
References
- 1.Oh J, Vidal-Jordana A, Montalban X (2018) Multiple sclerosis: clinical aspects. Curr Opin Neurol 31:752–9 [DOI] [PubMed] [Google Scholar]
- 2.Petzold A, Balcer L, Calabresi PA, Costello F, Frohman T, Frohman E, Martinez-Lapiscina EH, Green A, Kardon R, Outteryck O, Paul F, Schippling S, Vermersch P, Villoslada P, Balk L, Aktas O, Albrecht P, Ashworth J, Asgari N, Black G, Boehringer D, Behbehani R, Benson L, Bermel R, Bernard J, Brandt A, Burton J, Calabresi P, Calkwood J, Cordano C, Courtney A, Cruz-Herranz A, Diem R, Daly A, Dollfus H, Fasser C, Finke C, Frederiksen J, Garcia-Martin E, Suárez IG, Pihl-Jensen G, Graves J, Havla J, Hemmer B, Huang SC, Imitola J, Jiang H, Keegan D, Kildebeck E, Klistorner A, Knier B, Kolbe S, Korn T, LeRoy B, Leocani L, Leroux D, Levin N, Liskova P, Lorenz B, Preiningerova JL, Martínez-Lapiscina EH, Mikolajczak J, Montalban X, Morrow M, Nolan R, Oberwahrenbrock T, Oertel FC, Oreja-Guevara C, Osborne B, Papadopoulou A, Ringelstein M, Saidha S, Sanchez-Dalmau B, Sastre-Garriga J, Shin R, Shuey N, Soelberg K, Toosy A, Torres R, Vidal-Jordana A, Waldman A, White O, Yeh A, Wong S, Zimmermann H (2017) Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 16(10):797–812 [DOI] [PubMed] [Google Scholar]
- 3.Romahn EF, Wiltgen T, Bussas M, Aly L, Wicklein R, Noll C, Berthele A, Dehmelt V, Mardin C, Zimmer C, Korn T, Hemmer B, Kirschke JS, Mühlau M, Knier B (2023) Association of retinal vessel pathology and brain atrophy in relapsing–remitting multiple sclerosis. Front Immunol. 14:1284986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostan M, Pirvulescu R, Tiu C, Bujor I, Popa-Cherecheanu A (2023) OCT and OCT-A biomarkers in multiple sclerosis—review. Rom J Ophthalmol 67(2):107–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostan M, Li C, Sim YC, Bujor I, Wong D, Tan B, Ismail MB, Garhöfer G, Tiu C, Pirvulescu R, Schmetterer L, Popa-Cherecheanu A, Chua J (2023) Combining retinal structural and vascular measurements improves discriminative power for multiple sclerosis patients. Ann NY Acad Sci 1529(1):72–83 [DOI] [PubMed] [Google Scholar]
- 6.Khader S, Nawar A, Ghali A, Ghoneim A (2021) Evaluation of optical coherence tomography angiography findings in patients with multiple sclerosis. Indian J Ophthalmol 69(6):1457–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farci R, Carta A, Cocco E, Frau J, Fossarello M, Diaz G (2020) Optical coherence tomography angiography in multiple sclerosis: a cross-sectional study. PLoS One 15:e0236090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cennamo G, Carotenuto A, Montorio D, Petracca M, Moccia M, Melenzane A, Tranfa F, Lamberti A, Spiezia AL, Servillo G, De Angelis M, Petruzzo M, Criscuolo C, Lanzillo R, Brescia Morra V (2020) Peripapillary vessel density as early biomarker in multiple sclerosis. Front Neurol. 11:542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Gameiro GR, Liu Y, Lin Y, Hernandez J, Deng Y, Gregori G, Delgado S, Wang J (2020) Visual function and disability are associated with increased retinal volumetric vessel density in patients with multiple sclerosis. Am J Ophthalmol 213:34–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saidha S, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD, Prince JL, Pham D, Roy S, Van Zijl P, Balcer LJ, Frohman EM, Reich DS, Crainiceanu C, Calabresi PA (2015) Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 78(5):801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patil SA, Joseph B, Tagliani P, Sastre-Garriga J, Montalban X, Vidal-Jordana A, Galetta SL, Balcer LJ, Kenney RC (2023) Longitudinal stability of inter-eye differences in optical coherence tomography measures for identifying unilateral optic nerve lesions in multiple sclerosis. J Neurol Sci. 449:120669 [DOI] [PubMed] [Google Scholar]
- 12.Montorio D, Lanzillo R, Carotenuto A, Petracca M, Moccia M, Criscuolo C, Spiezia AL, Lamberti A, Perrotta F, Pontillo G, Cennamo G, Morra VB (2021) Retinal and choriocapillary vascular changes in early stages of multiple sclerosis: a prospective study. J Clin Med. 10(24):5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidal-Jordana A, Rovira A, Calderon W, Arrambide G, Castilló J, Moncho D, Rahnama K, Collorone S, Toosy AT, Ciccarelli O, Papadopoulou A, Cerdá-Fuertes N, Lieb JM, Ruggieri S, Tortorella C, Gasperini C, Bisecco A, Capuano R, Gallo A, de Barros A, Salerno A, Auger C, Sastre-Garriga J, Tintore M, Montalban X (2024) Adding the optic nerve in multiple sclerosis diagnostic criteria: a longitudinal, prospective, multicenter study. Neurology. 102(1):e200805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua J, Tan B, Wong D, Garhöfer G, Liew XW, Popa-Cherecheanu A, Loong Chin CW, Milea D, Li-Hsian Chen C, Schmetterer L (2024) Optical coherence tomography angiography of the retina and choroid in systemic diseases. Prog Retin Eye Res. 103(May):101292 [DOI] [PubMed] [Google Scholar]
- 15.Lanzillo R, Cennamo G, Moccia M, Criscuolo C, Carotenuto A, Frattaruolo N, Sparnelli F, Melenzane A, Lamberti A, Servillo G, Tranfa F, de Crecchio G, Brescia MV (2019) Retinal vascular density in multiple sclerosis: a 1-year follow-up. Eur J Neurol 26(1):198–201 [DOI] [PubMed] [Google Scholar]
- 16.Noll C, Hiltensperger M, Aly L, Wicklein R, Afzali AM, Mardin C, Gasperi C, Berthele A, Hemmer B, Korn T, Knier B (2022) Association of the retinal vasculature, intrathecal immunity, and disability in multiple sclerosis. Front Immunol. 13:997043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, Zhou WX, Sun X, Zhang X, Xiao H, Yang H, Lin H, Lu Y, Liu Z, Qiu W, Kermode AG, Yang X, Wang Y (2024) Combination of serum markers with optical coherence tomography angiography for evaluating neuromyelitis optica spectrum disorders and multiple sclerosis. Mult Scler Relat Disord. 85:105478 [DOI] [PubMed] [Google Scholar]
- 18.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoré M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–73 [DOI] [PubMed] [Google Scholar]
- 19.Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–52 [DOI] [PubMed] [Google Scholar]
- 20.Chua J, Bostan M, Li C, Sim YC, Bujor I, Wong D, Tan B, Yao X, Schwarzhans F, Garhöfer G, Fischer G, Vass C, Tiu C, Pirvulescu R, Popa-Cherecheanu A, Schmetterer L (2022) A multi-regression approach to improve optical coherence tomography diagnostic accuracy in multiple sclerosis patients without previous optic neuritis. NeuroImage Clin 34:103010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostan M, Chua J, Sim YC, Tan B, Bujor I, Wong D, Garhöfer G, Tiu C, Schmetterer L, Popa-Cherecheanu A (2022) Microvascular changes in the macular and parafoveal areas of multiple sclerosis patients without optic neuritis. Sci Rep 12:13366. 10.1038/s41598-022-17344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, Petzold A (2012) The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 7:e34823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aytulun A, Cruz-Herranz A, Aktas O, Balcer LJ, Balk L, Barboni P, Blanco AA, Calabresi PA, Costello F, Sanchez-Dalmau B, DeBuc DC, Feltgen N, Finger RP, Frederiksen JL, Frohman E, Frohman T, Garway-Heath D, Gabilondo I, Graves JS, Green AJ, Hartung HP, Havla J, Holz FG, Imitola J, Kenney R, Klistorner A, Knier B, Korn T, Kolbe S, Krämer J, Lagrèze WA, Leocani L, Maier O, Martínez-Lapiscina EH, Meuth S, Outteryck O, Paul F, Petzold A, Pihl-Jensen G, Preiningerova JL, Rebolleda G, Ringelstein M, Saidha S, Schippling S, Schuman JS, Sergott RC, Toosy A, Villoslada P, Wolf S, Yeh EA, Yu-Wai-Man P, Zimmermann HG, Brandt AU, Albrecht P (2021) APOSTEL 2.0 recommendations for reporting quantitative optical coherence tomography studies. Neurology. 97(2):68–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua J, Tan B, Ke M, Schwarzhans F, Vass C, Wong D, Nongpiur ME, Wei Chua MC, Yao X, Cheng CY, Aung T, Schmetterer L (2020) Diagnostic ability of individual macular layers by spectral-domain OCT in different stages of glaucoma. Ophthalmol Glaucoma. 3(5):314–326 [DOI] [PubMed] [Google Scholar]
- 25.Chua J, Schwarzhans F, Nguyen DQ, Tham YC, Sia JT, Lim C, Mathijia S, Cheung C, Tin A, Fischer G, Cheng CY, Vass C, Schmetterer L (2020) Compensation of retinal nerve fibre layer thickness as assessed using optical coherence tomography based on anatomical confounders. Br J Ophthalmol. 104(2):282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chua J, Hu Q, Ke M, Tan B, Hong J, Yao X, Hilal S, Venketasubramanian N, Garhöfer G, Cheung CY, Wong TY, Chen CLH, Schmetterer L (2020) Retinal microvasculature dysfunction is associated with Alzheimer’s disease and mild cognitive impairment. Alzheimer’s Res Ther 12(1):161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua J, Chin CWL, Hong J, Chee ML, Le TT, Ting DSW, Wong TY, Schmetterer L (2019) Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J Hypertens 37(3):572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bujor I, Chua J, Tan B, Iancu R, Pirvulescu R, Geamanu A, Bostan M, Toma E, Ionescu D, Schmetterer L, Popa-Cherecheanu A (2024) Comparing optical coherence tomography angiography metrics in healthy chinese and caucasian adults. J Pers Med 14(8):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeld PJ, Durbin MK, Roisman L, Zheng F, Miller A, Robbins G, Schaal KB, Gregori G (2016) ZEISS angioplex™ spectral domain optical coherence tomography angiography: technical aspects. Dev Ophthalmol 56:18–29 [DOI] [PubMed] [Google Scholar]
- 30.Lin E, Ke M, Tan B, Yao X, Wong D, Ong L, Schmetterer L, Chua J (2020) Are choriocapillaris flow void features robust to diurnal variations? A swept-source optical coherence tomography angiography (OCTA) study. Sci Rep. 10(1):11249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahabadi N, Al Khalili Y. Neuroanatomy, Retina [Internet]. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545310/; Available from: https://www.ncbi.nlm.nih.gov/books/NBK545310 [PubMed]
- 32.Cordano C, Werneburg S, Abdelhak A, Bennett DJ, Beaudry-Richard A, Duncan GJ, Oertel FC, Boscardin WJ, Yiu HH, Jabassini N, Merritt L, Nocera S, Sin JH, Samana IP, Condor Montes SY, Ananth K, Bischof A, Oksenberg J, Henry R, Baranzini S, Wilson M, Bove R, Cuneo R, Gupta S, Sabatino J, Guo J, Sacco S, Papinutto N, Hollenbach J, Gelfand J, Pleasure S, Zamvil S, Goodin D, Waubant E, Gomez R, Cerono G, Nourbakhsh B, Hauser SL, Cree BAC, Emery B, Schafer DP, Chan JR, Green AJ (2024) Synaptic injury in the inner plexiform layer of the retina is associated with progression in multiple sclerosis. Cell Reports Med. 5(4):101490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sotirchos ES, Gonzalez Caldito N, Filippatou A, Fitzgerald KC, Murphy OC, Lambe J, Nguyen J, Button J, Ogbuokiri E, Crainiceanu CM, Prince JL, Calabresi PA, Saidha S (2020) Progressive multiple sclerosis is associated with faster and specific retinal layer atrophy. Ann Neurol. 87(6):885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gernert JA, Böhm L, Starck M, Buchka S, Kümpfel T, Kleiter I, Havla J (2023) Inner retinal layer changes reflect changes in ambulation score in patients with primary progressive multiple sclerosis. Int J Mol Sci. 24(16):12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIlwaine G, Csincsik L, Coey R, Wang L, Fitzgerald D, Moffat J, Dubis AM, McDonnell G, Hughes S, Peto T, Lengyel I (2023) Reduced cone density is associated with multiple sclerosis. Ophthalmol Sci. 3(3):100308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wooff Y, Man SM, Aggio-Bruce R, Natoli R, Fernando N (2019) IL-1 family members mediate cell death, inflammation and angiogenesis in retinal degenerative diseases. Front Immunol 10:1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi S, Guo L, Cordeiro MF (2021) Retinal and brain microglia in multiple sclerosis and neurodegeneration. Cells. 10(6):1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huseyinoglu N, Ozben S, Ekinci M, Buyukuysal C, Yildirim M, Safak H, Huseyin H (2013) Optical coherence tomography in patients with relapsing–remitting multiple sclerosis without optic neuritis: a 20-month longitudinal study. Neuro-Ophthalmol 37(3):104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Louzi OA, Bhargava P, Newsome SD, Balcer LJ, Frohman EM, Crainiceanu C, Calabresi PA, Saidha S (2016) Outer retinal changes following acute optic neuritis. Mult Scler 22(3):362–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Mujaini AS, Al-Mujaini MS, Sabt BI (2021) Retinal nerve fiber layer thickness in multiple sclerosis with and without optic neuritis: a four-year follow-up study from Oman. BMC Ophthalmol. 21(1):1–6. 10.1186/s12886-021-02158-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordano C, Yiu HH, Oertel FC, Gelfand JM, Hauser SL, Cree BAC, Green AJ (2021) Retinal INL thickness in multiple sclerosis: a mere marker of neurodegeneration? Ann Neurol 89:192–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piri Çinar B, Güven Yorgun Y (2018) What we learned from the history of multiple sclerosis measurement: expanded disability status scale. Noropsikiyatri Arsivi. 55:23343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Martin E, Ara JR, Martin J, Almarcegui C, Dolz I, Vilades E, Gil-Arribas L, Fernandez FJ, Polo V, Larrosa JM, Pablo LE, Satue M (2017) Retinal and optic nerve degeneration in patients with multiple sclerosis followed up for 5 years. Ophthalmology 124(5):688–696 [DOI] [PubMed] [Google Scholar]
- 44.Lambe J, Fitzgerald KC, Murphy OC, Filippatou AG, Sotirchos ES, Kalaitzidis G, Vasileiou E, Pellegrini N, Ogbuokiri E, Toliver B, Luciano NJ, Davis S, Fioravante N, Kwakyi O, Risher H, Crainiceanu CM, Prince JL, Newsome SD, Mowry EM, Saidha S, Calabresi PA (2021) Association of spectral-domain OCT with long-term disability worsening in multiple sclerosis. Neurology 96(16):e2058–e2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toscano S, Chisari CG, Biondi A, Patti F (2024) Early reduction of retinal thickness predicts physical and cognitive disability in newly diagnosed multiple sclerosis patients: results from a cross-sectional study. Neurol Sci. 45(11):5385–94. 10.1007/s10072-024-07664-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee GI, Park KA, Oh SY, Min JH, Kim BJ (2021) Peripapillary and parafoveal microvascular changes in eyes with optic neuritis and their fellow eyes measured by optical coherence tomography angiography: an exploratory study. Acta Ophthalmol 99(3):288–298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.