Abstract
Non-essential amino acids are often overlooked in biomedical research; however, they are crucial components of organismal metabolism. One such metabolite that is integral to physiological function is serine. Serine acts as a pivotal link connecting glycolysis with one-carbon and lipid metabolism, as well as with pyruvate and glutathione syntheses. Interestingly, increasing evidence suggests that serine metabolism may impact the aging process, and supplementation with serine may confer benefits in safeguarding against aging and age-related disorders. This review synthesizes recent insights into the regulation of serine metabolism during aging and its potential to promote healthy lifespan and mitigate a spectrum of age-related diseases.
Keywords: Serine, Aging, Age-related diseases, Longevity, Oxidative stress, Metabolism
Introduction
Serine is classified as a nutritionally nonessential amino acid (NEAA); however, metabolically, serine plays critical roles in central metabolism. It is involved in the synthesis of essential biomolecules, including proteins, sphingosine, lipids, and nucleotides [1–3], and it affects numerous aspects of animal physiology. Its metabolic products are important for cell proliferation, tissue development, and specific organ functions. For example, serine is converted into intermediates used in glycolysis for energy production, and its catabolic products are essential for one-carbon metabolism, involving folate and methionine [2]. As a key one-carbon donor to the folate cycle, serine is also important to nucleotide synthesis, methylation reactions, and the generation of NADPH for antioxidant defense [4]. Therefore, serine is a metabolically essential amino acid, playing a significant role in a wide range of biological functions [5].
As an NEAA, serine can be derived exogenously in the diet and endogenously through the de novo serine synthesis pathway (SSP) [6, 7]. Increased serine can enhance intestinal immune and antioxidant functions [8, 9], regulate intestinal flora both in vitro and in vivo [10], and promote growth and development [11, 12]. Under oxidative stress or bacterial infection conditions, endogenous serine is often insufficient to meet cellular demands, thus necessitating exogenous serine supplementation to alleviate stress responses [10, 13]. In addition, aberrant metabolic fluxes associated with serine metabolism have emerged as potential risk factors for various age-related diseases (ARDs) and their comorbidities. For instance, impaired endogenous serine synthesis is associated with the development of ARDs, including Alzheimer’s disease [14], cardiovascular disease [15], diabetes [16], and fatty liver disease [17], and increasing evidence suggests that targeting serine metabolism may provide novel potential therapeutics to improve aging associated negative health outcomes, as demonstrated in various animal models [18–20].
Here, we review recent advances in the regulation of serine metabolism in the aging process, highlighting serine’s potential pro-longevity role and therapeutic promise for numerous age-related disorders. We first provide a comprehensive guide for researchers interested in understanding serine metabolism and its downstream metabolites. Then, we focus on the impact of serine on a wide range of biological functions in the aging process.
Serine sources
Serine biosynthesis from glucose
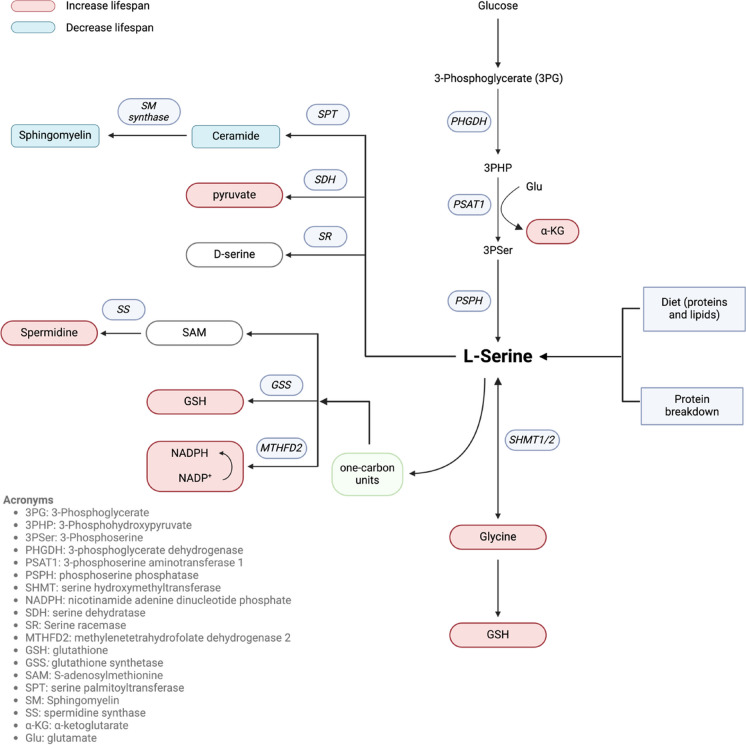
The serine synthesis pathway (SSP) utilizes glucose via diversion of the glycolytic intermediate 3-phosphoglycerate (3PG) to synthesize serine through a series of three enzymatic steps (Fig. 1). In the first step, 3PG is converted to 3-phosphohydroxypyruvate (3PHP) by phosphoglycerate dehydrogenase (PHGDH). Next, phosphoserine aminotransferase 1 (PSAT1) catalyzes 3PHP into 3-phosphoserine (3PSer). Lastly, phosphoserine phosphatase (PSPH) converts 3PSer into serine [4]. De novo serine biosynthesis from glucose is highly active in a wide variety of cell types such as astrocytes [14], macrophages [21], and epidermal stem cells [22], as well as specific organs including the central nervous system (CNS) [23], liver [24], and the kidney [25].
-
2.
Serine regeneration from glycine
Fig. 1.
Major sources, metabolites, and biosynthetic pathways for serine. Colors indicate if a metabolite has been shown to have pro-longevity (red) or longevity shortening (blue) effects. Metabolites lacking a color have unknown or conflicting effects on longevity. Figure produced in BioRender
L-serine can be synthesized de novo from glycine, a closely related NEAA. Serine hydroxymethyltransferase (SHMT) catalyzes the reversible conversion of glycine to serine. SHMT transfers a one-carbon unit from 5,10-methylene-tetrahydrofolate (5,10-MTHF) to glycine, producing tetrahydrofolate (THF) and L-serine [26, 27]. L-serine and glycine are rapidly converted in reactions catalyzed by two protein isoforms of SHMT located in the cytosol (SHMT1) and mitochondria (SHMT2) [28, 29]. In healthy humans, the synthesis of L-serine by SHMT constitutes approximately 41% of the total glycine flux throughout the body [30].
-
3.
Serine uptake and transport from the diet
The primary dietary source of L-serine is protein, with 2 to 5% of the amino acid content of plant-based and animal-based proteins being L-serine [31]. Lipids, which contain serine in the form of phosphatidylserine and sphingolipids, contribute minimally to dietary serine intake. This is due to the low phosphatidylserine content in the diet, and most sphingolipids are either used by the intestinal mucosa or excreted in feces [32]. Therefore, the average daily intake of serine from food sources for an adult is estimated to be approximately 3.5 g [33–35].
-
4.
Serine recycled from protein breakdown
Serine can also be produced through the degradation of serine-containing proteins in the lysosome, a process that breaks down deactivated or misfolded endogenous proteins during autophagy [36]. Assuming that the serine content in human muscle protein is approximately 2.3% [31] and recognizing the significant role of skeletal muscle in protein turnover, which is about 300 g per day in a 70 kg individual, it is estimated that approximately 6.9 g (65.1 mmol) per day of L-serine is released during the degradation of endogenous proteins. This estimation aligns closely with measurements of the rate of serine appearance following an overnight fast (~ 58 mmol per day) using stable isotope tracers [37].
Major metabolites of serine and their related physiological importance
In addition to serine itself, serine metabolism also produces multiple metabolites crucial for human health, which we describe in this section. Serine racemase (SR) catalyzes the conversion of L-serine to D-serine, which acts as a neuromodulator by binding to and activating the N-methyl-D-aspartate (NMDA) subtype of glutamate receptors [38].
As stated above, L-serine can be converted to glycine in both the cytosol (SHMT1) and mitochondria (SHMT2) [28, 29]. Glycine, a precursor for multiple metabolites including glutathione, porphyrins, purines, heme, and creatine, plays a significant physiological role in both humans and animals. It functions as a neurotransmitter in the CNS and exhibits various roles in peripheral and nervous tissues, such as acting as an antioxidant, anti-inflammatory agent, cryoprotectant, and immunomodulator [39].
L-serine is degraded to pyruvate via serine dehydratase (SDH) [40]. Pyruvate serves as a fuel input for the citric acid cycle (also known as the Krebs cycle or TCA cycle). These reactions produce ATP as well as intermediates essential for various major biosynthetic pathways including gluconeogenesis, nucleotide, and lipid synthesis [41]. Additionally, pyruvate acts as a protective antioxidant in the brain and other tissues susceptible to oxidative stress [42, 43].
Serine also contributes to the production of nicotinamide adenine dinucleotide phosphate (NADPH), glutathione (GSH), and S-adenosylmethionine (SAM) through participating in one-carbon metabolism, which includes the folate and methionine cycles [44]. NADPH, a coenzyme acting as an electron transporter, supports various anabolic processes and has strong antioxidant properties that help maintain cell growth [45]. GSH is ubiquitously present in all mammalian tissues as the most abundant non-protein thiol, preventing damage due to oxidative stress [46]. Additionally, GSH is essential for the detoxification of xenobiotics, and it regulates cell proliferation, apoptosis, immune function, and fibrogenesis [47]. SAM functions as the principal biological methyl donor for histone and DNA methylation processes [48]. SAM is converted into S-adenosyl-L-homocysteine (SAH), which in the liver, serves as a precursor for GSH through conversion into cysteine via the trans-sulfuration pathway [49]. Additionally, SAH donates an aminopropyl group for spermidine synthesis [50]. Spermidine, a natural polyamine, induces cytoprotective autophagy, facilitating the turnover of cytoplasmic organelles and long-lived proteins.
Serine can also lead to the production of ceramide lipids. A ceramide consists of sphingosine and a fatty acid linked by an amide bond. Ceramide acts as the backbone of sphingolipids such as sphingomyelin (SM), cerebrosides, and gangliosides and is therefore an important component of the plasma membrane in eukaryotic cells [51, 52]. As a bioactive lipid, ceramide is involved in numerous functions, including apoptosis, cell growth arrest, differentiation, cell senescence, cell migration, and cell adhesion [53]. SM is found in animal cell membranes, particularly in the myelin sheath that encases some nerve cell axons and in plasma lipoproteins [54]. SM plays a critical role in cellular signaling by serving as a reservoir for lipid signaling molecules and facilitating the formation of SM-enriched membrane domains. These domains regulate the clustering of signaling molecules, thereby promoting efficient signal transduction [55].
In addition to serine’s direct downstream metabolites, the serine biosynthesis pathway is crucial for generating alpha-ketoglutarate (α-KG), a key intermediate in the tricarboxylic acid (TCA) cycle. PSAT1 utilizes 3PHP, produced by PHGDH (Fig. 1), to facilitate the transamination of glutamate to produce α-KG [56, 57]. α-KG is integral to various metabolic processes, including amino acid synthesis, nucleotide production, and cellular energy generation [58, 59]. Beyond its metabolic role, α-KG is essential for maintaining mitochondrial homeostasis [60, 61], supporting antioxidative defenses [62], reducing inflammation [63], promoting cell proliferation [64], and even exerting tumor-suppressive effects [65]. These diverse functions underscore α-KG’s importance not only in energy metabolism but also in broader cellular homeostasis and defense mechanisms.
Serine metabolism in aging and aging-related diseases
Aging is characterized by a progressive decline of physiological integrity, leading to impaired function, heightened susceptibility to chronic diseases, and increased mortality risk [66]. This decline is often caused by and accompanied with overall nutrient deficiencies [67, 68], and nutritional interventions have potential to slow or reverse the aging processes across various organ systems, including the brain, cardiovascular system, gastrointestinal tract, skeletal structure, and skin [69]. Specifically, changes in amino acid metabolism have been increasingly recognized as key contributors to the aging process [70, 71]. While much of the research has traditionally focused on essential amino acids [72], recent studies have revealed that NEAAs, such as serine, may also play significant roles in late-life health and longevity. Understanding the complex relationship between serine metabolism and aging may potentially lead to the development of novel strategies to mitigate the adverse effects of aging and increase healthy lifespan. This section highlights the significant roles that serine metabolism plays in aging, longevity extension, and aging-related diseases, providing insights into potential therapeutic targets for longevity interventions, as well as denoting needed areas of future research.
Serine and aging and longevity
Recent studies on serine supplementation across various model organisms have revealed its potential role in extending lifespan. In C. elegans, supplementation with 10 mM serine has been shown to produce the greatest increase in lifespan—up to 22%—among the 20 proteogenic amino acids [73], indicating an important role of serine in lifespan extension. Moreover, serine supplementation alters mitochondrial TCA cycle metabolism and respiratory substrate utilization, contributing to the observed extended lifespan [74]. In yeast, L-serine supplementation extends chronological lifespan by mitigating acidification and working through the one-carbon metabolism pathway [75]. In addition, preclinical studies in rodents have also documented reductions in both circulating and hippocampal serine levels with age [76, 77].
In vitro studies utilizing human cells also underscore the importance of serine metabolism in the process of cellular senescence. Specifically, the inhibition of serine synthesis via CBR-5884, an inhibitor of phosphoglycerate dehydrogenase (PHGDH), in human primary Müller cells subjected to mild oxidative stress, results in a marked reduction in metabolic activity and a concomitant increase in cellular damage [78]. Corroborating these observations, overexpression of PHGDH in human vascular endothelial cells, which enhances de novo serine synthesis, effectively mitigates premature cellular senescence [79]. Similarly, in human dental pulp cells (DPCs), the inhibition of PHGDH-mediated serine biosynthesis triggers replicative senescence, highlighting the essential role of serine in sustaining cellular function and longevity [80]. Collectively, these preclinical studies demonstrate the potential involvement of serine metabolism in aging across diverse model organisms, as summarized in Table 1.
Table 1.
Preclinical studies for serine-relevant intervention increased lifespan
| Study | Organism | Intervention | Effect on lifespan | Mechanism |
|---|---|---|---|---|
| Serine supplementation in C. elegans [74] | C. elegans | Serine supplementation | Extended lifespan | Altered mitochondrial TCA cycle metabolism and respiratory substrate utilization |
| Glycine/serine supplementation in C. elegans [73] | C. elegans | Glycine/serine supplementation | 5 mM serine prolonged the lifespan of worms (+ 20.8% in median lifespan) | Serine-mediated longevity effect is dependent on the methionine cycle in a metr-1 and sams-1 dependent manner |
| Serine supplementation in yeast [81] | Budding yeast | Supplementation of serine in culture media | Extend the lifespan of budding yeast | Controls extracellular pH through catabolism into ammonium and acting as an energy source after glucose exhaustion |
| L-serine supplementation in C. elegans [82] | C. elegans | L-serine supplementation | 5 mM serine can increase the lifespan of wild type worms | De novo serine biosynthesis is required for longevity upon mitochondrial protein import system suppression (MitoMISS) |
| L-serine supplementation in budding yeast [75] | Budding yeast | L-serine supplementation | Extend the chronological lifespan of yeast | Supplementing L-serine into NR cultures extended chronological life span through a mechanism dependent on the one-carbon metabolism pathway |
| Inhibition de novo serine synthesis in human cells [78] | Human primary Müller cells | Inhibition de novo serine synthesis using CBR-5884 | Metabolic activities significantly reduced and cellular damage of Müller cells increased | De novo serine synthesis improved Müller cell survival by maintaining mitochondrial function and generating glutathione and NADPH to counteract ROS |
| Enhancement of de novo serine synthesis in human cells [79] | Human vascular endothelial cells | Overexpression of PHGDH in vascular endothelium (VE) | VE-specific overexpression of PHGDH prevents premature cellular senescence | L-serine attenuates senescence in endothelial cells by acting as a signal molecule to activate PKM2 |
| Inhibition de novo serine synthesis in human cells [80] | Human dental pulp cells | Inhibition of PHGDH in human dental pulp cells | Inhibition of PHGDH leads to the phenotype of replicative senescence | PHGDH-mediated serine biosynthesis reduces H3K36me3 levels by providing less methylation donor SAM |
| Long-term L-serine administration in aging mice [83] | Age-related obesity with C57BL/6 J mice | L-serine were administered to old mice for 6 months | 0.5% L-serine significantly reduced food intake and body weight gain | L-serine reduced body weight by decreasing orexigenic peptide expression and reduced oxidative stress and inflammation via Sirt1/NFκB pathway |
In addition to its potential lifespan extending properties in animal models and cell culture, there is evidence that dietary serine consumption may also be associated with improved late-life health outcomes in humans. It has been well described that circulating serine levels decline with age in humans [84, 85], and lower plasma serine levels are associated with an increased risk of mortality in COVID-19 patients [86], as well as across different age groups [87]. The Ogimi village population, in the “blue zone” Okinawa [88], has exceptional lifespan that may be partly attributed to the high serine content in their diet [89]. The Ogimi diet features foods such as tofu and seaweeds, which are rich in L-serine. Notably, Ogimi women consume more than 8 g of L-serine per day, in stark contrast to the average intake of 2.5 g per day for women over 70 years old in the USA [89]. Given the apparent scarcity of progressive neurodegenerative illnesses among the Ogimi villagers, the substantial intake of L-serine potentially supports their neurological health and contributes to their exceptional longevity [89], though more prospective research is needed. These findings indicate that serine supplementation could potentially offer benefits for longevity, though most previous studies are observational in nature.
Serine and age-related diseases
Age is one of the greatest risk factors for numerous pathologies, such as neurodegenerative diseases [90, 91], type 2 diabetes [92], cardiovascular disease [93], and cancers [94]. All of these are often termed “age-related diseases” (ARDs), and aging and ARDs may share a common set of fundamental biological mechanisms [95, 96]. Interestingly, accumulating evidence indicates that serine deficiency is associated with various ARDs [14–17, 97, 98]; there are multiple human clinical trials as well as basic studies to investigate the therapeutic potential of serine in various ARDs, which we describe below, and animal studies are overviewed in Table 2.
Table 2.
Studies of serine improving aspects of age-related diseases in diverse animal models
| Study | Animal models | Intervention | Effect on age-related pathologies | Mechanism |
|---|---|---|---|---|
| Maternal serine supplementation on high-fat diet-induced oxidative stress [99] | C57BL/6 J mouse dams during gestation with HF diet | 1% serine supplementation during pregnancy | Maternal serine prevents oxidative stress and fat accumulation in weanlings from dams fed high-fat diet | Serine alleviates HF diet-induced oxidative stress by epigenetically regulating glutathione synthesis |
| L-serine supplementation in diquat-induced oxidative stress in mice [100] | Mice model of diquat-induced oxidative stress | L-serine was supplemented in the drinking water for 14 days | Serine supplementation improved glutathione synthesis and alleviated oxidative stress | Serine alleviated oxidative stress via supporting glutathione synthesis and methionine cycle |
| Effects of serine on acute pancreatitis during diabetes in mice [16] | Mouse model of diabetes | 10% L-serine supplementation in diabetic mice | L-serine supplementation reduced the acinar tissue damage resulting from pancreatitis in diabetic mice | L-serine decreased the ROS production, ER stress and cellular apoptosis in acinar tissue |
| L‐serine in ALS/Parkinsonism dementia [101] | Rat model of ALS/Parkinsonism dementia complex | L-serine solution was injected intraperitoneally daily for 1 week into the model rats | Administration of L-serine enhanced cognitive function, and ameliorated electrophysiological abnormalities | L‐serine alleviated apoptotic and autophagic changes in the central nerve system |
| L-serine supplementation in autoimmune diabetes [102] | Autoimmune diabetic model in NOD mice | Supplementation of L-serine 85.7 g/L, or 280 mg/day/mouse in water | Supplementation of L-serine reduced diabetes incidence and insulitis score | L-serine protects against autoimmune diabetes by regulating pancreas sphingolipid composition |
| Long-term effects of L-serine supplementation in diabetic neuropathy [103] | db/db mice model for diabetic neuropathy | 5–20% oral L-serine for 6 months | L-serine treatment significantly improved functional neuropathy and sensory modalities | L-serine suppresses neurotoxic deoxysphingolipids (1-deoxySLs) in db/db mice |
| Serine one-carbon metabolism in myocardial ventricular function [15] | Ventricular pressure overload murine model | Cardiac-specific overexpression of CnAβ1 in transgenic mice | Activation of serine and one-carbon metabolism in transgenic mice reduced cardiac hypertrophy and improved cardiac function | Activation of the serine and one-carbon pathway leads to the production of antioxidant mediators that prevent mitochondrial protein oxidation and preserve ATP production |
| Effects of L-serine in hypertensive rats [104] | Spontaneously hypertensive rats | L-serine (0.3–3.0 mmol/kg) was administered intravenously | L-serine evoked a greater maximal fall in mean arterial pressure | The antihypertensive effect of L-serine is likely mediated through the activation of endothelial KCa channels |
| Antihypertensive effect of L-serine [105] | NO synthase-inhibited hypertensive rats | Acute intravenous infusion of L-serine in rats | L-serine evoked a dose-dependent decrease in mean arterial pressure without increasing heart rate | L-serine promotes vasodilatation in resistance arterioles via activation of apamin and charybdotoxin/TRAM-34-sensitive K(Ca) channels present on the endothelium |
| The role of serine in nonalcoholic fatty liver disease [106] | Mouse model of HFD-induced hepatic lipid accumulation and injury | 1% L-serine (wt/vol) was supplemented in drinking water for 8 weeks | Serine supplementation increased glucose tolerance and insulin sensitivity, and protected mice from hepatic lipid accumulation | Serine prevents HF diet-induced oxidative stress and steatosis by epigenetically modulating the expression of glutathione synthesis-related genes and through AMPK activation |
| Effects of L-serine on alcoholic fatty liver [107] | Ethanol-induced fatty liver model in mice and rat | Dietary 1% L-serine supplementation for 2 weeks | L-serine decreased hepatic neutral lipid accumulation and increased glutathione and S-adenosylmethionine | L-serine ameliorated alcoholic fatty liver by accelerating L-serine–dependent homocysteine metabolism |
| L-serine treatment in chronic liver injury [108] | CCl4-induced hepatic fibrosis mouse model | L-serine (100 g/L) treatment for 8 weeks | L-serine reduces inflammatory cell and collagen deposition and reduces hepatic fibrosis in the liver tissue | L-serine induces antioxidant production via the maintenance of NADPH production in the mitochondria |
| Effects of serine deficiency on hepatic fat accumulation in mice [5] | C57BL/6 J male mice | Mice fed a serine-deficient diet or PHGDH inhibitor NCT-503 | Both treatments increased body and liver weight and triglyceride content in the liver, and exacerbated hepatic inflammatory responses and oxidative stress | Serine deficiency leads to hepatic fat accumulation by affecting the gut-microbiota-liver axis |
| Dietary serine in effector T cell expansion [109] | C57BL/6 and OT-I mice with infection | Animals were fed a test diet lacking serine for 2 weeks prior to infection | Restricting dietary serine impairs pathogen-driven expansion of T cells in vivo | Serine supplies glycine and one-carbon units for de novo nucleotide biosynthesis in proliferating T cells |
Serine plays a pivotal role in neurological signaling to promote neuronal elongation, differentiation, and growth [110]. Additionally, L-serine serves as an essential neurotrophic factor and a precursor to neuroactive substances such as D-serine, glycine, and cysteine. These metabolic products are important for cell proliferation, neuronal development, and other functions within the mammalian CNS [111]. Recent research has highlighted the role of aberrant L-serine homeostasis as a potential modifier of Alzheimer’s disease (AD). For example, L-serine is reduced in brain tissue of AD patients as compared to normal controls [112], and a mouse model of AD exhibited decreased L-serine availability in the hippocampus, which was associated with synaptic deficits [14]. Treatment of AD patients with combined metabolic activators (CMA), where L-serine constituted 61.75% of the formulation, resulted in enhanced cognitive functions and improved clinical parameters [113]. L-serine has also been shown to protect against β-N-methylamino-L-alanine (L-BMAA)-induced neurotoxicity in both animal and cell culture models [114], and L-BMAA activates endoplasmic reticulum (ER) stress [115] and has been linked to neurodegenerative diseases [116, 117]. Cognitive decline, a common phenomenon among older adults, is often an early indicator of AD and related dementias [118, 119]. D-serine administration was shown to enhance cognitive function in older adults, suggesting a transfer of benefits to daily activities and overall health-related quality of life [120].
Moreover, studies deploying a mouse model of AD have shown that the de novo serine synthesis pathway (SSP) is disrupted in astrocytes [14]. In mice, the deletion of PHGDH, the first enzyme in the SSP, results in severe neurological defects and early postnatal death [121]. Furthermore, various serine-deficiency syndromes linked to mutations in the SSP have been identified in human patients, leading to neurological disorders that can be alleviated by substantially increasing dietary serine [122, 123]. Adeno-associated virus (AAV)-mediated inactivation of PHGDH in the brain led to reduced L-serine levels, impaired synaptic plasticity, and diminished spatial memory, demonstrating that reduced L-serine levels in the brain contribute to cognitive dysfunction [14]. Importantly, dietary L-serine supplementation in this model normalized L-serine and D-serine levels and improved spatial memory and cognitive function [14, 124]. These findings suggest that L-serine deficiency may contribute to the neuronal dysfunction associated with AD, and dietary L-serine supplementation may enhance cognitive function and mitigate the effects of AD.
In alignment with findings observed in AD, L-serine has also shown promising therapeutic effects in the treatment of amyotrophic lateral sclerosis (ALS), a progressive neurodegenerative disease characterized by the loss of cortical and lower motor neurons in the spinal cord and brainstem [125], leading to progressive muscle atrophy, fatigue, dysphagia, and eventually death [125, 126]. In a rat model of ALS/Parkinsonism dementia complex, administration of L-serine resulted in improvements in alleviating apoptotic and autophagic alterations, enhancing cognitive function, and ameliorating electrophysiological abnormalities [101]. Furthermore, in a clinical trial, ALS patients who received L-serine showed slowed ALS disease progression [127]. These findings suggest that L-serine may exert a neuroprotective effect in the treatment of ALS. Similarly, in hereditary sensory autonomic neuropathy type I (HSAN1), a slow progressive neurodegenerative disorder primarily affecting sensory nerves and often leading to severe disability, oral supplementation with high doses of L-serine was shown to significantly slow disease progression [128]. These studies suggest a therapeutic potential of L-serine as a general treatment for AD and other neurodegenerative diseases.
Serine supplementation may also hold therapeutic promise in addressing other age-related diseases and conditions. A notable association exists between advancing age and type 2 diabetes mellitus (T2DM) [129]. T2DM is primarily characterized by hyperglycemia, which arises from a progressive decline in insulin secretory capacity of β-cells, often coupled with varying degrees of insulin resistance [129]. Increasing evidence indicates that L-serine concentrations in both plasma and tissues are significantly diminished in individuals with T2DM [130–134]. In addition, a large study of 5181 non-diabetic men demonstrated that higher L-serine levels were associated with enhanced insulin secretion and sensitivity, thereby potentially reducing the risk of developing T2DM [135]. In an animal model of non-obese diabetes (NOD), supplementation with L-serine was shown to attenuate insulitis and decrease the incidence of diabetes compared to controls, which was linked to improved glucose tolerance, reduced insulin resistance, and a modest reduction in average blood glucose levels [102]. Additionally, in a murine model of streptozotocin (STZ)-induced diabetes, dietary L-serine supplementation reduced pancreatic acinar cell damage and lowered markers of pancreatitis, suggesting that L-serine may mitigate pancreatic injury [16]. Handzlik et al. [136] recently developed a novel model of serine-associated sensory neuropathy in diabetic mice, finding that serine depletion exacerbates lipid abnormalities and accelerates neurological decline. In addition, a serine-enriched diet reduced levels of neurotoxic 1-deoxysphingolipids in both liver and paw skin, while administration of myriocin, an SPT inhibitor, effectively decreased canonical sphingolipids in the liver. Both interventions alleviated sensory neuropathy progression, linking serine-associated neuropathy to sphingolipid metabolism and suggesting that targeting serine metabolism could be a promising therapeutic approach. The authors also introduced the “serine tolerance test” (STT), analogous to the oral glucose tolerance test, which holds the potential to identify patients at risk for sensory neuropathy due to serine dysregulation [136]. By linking insulin resistance, serine homeostasis, and lipid metabolism, the study underscores the therapeutic potential of serine-targeted interventions for diabetes-associated neuropathies. Furthermore, L-serine supplementation to mice fed a high-fat diet (HFD) enhanced glucose tolerance and insulin sensitivity [137]. Collectively, these findings indicate that L-serine supplementation may offer therapeutic benefits in the context of diabetes; however, future prospective studies are still needed.
Aging is also closely linked to the progression of chronic liver diseases, such as non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and alcoholic steatohepatitis [138], and dysregulation of serine metabolism may play a role in development and progression of these disorders. Notably, circulating serine levels are significantly reduced in patients with NAFLD [139], and serine deficiency has been implicated in the development of fatty liver disease [3]. Furthermore, animal models of HFD-induced steatosis and methionine-choline-deficient diet-induced steatohepatitis have reduced PHGDH expression [140], and overexpression of PHGDH in mice resulted in significantly reduced triglyceride accumulation in the liver, lower expression of lipogenic genes and proteins, and increased activity of SIRT1 [140, 141]. Interestingly, mice subjected to a serine-deficient diet or administered a PHGDH inhibitor exhibited increased body weight, liver weight, and triglyceride content in the liver [5]. Both interventions also exacerbated hepatic inflammatory responses and oxidative stress, suggesting that exogenous and endogenous serine deficiencies contribute to hepatic lipid accumulation and aggravate oxidative stress and inflammation in the liver [5]. Other potential therapeutic targets within the endogenous serine synthesis pathway, such as phosphoserine phosphatase (PSPH) and serine hydroxymethyltransferase 1 (SHMT1), have been identified for the treatment of NASH [3, 142]. In a mouse model of HFD-induced hepatic lipid accumulation and injury, L-serine supplementation improved glucose tolerance and insulin sensitivity and reduced hepatic lipid accumulation [106], and in an ethanol-induced animal model of alcoholic fatty liver disease, L-serine supplementation reduced hepatic neutral lipid accumulation and increased glutathione and S-adenosylmethionine levels [107]. In addition, in a mouse model of hepatic fibrosis, a common feature of most chronic liver diseases [143], L-serine supplementation reduced inflammatory cell infiltration, collagen deposition, and the extent of fibrosis in liver tissue [108]. Together, these findings underscore the beneficial role of both exogenous and endogenous serine in regulating hepatic lipid metabolism, thereby reducing the risk of age-related chronic liver diseases.
While the effects of serine on cardiovascular-related disorders have not been as well studied as other ARDs, there are promising preliminary results that suggest it may be a beneficial preventative or treatment for cardiovascular disorders. For example, an observational study identified an inverse relationship between serine concentration and coronary heart disease (CHD) in patients, suggesting a possible protective role of serine in CHD [144]. Furthermore, enhanced contractile function was observed in induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) from patients with genetic dilated cardiomyopathy (DCM) following treatment with agents that activate the L-serine biosynthetic pathway [145]. Notably, serine supplementation was found to reduce methionine-induced elevation of plasma homocysteine concentrations, a known risk factor for cardiovascular disease, suggesting potential cardiovascular benefits of serine supplementation [146]. Others further demonstrated that activation of the serine and one-carbon metabolism pathway increases ATP levels causing improved cardiac function and attenuated pathological remodeling following pressure overload [15]. Also, significant antihypertensive effects of L-serine were found in a rat model of hypertension, noting that it promotes vasodilation in resistance arterioles and reduces mean arterial pressure [104, 105]. Overall, these findings suggest that L-serine may play a beneficial role in promoting cardiovascular health, but more hypothesis-directed studies are needed.
Serine metabolism has probably been most studied in cancers, and findings are often contradictory to the benefits for other ARDs. We will not delve deeply into the role of serine metabolism in antitumor immunity and cancer and only highlight a couple of findings, as these topics have been extensively discussed in previous reviews [1, 44]. For example, de novo serine production is frequently elevated across various cancer cell types [56], and some studies have suggested that serine supplementation may enhance tumor growth [147], raising concerns regarding its nutritional application in humans. Moreover, emerging evidence links serine metabolism to antitumor immunity, as serine may directly influence adaptive immune responses by regulating T cell proliferation and supplying one-carbon units necessary for de novo nucleotide biosynthesis in proliferating T cells [109]. Lipopolysaccharide (LPS) has been shown to activate the serine synthesis pathway and one-carbon metabolism, driving SAM production in macrophages [148]. Thus, serine is vital for the proliferation and differentiation of immune cells in antitumor immunity. However, given the reliance on serine in both tumor cells and immune cells, therapeutic strategies involving serine restriction, supplementation, or inhibition of serine biosynthesis may yield distinct outcomes depending on the cancer type. Therefore, long-term studies and appropriate experimental models are required to rigorously evaluate the effects of serine intervention in cancer treatment before such approaches can be considered for clinical application.
Serine metabolites and serine-linked metabolic pathways in aging
Evidence suggests that serine significantly influences organismal lifespan, not just as a metabolite but also through its downstream metabolic pathways. NADPH levels decrease with age across various tissues, and the overexpression of NADPH-synthesizing enzymes has been associated with extended lifespans in multiple biological models [149]. In addition, depletion of GSH has been linked to numerous adverse effects, including impaired immune function and increased vulnerability to oxidative stress [150]. A decline in GSH levels has been correlated with aging and various age-related diseases, including neurodegenerative disorders [151]. Conversely, older adults with excellent physical and mental health have been reported to have elevated GSH, suggesting that higher GSH concentrations may contribute to increased healthspan and lifespan [152].
Moreover, L-serine can be converted into glycine, and evidence suggests that glycine may function as a pro-longevity molecule. Supplementation of this amino acid has been demonstrated to extend lifespan across various model organisms, including worms, flies, mice, and rats [73, 153–155], and it enhances health outcomes by inducing autophagy and exerting anti-inflammatory effects [156–159]. Additionally, glycine acts as an acceptor in the methylation reaction catalyzed by glycine N-methyltransferase (GNMT), an enzyme whose overexpression has been shown to extend lifespan in flies [153, 160]. In mammals, GNMT plays a role in methionine clearance [161], and mice deficient in GNMT are predisposed to developing hepatocellular carcinoma and steatosis [162]. In addition, glycine supplementation has been reported to reduce plasma methionine concentrations and protect against methionine toxicity in rats [163, 164]. Similarly, glycine supplementation has been found to decrease methionine levels in C. elegans [73]. Thus, glycine may also improve health and extend lifespan by mimicking the effects of methionine restriction.
Additionally, serine contributes to the synthesis of S-adenosylmethionine (SAM), which can extend lifespan by activating adenosine monophosphate-activated protein kinase (AMPK). AMPK serves as a central regulator in numerous nutrient-responsive pathways linked to longevity [165, 166] and has been identified as a key modulator of aging across various organismal models [167, 168]. For example, the activation of SAM synthesis stimulates Snf1, the yeast ortholog of AMPK, leading to lifespan extension in Saccharomyces cerevisiae [169]. AMPK activation also has been shown to enhance mitochondrial function, promote fatty acid and cholesterol synthesis, and inhibit inflammation, all of which play critical roles in regulating cellular senescence [170]. SAM also serves as a precursor to S-adenosyl-L-homocysteine (SAH), which donates an aminopropyl group for the synthesis of spermidine [171]. Tissue levels of spermidine have been reported to decline with age in both humans and model organisms [172–174], and spermidine has been recognized as a significant geroprotective compound found in food [175]. Remarkably, spermidine has been shown to significantly extend the lifespan of worms, yeast, flies, mice, and human immune cells [174, 176–178]. A recent epidemiological study further supports a positive association between dietary spermidine intake and increased human healthspan and lifespan [179]. Spermidine functions as a well-tolerated caloric restriction mimetic, increasing autophagy and extending cellular lifespan [180]. Additionally, spermidine exerts direct antioxidant and metabolic effects on arginine bioavailability and nitric oxide production [178]. These health-promoting mechanisms collectively may contribute to lifespan extension across diverse organisms.
Furthermore, serine is converted to pyruvate by cytosolic serine dehydratase. In C. elegans, increased pyruvate levels mimic lifespan extension typically induced by dietary restriction (DR) [181]. Pyruvate also exerts protective effects against aging phenotypes by preventing senescence in normal human dermal fibroblasts, partly through the increased production of NAD + during its conversion to lactate, and pyruvate deprivation leads to cell senescence in these fibroblasts [182]. Dysregulation of pyruvate metabolism has been implicated in the pathogenesis of age-related disorders including cancer, heart failure, and neurodegenerative diseases [41]. Taken together, nutritional interventions targeting serine metabolism may promote human longevity by enhancing the production of these health-promoting metabolites through serine-related metabolic pathways or serine supplementation.
An indirect metabolite of serine biosynthesis, α-KG, produced by PSAT1, is also significantly associated with aging, as circulating α-KG levels decline with age [183], and α-KG supplementation has been linked with lifespan extension in various model organisms. For example, in C. elegans, dietary supplementation with α-KG has been associated with increased autophagy and extended lifespan, mediated by the inhibition of ATP synthase and the mTOR pathway [57]. Additionally, α-KG supplementation has been shown to reduce systemic inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-2 (IL-2), and interleukin-6 (IL-6), and to extend lifespan in older mice [63].
While many serine metabolites appear to have beneficial effects in later ages, others, such as ceramides and sphingomyelin (SM), have been implicated in negative aging phenotypes. Ceramide, a central molecule in sphingolipid metabolism, has been shown to significantly accumulate in senescent cells [184]. Elevated ceramide levels are associated with mitochondrial dysfunction, oxidative stress, and the activation of pro-inflammatory pathways [53]. Furthermore, genetic disruptions in ceramide and sphingolipid synthesis pathways have been reported to extend lifespan in model organisms. For instance, RNA interference (RNAi)-mediated knockdown of the ceramide synthase gene hyl-1 increases lifespan in C. elegans [185]. Moreover, the loss of function of ceramide synthase genes hyl-1 and lagr-1 induces autophagy-dependent lifespan extension in worms [186]. Notably, exogenous ceramide has been found to cause a senescent phenotype in young human diploid fibroblasts [184]. Similarly, dysregulation of SM metabolism has been linked to neuroinflammation and neuronal cell death [187]. SM-ceramide signaling may play an important role in regulating cellular processes such as proliferation, differentiation, and survival, as well as modulating lifespan [188]. Given the diverse roles of serine metabolites and serine-linked metabolic pathways in aging, it is essential to investigate the specific mechanisms through which serine metabolism influences health and lifespan.
Concluding remarks and future perspectives
Traditionally regarded as a non-essential amino acid, serine has emerged as a significant, yet historically undervalued, component in aging research. Substantial progress has been made in elucidating the effects of serine on longevity and health across various model organisms, and the evidence for dysregulations of serine metabolism in neurological disorders and other age-related conditions suggests increasing serine levels may be a viable strategy to slow aging related phenotypes. The detailed examination of serine metabolism across diverse species in this review identifies critical targets for such serine interventions. Future studies that explore the combination of endogenous serine synthesis with exogenous dietary supplementation could lead to innovative therapeutic applications of serine, particularly in the context of aging and age-related diseases. Such advancements will deepen our understanding of serine as a functional amino acid and its broader implications for human health.
While the pro-longevity effects of L-serine are well-documented, some studies have produced conflicting results. For example, supplementation with D-serine, an enantiomer of L-serine, did not extend lifespan and even slightly reduced it at higher concentrations in C. elegans [74]. In yeast, inhibition of serine synthesis from 3-phosphoglycerate was shown to extend chronological lifespan [189], and serine may accelerate yeast aging by activating a phosphorylation signaling cascade involving Pkh1/2, Sch9 (the mammalian homolog of S6 kinase), and Rim15 protein kinases [190]. Furthermore, serine is involved in the production of sphingolipids such as ceramide and sphingomyelin, which have been implicated with mitochondrial dysfunction and impaired protein homeostasis, thus warranting further investigation.
Overall, a more comprehensive understanding of the mechanisms by which serine metabolism influences human pathologies associated with aging is essential. Future research should aim to elucidate the molecular mechanisms through which serine metabolism supports animal and human health, thereby enabling the development of therapeutic strategies targeting the serine metabolic pathways.
Acknowledgements
We would like to thank members of the Hoffman lab including Shelton Swint, Ryan Armant, Kelsey Patterson, and Eric Foreman for proofreading and editing.
Funding
This work was funded by R00AG059920 to JMH. The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shengshuai Shan, Email: sshan@augusta.edu.
Jessica M. Hoffman, Email: jehoffman@augusta.edu
References
- 1.Mattaini KR, Sullivan MR, Vander Heiden MG. The importance of serine metabolism in cancer. J Cell Biol. 2016;214(3):249–57. 10.1083/jcb.201604085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker SJ, Metallo CM. Chasing one-carbon units to understand the role of serine in epigenetics. Molecular Cell. 2016;61(2):185–6. 10.1016/j.molcel.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Mardinoglu A, Agren R, Kampf C, Asplund A, Uhlen M, Nielsen J. Genome-scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:3083. 10.1038/ncomms4083. [DOI] [PubMed] [Google Scholar]
- 4.Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16(10):650–62. 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 5.He L, Liu Y, Liu D, Feng Y, Yin J, Zhou X. Exogenous and endogenous serine deficiency exacerbates hepatic lipid accumulation. Oxid Med Cell Longev. 2021;2021:4232704. 10.1155/2021/4232704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stegen S, Loopmans S, Stockmans I, Moermans K, Carmeliet P, Carmeliet G. De novo serine synthesis regulates chondrocyte proliferation during bone development and repair. Bone Res. 2022;10(1):14. 10.1038/s41413-021-00185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krasovec G, et al. d-Serine controls epidermal vesicle release via NMDA receptor, allowing tissue migration during the metamorphosis of the chordate Ciona. Sci Adv. 2022;8(10):eabn3264. 10.1126/sciadv.abn3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F, Zhou H, Deng L, Wang L, Chen J, Zhou X. Serine deficiency exacerbates inflammation and oxidative stress via microbiota-gut-brain axis in D-galactose-induced aging mice. Mediators Inflamm. 2020;2020:5821428. 10.1155/2020/5821428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He L, Zhang H, Zhou X. Weanling offspring of dams maintained on serine-deficient diet are vulnerable to oxidative stress. Oxid Med Cell Longev. 2018;2018:8026496. 10.1155/2018/8026496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He L, Long J, Zhou X, Liu Y, Li T, Wu X. Serine is required for the maintenance of redox balance and proliferation in the intestine under oxidative stress. The FASEB Journal. 2022;34(3):4702–17. 10.1096/fj.201902690R. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Feng Y, Liu Y, He L, Zhou X, Yin Y. Serine supplementation in the diets of late gestating and lactating sows improves selenium nutritional status in sows and their offspring. Biol Trace Element Res. 2022;200(2):609–14. 10.1007/s12011-021-02661-x. [DOI] [PubMed] [Google Scholar]
- 12.He L, et al. Maternal serine supply from late pregnancy to lactation improves offspring performance through modulation of metabolic pathways. Food Funct. 2020;11(9):8089–98. 10.1039/d0fo01594f. [DOI] [PubMed] [Google Scholar]
- 13.Jiang J, Li B, He W, Huang C. Dietary serine supplementation: friend or foe?”. Curr Opinion Pharmacol. 2021;61:12–20. 10.1016/j.coph.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Le Douce J, et al. Impairment of glycolysis-derived l-serine production in astrocytes contributes to cognitive deficits in Alzheimer’s disease. Cell Metab. 2020;31(3):503-517.e8. 10.1016/j.cmet.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Padron-Barthe L, et al. Activation of serine one-carbon metabolism by calcineurin Abeta1 reduces myocardial hypertrophy and improves ventricular function. J Am Coll Cardiol. 2018;71(6):654–67. 10.1016/j.jacc.2017.11.067. [DOI] [PubMed] [Google Scholar]
- 16.Chen R, et al. Serine administration as a novel prophylactic approach to reduce the severity of acute pancreatitis during diabetes in mice. Diabetologia. 2022;63(9):1885–99. 10.1007/s00125-020-05156-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, et al. Serine catabolism generates liver NADPH and supports hepatic lipogenesis. Nat Metab. 2021;3(12):1608–20. 10.1038/s42255-021-00487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Zhang Y, Wu X, Wan D, Yin Y. Effects of dietary serine supplementation on intestinal integrity, inflammation and oxidative status in early-weaned piglets. Cell Physiol Biochem. 2018;48(3):993–1002. 10.1159/000491967. [DOI] [PubMed] [Google Scholar]
- 19.Ogbuagu NE, Ayo JO, Aluwong T, Akor-Dewu MB. L-serine improves lipid profile, performance, carcass weight and intestinal parameters in feed restricted broiler chickens during the hot-dry season. Trop Anim Health Prod. 2022;54(5):324. 10.1007/s11250-022-03318-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Icyuz M, Tollefsbol T, Cox PA, Banack SA, Sun LY. L-serine influences epigenetic modifications to improve cognition and behaviors in growth hormone-releasing hormone knockout mice. Biomedicines. 2022;11(1):104. 10.3390/biomedicines11010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez AE, et al. Serine metabolism supports macrophage IL-1β production. Cell Metab. 2019;29(4):1003-1011.e4. 10.1016/j.cmet.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baksh SC, et al. Extracellular serine controls epidermal stem cell fate and tumour initiation. Nat Cell Biol. 2020;22(7):779–90. 10.1038/s41556-020-0525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuya S. An essential role for de novo biosynthesis of L-serine in CNS development. Asia Pac J Clin Nutr. 2008;17(Suppl 1):312–5. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/18296366. Accessed 8 Nov 2024 [PubMed]
- 24.Narkewicz MR, Thureen PJ, Sauls SD, Tjoa S, Nikolayevsky N, Fennessey PV. Serine and glycine metabolism in hepatocytes from mid gestation fetal lambs. Pediatr Res. 1996;39(6):1085–90. 10.1203/00006450-199606000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Lowry M, Hall DE, Hall MS, Brosnan JT. Renal metabolism of amino acids in vivo: studies on serine and glycine fluxes. Am J Physiol. 1987;252(2 Pt 2):F304–9. 10.1152/ajprenal.1987.252.2.F304. [DOI] [PubMed] [Google Scholar]
- 26.Holmes WB, Appling DR. Cloning and characterization of methenyltetrahydrofolate synthetase from Saccharomyces cerevisiae. J Biol Chem. 2002;277(23):20205–13. 10.1074/jbc.M201242200. [DOI] [PubMed] [Google Scholar]
- 27.Stover P, Schirch V. Serine hydroxymethyltransferase catalyzes the hydrolysis of 5,10-methenyltetrahydrofolate to 5-formyltetrahydrofolate J Biol Chem 1990;265(24):14227. 10.1016/S0021-9258(18)77290-6. [PubMed]
- 28.Stover PJ, Chen LH, Suh JR, Stover DM, Keyomarsi K, Shane B. Molecular cloning, characterization, and regulation of the human mitochondrial serine hydroxymethyltransferase gene. J Biol Chem. 1997;272(3):1842–8. 10.1074/jbc.272.3.1842. [DOI] [PubMed] [Google Scholar]
- 29.MacFarlane AJ, et al. Cytoplasmic serine hydroxymethyltransferase regulates the metabolic partitioning of methylenetetrahydrofolate but is not essential in mice. J Biol Chem. 2008;283(38):25846–53. 10.1074/jbc.M802671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamers Y, Williamson J, Gilbert LR, Stacpoole PW, Gregory JF 3rd. Glycine turnover and decarboxylation rate quantified in healthy men and women using primed, constant infusions of [1,2-(13)C2]glycine and [(2)H3]leucine. J Nutr. 2007;137(12):2647–52. 10.1093/jn/137.12.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorissen SHM, et al. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50(12):1685–95. 10.1007/s00726-018-2640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohn JS, Kamili A, Wat E, Chung RW, Tandy S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients. 2010;2(2):116–27. 10.3390/nu2020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasaki M, et al. Validity of a self-administered food-frequency questionnaire for assessing amino acid intake in Japan: comparison with intake from 4-day weighed dietary records and plasma levels. J Epidemiol. 2016;26(1):36–44. 10.2188/jea.JE20150044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt JA, et al. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort. Eur J Clin Nutr. 2016;70(3):306–12. 10.1038/ejcn.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miura N, et al. Subchronic tolerance trials of graded oral supplementation with phenylalanine or serine in healthy adults. Nutrients. 2021;13(6):1976. 10.3390/nu13061976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galluzzi L, et al. Autophagy in malignant transformation and cancer progression. Embo J. 2015;34(7):856–80. 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalhan SC, Hanson RW. Resurgence of serine: an often neglected but indispensable amino acid. J Biol Chem. 2012;287(24):19786–91. 10.1074/jbc.R112.357194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder SH, Kim PM. D-amino acids as putative neurotransmitters: focus on D-serine. Neurochem Res. 2000;25(5):553–60. 10.1023/a:1007586314648. [DOI] [PubMed] [Google Scholar]
- 39.Razak MA, Begum PS, Viswanath B, Rajagopal S. Multifarious beneficial effect of nonessential amino acid, glycine: a review. Oxid Med Cell Longev. 2017;2017:1716701. 10.1155/2017/1716701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pamela C, Richard A, Denise R. Lippincott's illustrated reviews: biochemistry. 3rd ed. Lippincott Williams & Wilkins; 2005.
- 41.Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71(14):2577–604. 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yako H, et al. Role of pyruvate in maintaining cell viability and energy production under high-glucose conditions. Sci Rep. 2021;11(1):18910. 10.1038/s41598-021-98082-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Perez E, Liu R, Yan LJ, Mallet RT, Yang SH. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007;1132(1):1–9. 10.1016/j.brainres.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun S, Li J, Chen X, Wu Q. Serine and metabolism regulation: a novel mechanism in antitumor immunity and senescence. Aging Disease. 2020;11(6):1640. 10.14336/ad.2020.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L et al. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat Metab 2019;1:404–415. Available: https://www.ncbi.nlm.nih.gov/pubmed/31058257. Accessed 8 Nov 2024. [PMC free article] [PubMed]
- 46.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66(8):1499–503. 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 47.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–53. 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mentch SJ, Locasale JW. One-carbon metabolism and epigenetics: understanding the specificity. Ann N Y Acad Sci. 2016;1363(1):91–8. 10.1111/nyas.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mato JM, Alvarez L, Ortiz P, Pajares MA. S-adenosylmethionine synthesis: molecular mechanisms and clinical implications. Pharmacol Ther. 1997;73(3):265–80. 10.1016/s0163-7258(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 50.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1(5):228–37. 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 51.Quinville BM, Deschenes NM, Ryckman AE, Walia JS. A comprehensive review: sphingolipid metabolism and implications of disruption in sphingolipid homeostasis. Int J Mol Sci. 2021;22(11):5793. 10.3390/ijms22115793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis D, Kannan M, Wattenberg B. Orm/ORMDL proteins: gate guardians and master regulators. Adv Biol Regul. 2018;70:3–18. 10.1016/j.jbior.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–50. 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 54.Augé N, Nègre-Salvayre A, Salvayre R, Levade T. Sphingomyelin metabolites in vascular cell signaling and atherogenesis. Progress Lipid Res. 2000;39(3):207–29. 10.1016/S0163-7827(00)00007-2. [DOI] [PubMed] [Google Scholar]
- 55.Abe M, Kobayashi T. Imaging local sphingomyelin-rich domains in the plasma membrane using specific probes and advanced microscopy. Biochimica et Biophysica Acta (BBA) - Mole Cell Biol Lipids. 2014;1841(5):720–6. 10.1016/j.bbalip.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Amelio I, Cutruzzolá F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci. 2014;39(4):191–8. 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chin RM, et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510(7505):397–401. 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan Y, et al. α-Ketoglutaric acid ameliorates hyperglycemia in diabetes by inhibiting hepatic gluconeogenesis via serpina1e signaling. Sci Adv. 2022;8(18):eabn2879. 10.1126/sciadv.abn2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang LC, Chiang SK, Chen SE, Hung MC. Targeting 2-oxoglutarate dehydrogenase for cancer treatment. Am J Cancer Res. 2022;12(4):1436–55. https://www.ncbi.nlm.nih.gov/pubmed/35530286. Accessed 8 Nov 2024. [PMC free article] [PubMed]
- 60.Wei P, et al. Mitochondrial pyruvate supports lymphoma proliferation by fueling a glutamate pyruvate transaminase 2-dependent glutaminolysis pathway. Sci Adv. 2022;8(39):eabq0117. 10.1126/sciadv.abq0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun LY, et al. Nuclear receptor NR1D1 regulates abdominal aortic aneurysm development by targeting the mitochondrial tricarboxylic acid cycle enzyme aconitase-2. Circulation. 2022;146(21):1591–609. 10.1161/circulationaha.121.057623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He L, et al. Prevention of oxidative stress by α-ketoglutarate via activation of CAR signaling and modulation of the expression of key antioxidant-associated targets in vivo and in vitro. J Agric Food Chem. 2018;66(43):11273–83. 10.1021/acs.jafc.8b04470. [DOI] [PubMed] [Google Scholar]
- 63.AsadiShahmirzadi A, et al. Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 2020;32(3):447–4566. 10.1016/j.cmet.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.TeSlaa T, et al. alpha-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 2016;24(3):485–93. 10.1016/j.cmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris JP, et al. α-Ketoglutarate links p53 to cell fate during tumour suppression. Nature. 2019;573(7775):595–9. 10.1038/s41586-019-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rémond D, et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget. 2015;6(16):13858–98. 10.18632/oncotarget.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson SM. Improving nutrition to support healthy ageing: what are the opportunities for intervention? Proc Nutr Soc. 2018;77(3):257–64. 10.1017/s0029665117004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohajeri MH. Nutrition and aging. Int J Mol Sci. 2023;24(11):9265. 10.3390/ijms24119265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Timmerman KL, Volpi E. Amino acid metabolism and regulatory effects in aging. Curr Opin Clin Nutr Metab Care. 2008;11(1):45–9. 10.1097/MCO.0b013e3282f2a592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tian H, et al. Precise metabolomics reveals a diversity of aging-associated metabolic features. Small Methods. 2022;6(7): e2200130. 10.1002/smtd.202200130. [DOI] [PubMed] [Google Scholar]
- 72.Dillon EL. Nutritionally essential amino acids and metabolic signaling in aging. Amino Acids. 2013;45(3):431–41. 10.1007/s00726-012-1438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu YJ, et al. Glycine promotes longevity in Caenorhabditis elegans in a methionine cycle-dependent fashion. PLoS Genet. 2019;15(3): e1007633. 10.1371/journal.pgen.1007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edwards C, et al. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015;16(1):8. 10.1186/s12863-015-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Enriquez-Hesles E, et al. A cell-nonautonomous mechanism of yeast chronological aging regulated by caloric restriction and one-carbon metabolism. J Biol Chem. 2021;296:100125. 10.1074/jbc.RA120.015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Houtkooper RH, et al. The metabolic footprint of aging in mice. Sci Rep. 2011;1(1):134. 10.1038/srep00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Potier B, et al. Contribution of the d-serine-dependent pathway to the cellular mechanisms underlying cognitive aging. Front Aging Neurosci. 2010;2:1. 10.3389/neuro.24.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang T, et al. Disruption of de novo serine synthesis in Müller cells induced mitochondrial dysfunction and aggravated oxidative damage. Mol Neurobiol. 2018;55(8):7025–37. 10.1007/s12035-017-0840-8. [DOI] [PubMed] [Google Scholar]
- 79.Wu Y, et al. Phosphoglycerate dehydrogenase activates PKM2 to phosphorylate histone H3T11 and attenuate cellular senescence. Nat Commun. 2023;14(1):1323. 10.1038/s41467-023-37094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou S, Cui J, Shi Y. Serine metabolism regulates the replicative senescence of human dental pulp cells through histone methylation. Curr Issues Mol Biol 2024;46(4):2856–2870. 10.3390/cimb46040179. [DOI] [PMC free article] [PubMed]
- 81.Kawamukai A, Iwano A, Shibata M, Kishi Y, Matsuura A. Serine metabolism contributes to cell survival by regulating extracellular pH and providing an energy source in Saccharomyces cerevisiae. Yeast. 2023;40(2):59–67. 10.1002/yea.3840. [DOI] [PubMed] [Google Scholar]
- 82.Lionaki E, Gkikas I, Daskalaki I, Ioannidi MK, Klapa MI, Tavernarakis N. Mitochondrial protein import determines lifespan through metabolic reprogramming and de novo serine biosynthesis. Nat Commun. 2022;13(1):651. 10.1038/s41467-022-28272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou X, Zhang H, He L, Wu X, Yin Y. Long-term l-serine administration reduces food intake and improves oxidative stress and Sirt1/NFkappaB signaling in the hypothalamus of aging mice. Front Endocrinol (Lausanne). 2018;9:476. 10.3389/fendo.2018.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ploux E, Freret T, Billard JM. d-serine in physiological and pathological brain aging. Biochimica et Biophysica Acta (BBA) - Proteins Proteomics. 2021;1869(1):140542. 10.1016/j.bbapap.2020.140542. [DOI] [PubMed] [Google Scholar]
- 85.Darst BF, Koscik RL, Hogan KJ, Johnson SC, Engelman CD. Longitudinal plasma metabolomics of aging and sex. Aging (Albany NY). 2019;11(4):1262–82. 10.18632/aging.101837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bánfai G, et al. Plasma levels and renal handling of amino acids contribute to determination of risk of mortality or feed of ventilation in patients with COVID-19. Metabolites. 2022;12(6):486. 10.3390/metabo12060486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang F, et al. Plasma metabolomic profiles associated with mortality and longevity in a prospective analysis of 13,512 individuals. Nat Commun. 2023;14(1):5744. 10.1038/s41467-023-41515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamori Y, Miura A, Taira K. Implications from and for food cultures for cardiovascular diseases: Japanese food, particularly Okinawan diets. Asia Pac J Clin Nutr. 2001;10(2):144–5. 10.1111/j.1440-6047.2001.00227.x. [DOI] [PubMed] [Google Scholar]
- 89.Cox PA, Metcalf JS. Traditional food items in Ogimi, Okinawa: l-serine content and the potential for neuroprotection. Curr Nutr Rep. 2017;6(1):24–31. 10.1007/s13668-017-0191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pandya VA, Patani R. Decoding the relationship between ageing and amyotrophic lateral sclerosis: a cellular perspective. Brain. 2020;143(4):1057–72. 10.1093/brain/awz360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lau V, Ramer L, Tremblay M-È. An aging, pathology burden, and glial senescence build-up hypothesis for late onset Alzheimer’s disease. Nature Communications. 2023;14(1):1670. 10.1038/s41467-023-37304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilkerson HL. Problems of an aging population : public health aspects of diabetes. Am J Public Health Nations Health 1947;37(2):177–188. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/18016480. Accessed 8 Nov 2024. [PMC free article] [PubMed]
- 93.Yan M, et al. Cardiac aging: from basic research to therapeutics. Oxid Med Cell Longev. 2021;2021:9570325. 10.1155/2021/9570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berben L, Floris G, Wildiers H, Hatse S. Cancer and aging: two tightly interconnected biological processes. Cancers (Basel). 2021;13(6):1400. 10.3390/cancers13061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Franceschi C, et al. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med (Lausanne). 2018;5:61. 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fried, L.P., Ferrucci, L. Etiological role of aging in chronic diseases: from epidemiological evidence to the New Geroscience. In: Sierra F, Kohanski R, editors. Advances in Geroscience. Springer: Cham; 2016. pp. 37–51. 10.1007/978-3-319-23246-1_2
- 97.Locasale JW, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43(9):869–74. 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen L, et al. Serine metabolism antagonizes antiviral innate immunity by preventing ATP6V0d2-mediated YAP lysosomal degradation. Cell Metab. 2021;33(5):971-987.e6. 10.1016/j.cmet.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 99.Cao G, Tao F, Xin L, Li Z, Zhou X. Effects of maternal serine supplementation on high-fat diet-induced oxidative stress and epigenetic changes in promoters of glutathione synthesis-related genes in offspring. J Funct Foods. 2018;47:316–24. 10.1016/j.jff.2018.05.067. [Google Scholar]
- 100.Zhou X, He L, Wu C, Zhang Y, Wu X, Yin Y. Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol Nutr Food Res. 2017;61(11):1700262. 10.1002/mnfr.201700262. [DOI] [PubMed] [Google Scholar]
- 101.Cai HY, Tian KW, Zhang YY, Jiang H, Han S. Angiopoietin-1 and alphanubeta3 integrin peptide promote the therapeutic effects of L-serine in an amyotrophic lateral sclerosis/Parkinsonism dementia complex model. Aging (Albany NY). 2018;10(11):3507–27. 10.18632/aging.101661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holm LJ, Haupt-Jorgensen M, Larsen J, Giacobini JD, Bilgin M, Buschard K. L-serine supplementation lowers diabetes incidence and improves blood glucose homeostasis in NOD mice. PLoS ONE. 2018;13(3): e0194414. 10.1371/journal.pone.0194414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xia C, et al. Long-term effects of l-serine supplementation upon a mouse model of diabetic neuropathy. J Diabetes Complications. 2023;37(2):108383. 10.1016/j.jdiacomp.2022.108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mishra RC, et al. L-serine lowers while glycine increases blood pressure in chronic L-NAME-treated and spontaneously hypertensive rats. J Hypertens. 2008;26(12):2339–48. 10.1097/hjh.0b013e328312c8a3. [DOI] [PubMed] [Google Scholar]
- 105.Mishra RC, et al. Nitric oxide synthase inhibition promotes endothelium-dependent vasodilatation and the antihypertensive effect of L-serine. Hypertension. 2008;51(3):791–6. 10.1161/hypertensionaha.107.099598. [DOI] [PubMed] [Google Scholar]
- 106.Zhou X, et al. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochimica et Biophysica Acta (BBA) - Mole Basis Dis. 2018;1864(2):488–98. 10.1016/j.bbadis.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 107.Sim WC, et al. L-serine supplementation attenuates alcoholic fatty liver by enhancing homocysteine metabolism in mice and rats. J Nutr. 2015;145(2):260–7. 10.3945/jn.114.199711. [DOI] [PubMed] [Google Scholar]
- 108.Yun HH, et al. Effects of losartan and l-serine in a mouse liver fibrosis model. Life Sciences. 2021;278:119578. 10.1016/j.lfs.2021.119578. [DOI] [PubMed] [Google Scholar]
- 109.Ma EH, et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 2017;25(2):345–57. 10.1016/j.cmet.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 110.Murtas G, Marcone GL, Sacchi S, Pollegioni L. L-serine synthesis via the phosphorylated pathway in humans. Cell Mole Life Sci. 2020;77(24):5131–48. 10.1007/s00018-020-03574-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ye L, Sun Y, Jiang Z, Wang G. L-serine, an endogenous amino acid, is a potential neuroprotective agent for neurological disease and injury. Front Mol Neurosci. 2021;14: 726665. 10.3389/fnmol.2021.726665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maffioli E, et al. Insulin and serine metabolism as sex-specific hallmarks of Alzheimer’s disease in the human hippocampus. Cell Rep. 2022;40(10):111271. 10.1016/j.celrep.2022.111271. [DOI] [PubMed] [Google Scholar]
- 113.Yulug B, et al. Combined metabolic activators improve cognitive functions in Alzheimer’s disease patients: a randomised, double-blinded, placebo-controlled phase-II trial”. Transl Neurodegener. 2023;12(1):4. 10.1186/s40035-023-00336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dunlop RA, Powell JT, Metcalf JS, Guillemin GJ, Cox PA. L-serine-mediated neuroprotection includes the upregulation of the ER stress chaperone protein disulfide isomerase (PDI). Neurotox Res. 2018;33(1):113–22. 10.1007/s12640-017-9817-7. [DOI] [PubMed] [Google Scholar]
- 115.Okle O, Stemmer K, Deschl U, Dietrich DR. L-BMAA induced ER stress and enhanced caspase 12 cleavage in human neuroblastoma SH-SY5Y cells at low nonexcitotoxic concentrations. Toxicol Sci. 2013;131(1):217–24. 10.1093/toxsci/kfs291. [DOI] [PubMed] [Google Scholar]
- 116.Cox PA, Davis DA, Mash DC, Metcalf JS, Banack SA. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc Royal Soc B: Biol Sci. 2016;283(1823):20152397. 10.1098/rspb.2015.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bradley WG, Mash DC. Beyond Guam: the cyanobacteria/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases. Amyotroph Lateral Scler. 2009;10(Suppl 2):7–20. 10.3109/17482960903286009. [DOI] [PubMed] [Google Scholar]
- 118.Jessen F, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10(6):844–52. 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alzheimer’s Association. 2018 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dement. 2018;14(3):367–429. 10.1016/j.jalz.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 120.Avellar M, et al. The effect of D-serine administration on cognition and mood in older adults. Oncotarget. 2016;7(11):11881–8. 10.18632/oncotarget.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoshida K, et al. Targeted disruption of the mouse 3-phosphoglycerate dehydrogenase gene causes severe neurodevelopmental defects and results in embryonic lethality. J Biol Chem. 2004;279(5):3573–7. 10.1074/jbc.C300507200. [DOI] [PubMed] [Google Scholar]
- 122.Jaeken J, et al. 3-Phosphoglycerate dehydrogenase deficiency and 3-phosphoserine phosphatase deficiency: inborn errors of serine biosynthesis. J Inherit Metab Dis. 1996;19(2):223–6. 10.1007/bf01799435. [DOI] [PubMed] [Google Scholar]
- 123.Hart CE, et al. Phosphoserine aminotransferase deficiency: a novel disorder of the serine biosynthesis pathway. Am J Hum Genet. 2007;80(5):931–7. 10.1086/517888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bonvento G, Oliet SHR, Panatier A. Glycolysis-derived L-serine levels versus PHGDH expression in Alzheimer’s disease. Cell Metab. 2022;34(5):654–5. 10.1016/j.cmet.2022.04.002. [DOI] [PubMed] [Google Scholar]
- 125.Kiernan MC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–55. 10.1016/s0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 126.Mehta P, et al. Prevalence of amyotrophic lateral sclerosis - United States, 2014. MMWR Morb Mortal Wkly Rep. 2018;67(7):216–8. 10.15585/mmwr.mm6707a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Levine TD, et al. Phase I clinical trial of safety of L-serine for ALS patients. Amyotrophic Lat Sclerosis Frontotemp Degen. 2017;18(1–2):107–11. 10.1080/21678421.2016.1221971. [DOI] [PubMed] [Google Scholar]
- 128.Fridman V, et al. Randomized trial of l-serine in patients with hereditary sensory and autonomic neuropathy type 1. Neurology. 2019;92(4):e359–70. 10.1212/WNL.0000000000006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. 2021;17(9):534–48. 10.1038/s41574-021-00512-2. [DOI] [PubMed] [Google Scholar]
- 130.Bertea M, et al. Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis. 2010;9:84. 10.1186/1476-511x-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Drábková P, Šanderová J, Kovařík J, Kanďár R. An assay of selected serum amino acids in patients with type 2 diabetes mellitus. Adv Clin Exp Med. 2015;24(3):447–51. 10.17219/acem/29223. [DOI] [PubMed] [Google Scholar]
- 132.Kamaura M, Nishijima K, Takahashi M, Ando T, Mizushima S, Tochikubo O. Lifestyle modification in metabolic syndrome and associated changes in plasma amino acid profiles. Circ J. 2010;74(11):2434–40. 10.1253/circj.cj-10-0150. [DOI] [PubMed] [Google Scholar]
- 133.Mook-Kanamori DO, et al. Type 2 diabetes is associated with postprandial amino acid measures. Arch Biochem Biophys. 2016;589:138–44. 10.1016/j.abb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 134.Enquobahrie DA, Denis M, Tadesse MG, Gelaye B, Ressom HW, Williams MA. Maternal early pregnancy serum metabolites and risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2015;100(11):4348–56. 10.1210/jc.2015-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vangipurapu J, Stancáková A, Smith U, Kuusisto J, Laakso M. Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5,181 Finnish men. Diabetes. 2019;68(6):1353–8. 10.2337/db18-1076. [DOI] [PubMed] [Google Scholar]
- 136.Handzlik MK, et al. Insulin-regulated serine and lipid metabolism drive peripheral neuropathy. Nature. 2023;614(7946):118–24. 10.1038/s41586-022-05637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou X, et al. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochim Biophys Acta Mol Basis Dis. 2018;1864(2):488–98. 10.1016/j.bbadis.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 138.Radonjić T, et al. Aging of liver in its different diseases. Int J Mol Sci. 2022;23(21):13085. 10.3390/ijms232113085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bianchi G, et al. Impaired insulin-mediated amino acid plasma disappearance in non-alcoholic fatty liver disease: a feature of insulin resistance. Dig Liver Dis. 2003;35(10):722–7. 10.1016/s1590-8658(03)00416-x. [DOI] [PubMed] [Google Scholar]
- 140.Sim WC, et al. Downregulation of PHGDH expression and hepatic serine level contribute to the development of fatty liver disease. Metabolism. 2020;102: 154000. 10.1016/j.metabol.2019.154000. [DOI] [PubMed] [Google Scholar]
- 141.Lopez-Gonzales E, et al. L-serine supplementation blunts fasting-induced weight regain by increasing brown fat thermogenesis. Nutrients. 2022;14(9):1922. 10.3390/nu14091922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–64. 10.1016/j.cell.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–18. 10.1172/jci24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fan F, et al. Association between serine concentration and coronary heart disease: a case-control study. Int J Gen Med. 2024;17:2955–65. 10.2147/ijgm.S467320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Perea-Gil I, et al. Serine biosynthesis as a novel therapeutic target for dilated cardiomyopathy. Eur Heart J. 2022;43(36):3477–89. 10.1093/eurheartj/ehac305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Verhoef P, Steenge GR, Boelsma E, van Vliet T, Olthof MR, Katan MB. Dietary serine and cystine attenuate the homocysteine-raising effect of dietary methionine: a randomized crossover trial in humans123. Am J Clin Nutr. 2004;80(3):674–9. 10.1093/ajcn/80.3.674. [DOI] [PubMed] [Google Scholar]
- 147.Muthusamy T, et al. Serine restriction alters sphingolipid diversity to constrain tumour growth. Nature. 2020;586(7831):790–5. 10.1038/s41586-020-2609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu W, et al. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol Cell. 2019;75(6):1147-1160.e5. 10.1016/j.molcel.2019.06.039. [DOI] [PubMed] [Google Scholar]
- 149.Bradshaw PC. Cytoplasmic and mitochondrial NADPH-coupled redox systems in the regulation of aging. Nutrients. 2019;11(3):504. 10.3390/nu11030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sinha R, et al. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur J Clin Nutr. 2018;72(1):105–11. 10.1038/ejcn.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Homma T, Fujii J. Application of glutathione as anti-oxidative and anti-aging drugs. Curr Drug Metab. 2015;16(7):560–71. 10.2174/1389200216666151015114515. [DOI] [PubMed] [Google Scholar]
- 152.Lapenna D. Glutathione and glutathione-dependent enzymes: from biochemistry to gerontology and successful aging. Ageing Res Rev. 2023;92:102066. 10.1016/j.arr.2023.102066. [DOI] [PubMed] [Google Scholar]
- 153.Tain LS, et al. Longevity in response to lowered insulin signaling requires glycine N-methyltransferase-dependent spermidine production. Aging Cell. 2020;19(1):e13043. 10.1111/acel.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miller RA, et al. Glycine supplementation extends lifespan of male and female mice. Aging Cell. 2019;18(3):e12953. 10.1111/acel.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brind J, et al. Dietary glycine supplementation mimics lifespan extension by dietary methionine restriction in Fisher 344 rats. The FASEB Journal. 2011;25(S1):528.2-528.2. 10.1096/fasebj.25.1_supplement.528.2. [Google Scholar]
- 156.Ham DJ, Murphy KT, Chee A, Lynch GS, Koopman R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin Nutr. 2014;33(3):448–58. 10.1016/j.clnu.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 157.Ullah R, et al. Glycine, the smallest amino acid, confers neuroprotection against D-galactose-induced neurodegeneration and memory impairment by regulating c-Jun N-terminal kinase in the mouse brain. J Neuroinflammation. 2020;17(1):303. 10.1186/s12974-020-01989-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen J, et al. Dietary supplementation with glycine enhances intestinal mucosal integrity and ameliorates inflammation in C57BL/6J mice with high-fat diet–induced obesity. J Nutr. 2021;151(7):1769–78. 10.1093/jn/nxab058. [DOI] [PubMed] [Google Scholar]
- 159.Kumar P, Osahon OW, Sekhar RV. GlyNAC (glycine and N-acetylcysteine) supplementation in old mice improves brain glutathione deficiency, oxidative stress, glucose uptake, mitochondrial dysfunction, genomic damage, inflammation and neurotrophic factors to reverse age-associated cognitive decline: implications for improving brain health in aging. Antioxidants (Basel). 2023;12(5):1042. 10.3390/antiox12051042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Obata F, Miura M. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat Commun. 2015;6(1):8332. 10.1038/ncomms9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luka Z, Capdevila A, Mato JM, Wagner C. A glycine N-methyltransferase knockout mouse model for humans with deficiency of this enzyme. Transgenic Res. 2006;15(3):393–7. 10.1007/s11248-006-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martinez-Chantar ML, et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47(4):1191–9. 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Benevenga NJ, Harper AE. Effect of glycine and serine on methionine metabolism in rats fed diets high in methionine. J Nutr. 1970;100(10):1205–14. 10.1093/jn/100.10.1205. [DOI] [PubMed] [Google Scholar]
- 164.Sugiyama K, Kushima Y, Muramatsu K. Effect of dietary glycine on methionine metabolism in rats fed a high-methionine diet. J Nutr Sci Vitaminol (Tokyo). 1987;33(3):195–205. 10.3177/jnsv.33.195. [DOI] [PubMed] [Google Scholar]
- 165.Mair W, et al. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470(7334):404–8. 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hwang AB, et al. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2014;111(42):E4458–67. 10.1073/pnas.1411199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Greer EL, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17(19):1646–56. 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh PP, Demmitt BA, Nath RD, Brunet A. The genetics of aging: a vertebrate perspective. Cell. 2019;177(1):200–20. 10.1016/j.cell.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ogawa T, et al. Stimulating S-adenosyl-l-methionine synthesis extends lifespan via activation of AMPK. Proc Natl Acad Sci U S A. 2016;113(42):11913–8. 10.1073/pnas.1604047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ge Y, Zhou M, Chen C, Wu X, Wang X. Role of AMPK mediated pathways in autophagy and aging. Biochimie. 2022;195:100–13. 10.1016/j.biochi.2021.11.008. [DOI] [PubMed] [Google Scholar]
- 171.Soda K. Polyamine metabolism and gene methylation in conjunction with one-carbon metabolism. Int J Mol Sci. 2018;19(10):3106. 10.3390/ijms19103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Scalabrino G, Ferioli ME. Polyamines in mammalian ageing: an oncological problem, too? A review. Mech Ageing Dev. 1984;26(2–3):149–64. 10.1016/0047-6374(84)90090-3. [DOI] [PubMed] [Google Scholar]
- 173.Gupta VK, et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci. 2013;16(10):1453–60. 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
- 174.Eisenberg T, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305–14. 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 175.Madeo F, Carmona-Gutierrez D, Kepp O, Kroemer G. Spermidine delays aging in humans. Aging (Albany NY). 2018;10(8):2209–11. 10.18632/aging.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Madeo F, Eisenberg T, Büttner S, Ruckenstuhl C, Kroemer G. Spermidine: a novel autophagy inducer and longevity elixir. Autophagy. 2010;6(1):160–2. 10.4161/auto.6.1.10600. [DOI] [PubMed] [Google Scholar]
- 177.Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359:6374. 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- 178.Eisenberg T, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22(12):1428–38. 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kiechl S, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr. 2018;108(2):371–80. 10.1093/ajcn/nqy102. [DOI] [PubMed] [Google Scholar]
- 180.Madeo F, Bauer MA, Carmona-Gutierrez D, Kroemer G. Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy. 2019;15(1):165–8. 10.1080/15548627.2018.1530929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mouchiroud L, et al. Pyruvate imbalance mediates metabolic reprogramming and mimics lifespan extension by dietary restriction in Caenorhabditis elegans. Aging Cell. 2011;10(1):39–54. 10.1111/j.1474-9726.2010.00640.x. [DOI] [PubMed] [Google Scholar]
- 182.Kim JY, et al. Pyruvate protects against cellular senescence through the control of mitochondrial and lysosomal function in dermal fibroblasts. J Investigative Dermatol. 2018;138(12):2522–30. 10.1016/j.jid.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 183.Tian Q, et al. Dietary alpha-ketoglutarate promotes beige adipogenesis and prevents obesity in middle-aged mice. Aging Cell. 2020;19(1): e13059. 10.1111/acel.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270(51):30701–8. 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 185.Tedesco P, Jiang J, Wang J, Jazwinski SM, Johnson TE. Genetic analysis of hyl-1, the C. elegans homolog of LAG1/LASS1. Age (Dordr). 2008;30(1):43–52. 10.1007/s11357-008-9046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mosbech MB, et al. Functional loss of two ceramide synthases elicits autophagy-dependent lifespan extension in C. elegans. PLoS One. 2013;8(7):e70087. 10.1371/journal.pone.0070087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mencarelli C, Martinez-Martinez P. Ceramide function in the brain: when a slight tilt is enough. Cell Mol Life Sci. 2013;70(2):181–203. 10.1007/s00018-012-1038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cutler RG, Mattson MP. Sphingomyelin and ceramide as regulators of development and lifespan. Mech Ageing Dev. 2001;122(9):895–908. 10.1016/S0047-6374(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 189.Jung PP, et al. Natural variation of chronological aging in the Saccharomyces cerevisiae species reveals diet-dependent mechanisms of life span control. NPJ Aging Mech Dis. 2018;4:3. 10.1038/s41514-018-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mirisola MG, Taormina G, Fabrizio P, Wei M, Hu J, Longo VD. Serine- and threonine/valine-dependent activation of PDK and Tor orthologs converge on Sch9 to promote aging. PLoS Genet. 2014;10(2):e1004113. 10.1371/journal.pgen.1004113. [DOI] [PMC free article] [PubMed] [Google Scholar]