Abstract
Background/Aims: Helicobacter pylori induces the apoptosis of gastric epithelial cells in vivo and in vitro. However, the molecular mechanism has not been clarified. The aim of this study was to investigate the effect of H pylori on the apoptosis of gastric epithelial cells and the expression of apoptosis related genes in vitro.
Methods: Human gastric adenocarcinoma SGC-7901 cells were co-cultured with a cytotoxic H pylori strain, NCTC 11637, at various densities ranging from 3.2 × 104 to 1.0 × 108 colony forming units (CFU)/ml for 48 hours. Apoptosis in gastric cells was determined by transmission electron microscopy, Hoechst 33258 fluorochrome staining, and flow cytometry. The expression of apoptosis related proteins, Bcl-2, Bax, and c-Myc, was measured by an immunohistochemical method, and c-Myc mRNA expression was determined by the reverse transcription-polymerase chain reaction.
Results: Helicobacter pylori induces morphological changes typical of apoptosis. Both fluorochrome staining and flow cytometry showed that the apoptotic index began to increase when H pylori were at a density of > 1.6 × 104 CFU/ml, and in a density dependent manner (p < 0.01; one way ANOVA). The expression of the Bax and c-Myc proteins and of c-Myc mRNA was increased, whereas Bcl-2 expression was decreased after co-culture for 48 hours.
Conclusions: Helicobacter pylori induced apoptosis in gastric epithelial cells is mediated by altered expression of the products of the Bcl-2, Bax, and c-Myc genes.
Keywords: Helicobacter pylori, gastric epithelial cells, apoptosis, Bcl-2, Bax, c-Myc
Helicobacter pylori infection in the human stomach is common, particularly in developing countries, where most adults are infected.1 Epidemiological data, clinical studies, and experimental studies have shown that H pylori is a major aetiological agent for gastritis, peptic ulcer disease, and gastric malignancy.2,3 However, the pathogenic mechanisms by which the organism causes the diseases have not been fully defined.
Gastric mucosal integrity is maintained by a balance between the rate of cell loss and the rate of epithelial cell regeneration.4 Cell proliferation and apoptosis (programmed cell death) are essential events involved in the cellular turnover of epithelial tissue.4–7 Apoptosis is a physiological suicide mechanism that occurs during normal tissue turnover and is involved in tissue homoeostasis.4,5 In the gastric epithelium, apoptosis plays an essential role in maintaining tissue integrity. Normally, the rate of cell loss by apoptosis is matched by the rate of new cell production by proliferation.6 However, this balance may be affected by H pylori infection, leading to various gastroduodenal diseases. It has been shown that H pylori infection induces apoptosis in gastric epithelial cells,8–20 and subsequently results in an increase in cell proliferation as a host response to apoptosis.4,21,22 However, apoptosis induced by H pylori infection that is not accompanied by a matched increase in cell proliferation will result in the loss of mucosal integrity, leading to gastric erosion and ulceration, or the loss of gastric glands, leading to gastric atrophy.13,23,24 Alternatively, if the response to apoptosis is greatly increased cell proliferation, this will result in increased proliferation of the gastric mucosa, which is believed to increase the risk of the development of gastric neoplasia.4,7
“In the gastric epithelium, apoptosis plays an essential role in maintaining tissue integrity”
Whether or not a cell undergoes apoptosis is controlled by the products of a large number of oncogenes and tumour suppressor genes, some of which are aberrantly expressed in a proportion of gastric carcinomas.7,25 These products include proapoptotic proteins, such as c-Myc, p53, c-Fos, c-Jun, and some of the Bcl-2 family members (for example, Bak, Bad, Bcl-xs, etc), and antiapoptotic proteins, such as Mcl-1, c-Abl, retinoblastoma protein (pRb), and some of the Bcl-2 members (for example, Bcl-2, Bcl-xl, etc).7 However, the genetic mechanism that regulates H pylori induced apoptosis has not been fully elucidated. There are a few reports regarding an association between the Bcl-2 family and H pylori induced apoptosis,14,26,27 but most of these studies have been carried out in vivo. A recent study showed that Bcl-2 is associated with the release of mitochondrial cytochrome c, which is a major mediator of H pylori induced apoptosis.28 Studies have shown that c-Myc has a dual role in regulating apoptosis and proliferation, but its role in H pylori induced apoptosis is unknown.29 We hypothesise that H pylori infection induces apoptosis via pathways mediated by the proteins of the Bcl-2 family and/or the c-Myc gene. Therefore, this study was conducted to determine the effect of H pylori on cell apoptosis and the expression of apoptosis regulating proteins including Bcl-2, Bax, and c-Myc in an in vitro model.
MATERIALS AND METHODS
Helicobacter pylori culture
Experiments were performed with a cytotoxic (CagA+ and VacA+) reference strain of H pylori, NCTC 11637 (National Collection of Type Cultures, Public Health Laboratory, London, UK). Helicobacter pylori were grown for 48–72 hours at 37°C in an atmosphere with 5% CO2 on nutrition agar plates (supplemented with 5% sheep’s blood), harvested, and resuspended in RPMI 1640 (Gibco Company, New York, USA) medium, which was supplemented with 2% fetal calf serum (FCS). The bacterial densities were adjusted by the optical density (OD) measurement at 660 nm—that is, 1 OD660 = 108 colony forming units (CFU)/ml.
Cell culture
The cell line SGC-7901, derived from a human gastric adenocarcinoma, was used in our study (Institute of Cytobiology of Chinese Academy of Sciences, Shanghai, China). Cells were grown in culture flasks containing RPMI 1640 culture medium supplemented with 10% FCS at 37°C in a humidified atmosphere with 5% CO2. For the evaluation of apoptosis and gene expression by fluorescence microscopy and immunohistochemistry, the cells were cultured on chamber slides in RPMI 1640 medium.
Co-culture of the gastric cells and H pylori
The gastric cells were seeded into flasks or the wells of microtitre plates at a density of 5 × 105 cells/ml or 5 × 104 cells/well, respectively. The flasks or microtitre plates were incubated overnight, and washed three times in RPMI 1640 medium. Then, medium containing intact, viable H pylori cells, ranging from 3.2 × 104 to 1.0 × 108 CFU/ml, was added, in quadruplicate, to corresponding flasks or wells. Co-culture of the gastric cells and H pylori was performed for 48 hours before the determination of cellular apoptosis and the expression of apoptosis related proteins.26,30 The mean (SD) of the quadruplicate tests was used to assess the effect of H pylori on apoptosis and the expression of apoptosis related proteins at certain densities of H pylori cells.
Assessment of apoptosis
Apoptosis was assessed by three independent methods, namely: transmission electron microscopy, DNA specific fluorochrome staining, and flow cytometry.
Transmission electron microscopy
The gastric cells co-cultured with H pylori for 48 hours were harvested with a rubber policeman. The cell pellets were fixed with 2.5% glutaraldehyde for two hours, and then with 1% OsO4 for 90 minutes. Next, the cells were dehydrated in an ethanol gradient, and embedded and polymerised at 60°C for 24 hours. Finally, ultrathin sections (40–80 nm) were prepared, double stained with 1% uranyl acetate and lead citrate, and examined by transmission electron microscopy (H-600; Hitachi Corporation, Tokyo, Japan).
DNA specific fluorochrome staining
The gastric cells co-cultured with H pylori on chamber slides for 48 hours were fixed with glacial acetic acid/methanol (3/1) for 10 minutes. Then the cells were incubated with Hoechst 33258 (Sigma Company, Saint Louis, Missouri, USA; 0.5 μg/ml) for one hour at room temperature. The slides were examined under fluorescence microscopy, and cells with condensed and fragmented chromatin in their nuclei were considered apoptotic. The apoptotic index of gastric cells was determined by the percentage of apoptotic cells over the total number of cells counted. At least 300 cells were counted in each experiment.
Flow cytometric analysis
The gastric cells co-cultured with H pylori in flasks for 48 hours were harvested and fixed with 70% alcohol for 12 hours at −20°C. Next, the cells were incubated with propidium iodide (50 μg/ml) and RNase A (5 μg/ml) for 30 minutes at room temperature in the dark. The cells were analysed by flow cytometry (BD Company, New York, USA) for cell apoptosis.
Assessing the expression of the Bcl-2, Bax, and c-Myc proteins
Gastric cells co-cultured with H pylori on chamber slides for 48 hours were fixed with glacial acetic acid/methanol (3/1), and then used to detect the expression of the Bcl-2, Bax, and c-Myc proteins by means of immunohistochemistry. Briefly, the slides were treated with 3% hydrogen peroxide for 60 minutes at 37°C, and incubated with rabbit monoclonal antihuman Bax antibody for 30 minutes, or with mouse monoclonal antihuman Bcl-2 or c-Myc antibody for 90 minutes at 37°C, followed by the application of biotinylated goat antirabbit or antimouse immunoglobins for 10 minutes at 37°C. Next, the slides were stained with the avidin–biotin complex and developed with diaminobenzidene-hydrogen peroxidase substrate (DAB; Maxim Biotect, Fuzhou, China), and lightly counterstained with haematoxylin. All antibodies and the avidin–biotin complex were purchased from Maxim Biotect and were ready to use without further dilution.
For each slide, cells were counted for immunostaining in five randomly chosen fields with at least 100 cells in each field. The intensity of immunostaining was scored as follows: 0, negative; 1+, weak; 2+, moderate; and 3+, strong. Cells with moderate or strong immunostaining were defined as positively stained. The percentage of positively stained cells over the total number of cells counted was calculated to provide the positive rate.
Measurement of c-Myc mRNA
The gastric cells co-cultured with H pylori in flasks for 48 hours were harvested. c-Myc mRNA in the cells was measured by the reverse transcription-polymerase chain reaction (RT-PCR). The primers specific for c-Myc were c1 (5′-GCATCCACGAAACTT-3′) and c2 (5′-AACGTTGAGGGGCAT-3′). The primers specific for β actin (as an internal reference) were β1 (5′-CTACAATGAGCTGCGTGTGGC-3′ and β2 (5′-CAGGTCCAGACGCAGGATGGC-3′).
RNA was extracted from the cells using a total RNA extraction kit (SABC, Shanghai, China). Contaminated DNA was removed by treating the samples with RNase free DNase I (Promega, Madison, Wisconsin, USA). RT-PCR was performed using a reverse transcription system (Promega), according to the manufacturer’s instructions. Briefly, the first strand cDNA was synthesised using the c2 and β2 primers and avian myeloblastosis virus reverse transcriptase (Promega), followed by PCR amplification using the c1, c2, β1, and β2 primers. PCR amplification was performed for 30 cycles. The PCR products were electrophoresed on a 40% polyacrylamide gel and stained with AgNO3. The average grey density of the c-Myc RT-PCR products in the polyacrylamide gel electrophoresis (PAGE) bands, which reflects the extent of c-Myc mRNA expression, was analysed using an image analysis system (IAS-1000; Quality Engineering Associates, Burlington, USA ).
Statistical analysis
The t test for independent samples was used to determine the difference in the apoptotic index, and the expression of the Bcl-2, Bax, and c-Myc proteins and c-Myc mRNA between each study group and the control. One way ANOVA was used to determine the effect of increasing numbers of H pylori on apoptosis and the expression of the Bcl-2, Bax, and c-Myc proteins and c-Myc mRNA. All p values calculated were two tailed; the α level of significance was set at p < 0.05.
RESULTS
Effect of H pylori on apoptosis
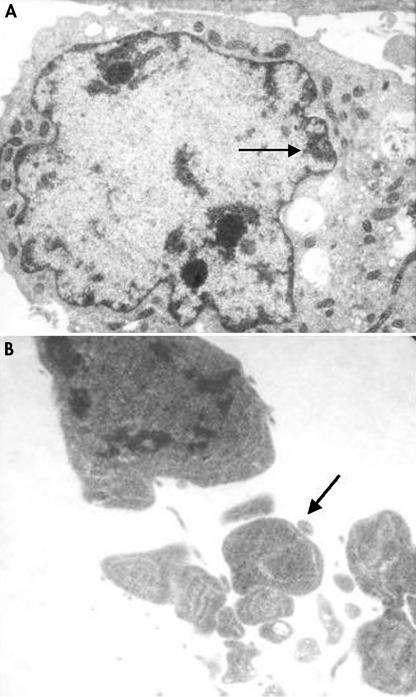
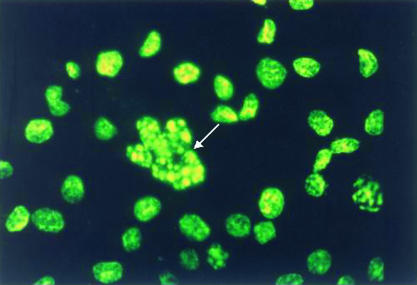
The ultrastructural changes associated with apoptosis in SGC-7901 cells after co-culture with H pylori for 48 hours are shown in fig 1A and 1B ▶. These changes included condensation beneath the nuclear membrane of irregular, crescent shaped, highly osmiophilic chromatin and the formation of membrane bound fragments (apoptotic bodies), as described previously.7 DNA specific fluorochrome staining also demonstrated the typical morphological changes of apoptosis in the gastric cells after incubation with H pylori for 48 hours—that is, chromatin condensation and nuclear fragmentation (fig 2 ▶). There was no significant difference between the apoptotic index obtained with a bacterial density of 3.2 × 104 CFU/ml and that seen in the control group (where no bacterial cells were added). However, the apoptotic index increased significantly when a bacterial density of 1.6 × 105 CFU/ml or greater was used, and this occurred in a density dependent manner (table 1 ▶). Flow cytometric analysis showed similar results to the fluorochrome staining—the apoptotic index of the gastric cells started to increase at a bacterial density of 1.6 × 105 CFU/ml, in a density dependent manner (table 1 ▶). However, a large number of necrotic cells were seen when the cells were co-cultured with H pylori at densities of 2 × 107 and 1 × 108 CFU/ml, so that results obtained at these densities were considered inaccurate, and not included in the analysis.
Figure 1.
Transmission electron microscopy showing the ultrastructural apoptotic changes of SGC-7901 cells after co-culture with Helicobacter pylori for 48 hours: (A) condensation of irregular nuclear chromatin (arrow) (original magnification, ×10 000); and (B) formation of apoptotic bodies (arrow) (original magnification, ×20 000).
Figure 2.
DNA specific fluorochrome staining showing condensation and fragmentation of nuclei of SGC-7901 cells (arrow) after co-culture with Helicobacter pylori (1.6 × 105 colony forming units/ml) for 48 hours (original magnification, ×400).
Table 1.
Apoptotic index (AI) of SGC-7901 cells after co-culture with increasing concentrations of Helicobacter pylori for 48 hours using DNA specific fluorochrome staining and flow cytometry
| Mean apoptotic index (SD) | ||
| Concentration of H pylori (CFU/ml) | Fluorochrome staining | Flow cytometry |
| 0 (control) | 1.68 (0.13) | 1.70 (0.12) |
| 3.2×104 | 2.35 (0.44) | 2.30 (0.27) |
| 1.6×105 | 3.55 (0.24)* | 3.56 (0.11)* |
| 8.0×105 | 5.35 (0.37)* | 5.31 (0.75)* |
| 4.0×106 | 9.53 (0.15)*† | 9.49 (0.17)*† |
Apoptotic index of gastric cells was defined by the percentage of apoptotic cells over the total number of cells counted. *p<0.01, compared with control; †one way ANOVA, p<0.01.
CFU, colony forming units.
Effect of H pylori on the expression of Bcl-2, Bax, and c-Myc
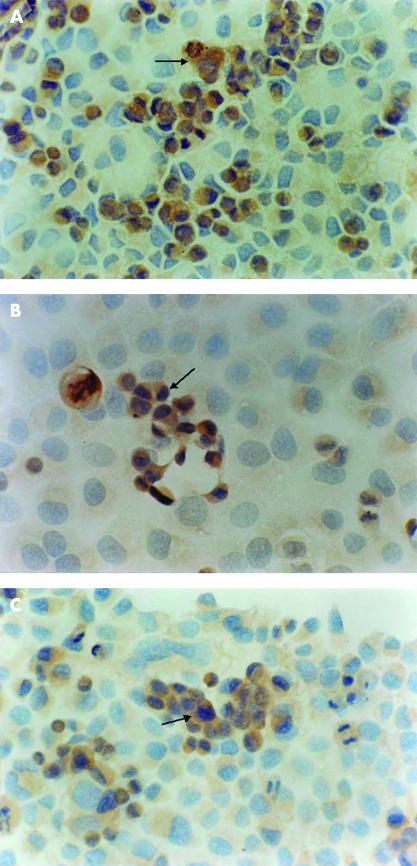
SGC-7901 cells constitutively express Bcl-2, Bax, and c-Myc in their cytoplasm; 40%, 23.3%, and 2.2% of cells were immunostained for Bcl-2, Bax, and c-Myc, respectively, with moderate or strong intensity (table 2 ▶). The expression of the Bax and c-Myc proteins was increased (fig 3A ▶, B), whereas the expression of the Bcl-2 protein was decreased after co-culture of the gastric cells with H pylori for 48 hours (fig 3C ▶). These changes were also dependent on the density of H pylori (p < 0.01; one way ANOVA; table 2 ▶).
Table 2.
Positive rate of Bcl-2, Bax, and c-Myc proteins of SGC-7901 cells after co-culture with increasing concentrations of Helicobacter pylori for 48 hours as determined using immunohistochemistry
| Positive rate (% (SD)) | |||
| Concentration of H pylori (CFU/ml) | Bcl-2 | Bax | c-Myc |
| 0 (control) | 40.00 (2.94) | 23.25 (1.26) | 2.20 (0.65) |
| 3.2×104 | 36.75 (1.71) | 24.25 (1.26) | 2.78 (0.97) |
| 1.6×105 | 25.25 (1.71)* | 34.50 (2.38)* | 5.65 (1.07)* |
| 8.0×105 | 14.50 (2.65)* | 43.75 (1.71)* | 9.23 (0.95)* |
| 4.0×106 | 7.75 (1.71)*† | 53.75 (2.63)*† | 12.88 (0.74)*† |
*p<0.01, compared with control; †one way ANOVA, p<0.01.
CFU, colony forming units.
Figure 3.
Positive immunohistochemical staining for (A) Bax (original magnification, ×200), (B) c-Myc (original magnification, ×400), and (C) Bcl-2 (original magnification, ×200) proteins in the cytoplasm of SGC-7901 cells (arrows) after co-culture with Helicobacter pylori (1.6 × 105 colony forming units/ml) for 48 hours.
Effect of H pylori on the expression of c-Myc mRNA
The c1 and c2 primers amplified a 237 bp section of the c-Myc gene. The average grey density of the c-Myc RT-PCR product in PAGE bands was increased after co-culture of the gastric cells with H pylori for 48 hours; these changes were also H pylori density dependent (p < 0.01; one way ANOVA; table 3 ▶).
Table 3.
Mean grey density of c-Myc RT-PCR products of SGC-7901 cells in PAGE bands after co-culture with increasing concentrations of Helicobacter pylori for 48 hours
| Concentration of H pylori (CFU/ml) | Mean (SD) grey density |
| 0 (control) | 156.2 (7.6) |
| 3.2×104 | 172.4 (4.2)* |
| 1.6×105 | 203.7 (3.7)* |
| 8.0×105 | 219.4 (1.4)* |
| 4.0×106 | 226.8 (3.2)*† |
*p<0.01, compared with control; †one way ANOVA, p<0.01.
CFU, colony forming units; PAGE, polyacrylamide gel electrophoresis; RT-PCR, reverse transcription polymerase chain reaction.
DISCUSSION
Most previous studies evaluating the association between H pylori infection and apoptosis have used the terminal uridine deoxynucleotide nick end labelling method to identify apoptotic cells in gastric biopsy specimens.8,12–14,18–20 Although this technique is an important method for visualising apoptotic cells, it has the limitation of being unable to discriminate between apoptosis and necrosis.31 In our present study, three different techniques—namely, transmission electron microscopy, fluorochrome staining, and flow cytometry—were used to determine apoptosis. Our findings confirmed that H pylori induces apoptosis in gastric cells in vitro, consistent with previous studies carried out both in vitro and in vivo.8–20
The mechanism by which H pylori induces apoptosis has yet to be elucidated. Both bacterial factors and the host response may be involved in the induction of apoptosis.4 The bacterial factors may include the VacA cytotoxin and lipopolysaccharide.12,14,16,18,21,27,32–35 In addition, the production of reactive oxygen metabolites, inducible nitric oxide synthase, and various cytokines such as tumour necrosis factor α, interferon γ, interleukin 2, and interleukin 1 during H pylori infection, as part of the host response, may also contribute to H pylori induced apoptosis.9,10,16,17,36–38 However, knowledge of the genetic background of H pylori induced apoptosis is scarce. Previous studies have reported that p53 is involved in H pylori associated apoptosis of gastric mucosa,11,15 although other studies suggest that H pylori induced apoptosis is mediated by multiple eukaryotic signalling cascades that are not dependent upon increased p53 concentrations.16,17,21,26,32,37 A few studies have investigated the role of the Bcl-2 family in H pylori induced apoptosis. It was reported that in cell culture, H pylori induced apoptosis was accompanied by an increased expression of Bak (Bcl-2 associated killer gene), with little change in the expression of other Bcl-2 family members, such as Bcl-2, Bcl-xL, or Bax.39 These findings were consistent with in vivo observations that the expression of Bak was increased in gastric epithelial cells in H pylori positive patients compared with H pylori negative patients, but there was no difference in the expression of Bcl-2.39–41 These data suggest that H pylori induced gastric epithelial cell apoptosis is at least partially mediated by a Bak dependent pathway.39 In addition, in our present study, we showed that co-culture with H pylori resulted in the overexpression of Bax, and suppressed the expression of Bcl-2. These findings are in agreement with in vivo observations that H pylori infection induces apoptosis associated with an upregulation of Bax and downregulation of Bcl-2.14 In contrast, an in vitro study reported that both toxic and non-toxic H pylori strains induced the expression of the Bcl-2, Bax, and Bcl-xS proteins in p53 deleted or mutated gastric epithelial cell lines at 24 hours when apoptosis did not occur.26 However, the expression of Bcl-2 and Bax declined, whereas that of Bcl-xS became raised at 48 hours when apoptosis increased significantly, implying that Bcl-xS may be an important mediator in H pylori induced apoptosis.26 Recently, Shibayama et al reported overexpression of two other proapoptotic Bcl-2 family members, Bad and Bid.27 Overall, these observations suggest that the overexpression of proapoptotic proteins and the underexpression of antiapoptotic proteins among the Bcl-2 family may play an important part in H pylori induced apoptosis.
“Our findings are in agreement with in vivo observations that H pylori infection induces apoptosis associated with an upregulation of Bax and downregulation of Bcl-2”
In a previous study, Nardone et al detected the overexpression of the c-Myc protein in a substantial proportion (36%) of patients with gastric carcinoma, and in some patients with H pylori associated atrophic gastritis (15%), whereas this protein was not found in normal controls.42 However, no data on apoptosis were given, and the association between c-Myc and H pylori induced apoptosis remains unknown. Therefore, our present study was the first, to our knowledge, to explore this issue. Our results clearly showed that co-culture of SGC-7901 cells with H pylori for 48 hours led to an increase in cell apoptosis, which was accompanied by the increased expression of the c-Myc protein and mRNA. This indicates that the c-Myc gene, in addition to the Bcl-2 family of genes, may be involved in regulating H pylori induced apoptosis, although in vivo studies are required to confirm this observation.
Take home messages.
Helicobacter pylori induces morphological changes typical of apoptosis
The apoptotic index began to increase when H pylori were at a density of > 1.6 × 104 colony forming units/ml, in a density dependent manner
The expression of the Bax and c-Myc proteins and of c-Myc mRNA was increased, whereas Bcl-2 expression was decreased after co-culture for 48 hours
Thus, H pylori induced apoptosis in gastric epithelial cells is mediated by the altered expression of the Bcl-2, Bax, and c-Myc genes
One limitation of our study was that the cells tested were derived from a human gastric cancer, which may not respond to H pylori in the same manner as intact gastric epithelial cells. It has been shown that cancer cell lines undergo about 50% less apoptosis in response to H pylori than cells in primary culture.16 However, non-transformed gastric cell lines are rarely available, and primary cultures are difficult to achieve. For these reasons, almost all studies evaluating the effect of H pylori on apoptosis of gastric epithelial cells in vitro have used gastric cancer cell lines. In our present study, a model of co-culture of H pylori with the gastric cancer cell line, SGC-7901, was established, which produced similar results obtained with other gastric cancer cells including AGS, a commonly used cell line.
In conclusion, H pylori induced apoptosis in gastric epithelial cells is mediated by the altered expression of the Bcl-2, Bax, and c-Myc genes.
Abbreviations
CFU, colony forming units
OD, optical density
PAGE, polyacrylamide gel electrophoresis
RT-PCR, reverse transcription-polymerase chain reaction
REFERENCES
- 1.Xia HH-X, Talley NJ. Natural acquisition and spontaneous elimination of Helicobacter pylori infection. Am J Gastroenterol 1997;92:1780–7. [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. Schistosomes, liver flukes and Helicobacter pylori. In: IARC monographs on the evaluation of carcinogenic risks to humans. IARC monograph, Vol. 61. Lyon: IARC, 1994:177–241. [PMC free article] [PubMed]
- 3.Xia HH-X, Wong BCY, Lam SK. Helicobacter pylori infection and gastric cancer. Asian J Surg 2001;24:217–21. [Google Scholar]
- 4.Xia HH-X, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol 2001;96:16–26. [DOI] [PubMed] [Google Scholar]
- 5.Jones BA, Gores GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas and intestine. Am J Physiol 1997;237:G1174–88. [DOI] [PubMed] [Google Scholar]
- 6.Hall PA, Coates PJ, Ansari A, et al. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci 1994;107:3569–77. [DOI] [PubMed] [Google Scholar]
- 7.Staunton MJ, Gaffney EF. Apoptosis. Basic concepts and potential significance in human cancer. Arch Pathol Lab Med 1998;122:310–19. [PubMed] [Google Scholar]
- 8.Moss SF, Calam J, Agarwal B, et al. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 1996;38:498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannick EE, Bravo LE, Zarama G, et al. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res 1996;56:3238–43. [PubMed] [Google Scholar]
- 10.Hahm KB, Lee KJ, Choi SY, et al. Possibility of chemoprevention by the eradication of Helicobacter pylori: oxidative DNA damage and apoptosis in H. pylori infection. Am J Gastroenterol 1997;92:1853–7. [PubMed] [Google Scholar]
- 11.Kodama M, Fujioka T, Kodama R, et al. p53 expression in gastric mucosa with Helicobacter pylori infection. J Gastroenterol Hepatol 1998;13:215–19. [DOI] [PubMed] [Google Scholar]
- 12.Zhu GH, Yang XL, Lai KC, et al. Nonsteroidal antiinflammatory drugs could reverse Helicobacter pylori-induced apoptosis and proliferation in gastric epithelial cells. Dig Dis Sci 1998;43:1957–63. [DOI] [PubMed] [Google Scholar]
- 13.Kohda K, Tanaka K, Aiba Y, et al. Role of apoptosis induced by Helicobacter pylori infection in the development of duodenal ulcer. Gut 1999;44:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konturek PC, Pierzchalski P, Konturek SJ, et al. Helicobacter pylori induces apoptosis in gastric mucosa through an upregulation of Bax expression in humans. Scand J Gastroenterol 1999;34:375–83. [DOI] [PubMed] [Google Scholar]
- 15.Jones NL, Shannon PT, Cutz E, et al. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol 1997;151:1695–703. [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner S, Beil W, Westermann J, et al. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology 1997;113:1836–47. [DOI] [PubMed] [Google Scholar]
- 17.Fan X, Crowe SE, Behar S, et al. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med 1998;10:1659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peek RM, Moss SF, Tham KT, et al. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst 1997;89:863–8. [DOI] [PubMed] [Google Scholar]
- 19.Anti M, Armuzzi A, Iascone E, et al. Epithelial-cell apoptosis and proliferation in Helicobacter pylori-related chronic gastritis. Ital J Gastroenterol Hepatol 1998;30:153–9. [PubMed] [Google Scholar]
- 20.Houghton J, Macera-Bloch LS, Harrison L, et al. Tumor necrosis factor alpha and interleukin 1β up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection. Infect Immun 2000;68:1189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirin AH, Sordillo EM, Oh SH, et al. Helicobacter pylori inhibits the G(1) to S transition in AGS gastric epithelial cells. Cancer Res 1999;59:2277–81. [PubMed] [Google Scholar]
- 22.Yamaguchi T, Nakajima N, Kuwayama H, et al. Gastric epithelial cell proliferation and apoptosis in Helicobacter pylori-infected mice. Aliment Pharmacol Ther 2000;14(suppl 1):68–73. [DOI] [PubMed] [Google Scholar]
- 23.Leung WK, To KF, Chan FK, et al. Interaction of Helicobacter pylori eradication and non-steroidal anti-inflammatory drugs on gastric epithelial apoptosis and proliferation: implications on ulcerogenesis. Aliment Pharmacol Ther 2000;14:879–85. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura T, Shimoyama T, Tanaka M, et al. Gastric mucosal inflammation and epithelial cell turnover are associated with gastric cancer in patients with Helicobacter pylori infection. J Clin Pathol 2000;53:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Chi DS, Kalin GB, et al. Helicobacter pylori infection and oncogene expression in gastric carcinoma and its precursor lesions. Dig Dis Sci 2002;47:107–13. [DOI] [PubMed] [Google Scholar]
- 26.Takagi A, Watanabe S, Igarashi M, et al. Helicobacter pylori induced cell death is associated with expression of Bcl-xS in gastric epithelial cells [abstract]. Gastroenterology 1999;116:A310. [Google Scholar]
- 27.Shibayama K, Doi Y, Shibata N, et al. Apoptotic signaling pathway activated by Helicobacter pylori infection and increase of apoptosis-inducing activity under serum-starved conditions. Infect Immun 2001;69:3181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda S, Yoshida H, Mitsuno Y, et al. Analysis of apoptotic and antiapoptotic signaling pathways induced by Helicobacter pylori. Gut 2002;50:771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamerson MH, Johnson MD, Dickson RB. Dual regulation of proliferation and apoptosis: c-Myc in bitransgenic murine mammary tumor models. Oncogene 2000;19:1065–71 . [DOI] [PubMed] [Google Scholar]
- 30.Chiou CC, Chan CC, Sheu DL, et al. Helicobacter pylori infection induced alteration of gene expression in human gastric cells. Gut 2001;48:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahelin BJ, Marti U, Solioz M, et al. False positive staining in the TUNEL assay to detect apoptosis in liver and intestine is caused by endogenous nucleases and inhibited by diethyl pyrocarbonate. Gut 1998;51:204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peek RM, Blaser MJ, Mays DJ, et al. Helicobacter pylori strain-specific genotypes and modulations of the gastric epithelial cell cycle. Cancer Res 1999;59:6124–31. [PubMed] [Google Scholar]
- 33.Moss SF, Sordillo EM, Abdalla AM, et al. Increased gastric epithelial cell apoptosis associated with colonization with cagA+ Helicobacter pylori strains. Cancer Res 2001;61:1406–11. [PubMed] [Google Scholar]
- 34.Piotrowski J, Piotrowski E, Skrodzka D, et al. Induction of acute gastritis and epithelial apoptosis by Helicobacter pylori lipopolysaccharide. Scand J Gastroenterol 1997;32:203–11. [DOI] [PubMed] [Google Scholar]
- 35.Igarashi M, Kitada Y, Yoshiyama H, et al. Ammonia as an accelerator of tumor necrosis factor alpha-induced apoptosis of gastric epithelial cells in Helicobacter pylori infection. Infect Immun 2001;69:816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe S, Takagi A, Hoga Y, et al. Helicobacter pylori induces apoptosis in gastric epithelial cells through inducible nitric oxide. J Gastroenterol Hepatol 2000;15:168–74. [DOI] [PubMed] [Google Scholar]
- 37.Rudi J, Kuck D, Strand S, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Invest 1998;102:1506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata J, Goto H, Arisawa T, et al. Regulation of tumour necrosis factor (TNF) induced apoptosis by soluble TNF receptors in Helicobacter pylori infection. Gut 1999;45:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G, Sordillo EM, Ramey WG, et al. Apoptosis in gastric epithelial cells is induced by Helicobacter pylori and accompanied by increased expression of BAK. Biochem Biophys Res Commun 1997;239:626–32. [DOI] [PubMed] [Google Scholar]
- 40.Moss SF, Alam S, Pou R, et al. Increased expression of the pro-apoptotic Bcl-2 homologue, Bak, in H. pylori-infected gastric mucosa [abstract]. Gastroenterology 1997;112:A225. [Google Scholar]
- 41.Xia HHX, Zhang G-S, Talley NJ, et al. Topographic association of gastric epithelial expression of Ki-67, Bax and Bcl-2 with antralization in the gastric incisura, body and fundus. Am J Gastroenterol [In press.] [DOI] [PubMed]
- 42.Nardone G, Staibano S, Rocco A, et al. Effect of Helicobacter pylori infection and its eradication on cell proliferation, DNA status, and oncogene expression in patients with chronic gastritis. Gut 1999;44:789–9. [DOI] [PMC free article] [PubMed] [Google Scholar]