Abstract
Aim: Transforming growth factor β (TGF-β) is involved in the control of autoimmune reactions, cell proliferation, and the accumulation of lymphocytes within organs. The aim of this study was to determine the expression of TGF-β in salivary glands from patients with primary Sjogren’s syndrome (SS) and benign lymphoepithelial lesions (BLEL) with emphasis on ductal epithelium.
Methods: Immunoperoxidase staining for TGF-β isoforms and Ki67 antigen was performed on formalin fixed sections of labial glands from patients with primary SS (n = 15) and controls (n = 5) and parotid glands reported as BLEL (n = 5) or normal (n = 5). Ductal expression of TGF-β was quantified by absorbance measurements using image analysis. The specificity of staining was confirmed by peptide blocking studies.
Results: All TGF-β isoforms were detected within the cytoplasm of most lymphocytes, endothelial cells, and ducts in all specimens. Acinar expression was variable and weaker than that seen in ducts. Absorbance measurements revealed that the expression of all isoforms was greater in ducts within primary SS glands than in control glands. Ductal expression in control parotid glands was greater than that seen in BLEL glands, irrespective of the presence of adjacent lymphoid infiltrates. Comparisons between control specimens showed that ductal expression of all isoforms was highest in parotid glands, whereas no differences were detected between primary SS and BLEL glands. Ki67 positive lymphocytes and duct cells were mainly restricted to pathological specimens, with BLEL glands containing larger populations of positive cells than primary SS glands.
Conclusion: These results demonstrate complex and variable changes in ductal expression of TGF-β in primary SS and BLEL, which may be important in the control of lymphoid infiltration and the proliferation of lymphocytes and ductal epithelium.
Keywords: Sjogren’s syndrome, benign lymphoepithelial lesion, transforming growth factor β isoforms, salivary glands
Sjogren’s syndrome (SS) is an autoimmune disease of the salivary and lachrymal glands and is characterised microscopically by the infiltration and replacement of the secretory acini by lymphoid cells.1–3 In addition, salivary ducts may be modified and become dilated or form islands of epithelial cells.4–7 Lymphoid infiltration associated with epithelial changes, variously termed benign lymphoepithelial lesion (BLEL), lymphoepithelial sialadenitis, or myoepithelial sialadenitis (MESA), is relatively uncommon in minor salivary glands and is more often a feature of major, parotid gland involvement in SS.6–8 Originally, the term benign lymphoepithelial lesion was used to describe lymphoepithelial changes in major salivary glands associated with unilateral or bilateral gland enlargement in the absence of the clinical signs of SS (dry eyes and mouth).9,10 However, it is now recognised that lymphoepithelial lesions of salivary glands include a spectrum of disorders ranging from reactive lymphoid proliferations to overt B cell lymphomas of mucosal associated lymphoid tissue type.6–8 Although these lesions are most often associated with SS and/or other connective tissue disease, they can occur as isolated salivary gland enlargements in patients without other associated disease.6
The transforming growth factor β (TGF-β) isoforms are a family of multifunctional polypeptides that regulate the proliferation, differentiation, and death of a wide variety of cell types.11,12 A crucial function of TGF-β in the immune system is the suppression of lymphocyte proliferation and differentiation, thereby preventing inappropriate autoimmune responses. Thus, the absence of TGF-β1 in “knockout” mice leads to a self targeting multifocal inflammatory response in many organs including, the heart, lung, liver, kidneys, stomach, pancreas, and salivary glands.13,14 Histologically, the lymphocyte infiltration of the salivary glands in such knockout mice is similar to that seen in SS.15 In normal circumstances, TGF-β facilitates the resolution of inflammation and promotes tissue repair.16,17 However, resolution of inflammation is crucially dependent upon the concentration of the growth factor, with excess TGF-β1 within a lesion also being associated with unresolved inflammation.18 Because SS is a chronic condition, the degree of expression of TGF-β within the exocrine glands could be an important factor. In situ, TGF-β1 is also known to regulate the proliferation of epithelial cells.19–22 Thus, reduced expression could also result in lack of control of epithelial proliferation and could play a role in the formation of dilated ducts and epithelial islands, which are characteristic of both SS and BLEL.
“A crucial function of transforming growth factor β in the immune system is the suppression of lymphocyte proliferation and differentiation, thereby preventing inappropriate autoimmune responses”
Published immunohistochemical studies of the expression of TGF-β by the glandular epithelium of salivary glands have been limited to TGF-β1 and TGF-β2, have yielded conflicting results, and have been directed exclusively at minor gland lesions in SS. Thus, the complete absence of staining in glands from patients with SS and controls for TGF-β223 has been reported, as have both increased24 and decreased25,26 expression of TGF-β1 in specimens from patients. Furthermore, attempts to investigate TGF-β expression at the mRNA level by in situ hybridisation27 and the polymerase chain reaction (PCR)28,29 have yielded similar conflicting data.
To date, there have been no studies of the glandular expression of all three TGF-β isoforms in SS, even though they have known roles in the control of autoimmune reactions, cell proliferation, and the accumulation of lymphocytes within organs, including the salivary glands. Thus, the purpose of our immunohistochemical study was to determine the presence and distribution of all three isoforms of TGF-β in labial salivary glands from healthy controls and patients with primary SS to investigate possible differences in growth factor expression, which might be important in pathogenesis. In addition, because of the possibility that the epithelial expression of TGF-β might be related to the ductal changes characteristic of BLEL, growth factor expression was also examined in a small group of BLEL lesions within parotid glands.
MATERIALS AND METHODS
Patients and tissues
Paraffin wax blocks of labial glands (n = 15) showing the characteristic histopathological features of SS were obtained from the files of the unit of oral pathology, University of Birmingham, UK. All patients (mean age, 45.9 years) fulfilled the European criteria for the diagnosis of primary SS (table 1 ▶).3 Although all specimens contained moderate to extensive periductal lymphocyte foci, only one specimen showed the ductal changes consistent with a histopathological diagnosis of BLEL/MESA (fig 1 ▶). For comparison, labial glands were excised from five healthy volunteers (mean age, 42.2 years). Five paraffin wax blocks of excision specimens of parotid salivary glands, which had been histologically reported as benign lymphoepithelial lesions in the 1980s, were retrieved from the files of the unit of oral pathology, University of Birmingham. These tissues were from female patients with a mean age of 63.4 years (range, 47–77). Two of the patients fulfilled the European criteria for a diagnosis of SS. The three other patients presented with parotid gland enlargement in the absence of sicca symptoms. In the 10 to 20 years since diagnosis, none of the patients had developed lymphoma, although three had died from unrelated causes. In situ hybridisation for κ and λ light chain mRNA expression within these tissues revealed polyclonal B cell populations (κ :λ ratios of 0.9 to 3.4 : 1) in four specimens and a monoclonal population in one specimen (κ : λ ratio of 22.1 : 1) (GI Mason and JB Matthews, 2000, unpublished observations). This last patient had SS and was known to have had an IgG paraprotein at the time of diagnosis.30 For control purposes, five paraffin wax blocks of parotid gland that had been reported as showing normal histology were also retrieved from the pathology files (mean age, 50.2 years). These glands had been excised because of recurrent swelling. None of these patients had clinical signs or symptoms of dry eyes or mouth and none fulfilled the European criteria for a diagnosis of SS. All specimens were fixed in neutral buffered formalin (18–24 hours) and processed via ethanol and xylene to paraffin wax.
Table 1.
Clinical features of the patients with primary Sjogren’s syndrome
| European criteria | ||||||||
| Patient | Sex | Age | Ocular symptoms | Ocular signs | Oral symptoms | Histopathology | Gland involvement | Autoantibodies |
| L1 | F | 63 | NK | NK | + | + | + | ANF |
| L2 | F | 65 | + | NK | + | + | + | NK |
| L3 | F | 68 | + | NK | + | + | NK | Ro, La, ANF, RhF |
| L4 | F | NK | + | NK | + | + | + | ANF, RhF |
| L5 | F | 46 | + | + | + | + | + | Ro, La |
| L6 | F | 44 | + | NK | + | + | NK | Ro, La |
| L7 | M | 42 | + | NK | + | + | + | NK |
| L8 | F | 17 | + | NK | + | + | + | Ro, La, ANF |
| L9 | F | 52 | + | NK | + | + | NK | ANF |
| L10 | F | 35 | + | + | + | + | + | NK |
| L11 | F | 35 | + | + | + | + | + | Ro, La |
| L12 | F | 49 | + | + | + | + | NK | Ro |
| L13 | F | 36 | + | NK | + | + | NK | Ro, La |
| L14 | F | 68 | + | NK | + | + | NK | Ro, La |
| L15 | F | 58 | + | NK | + | + | + | NK |
Occular symptoms: dry eyes; ocular signs: diminished tear flow and/or measurement of punctate keratitis of the cornea; oral symptoms: dry mouth; histopathology: focal lymphocyte infiltration of the salivary gland; gland involvement: diminished saliva flow and/or changes in salivary ducts. Autoantibodies: ANF, antinuclear factor; RhF, rheumatoid factor.
NK, not known.
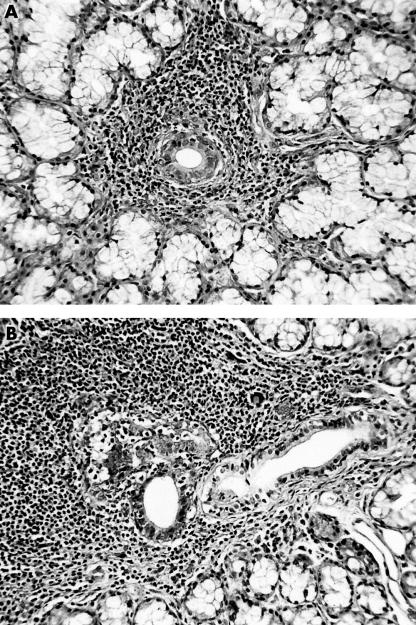
Figure 1.
Haematoxylin and eosin stained section of a labial gland from a patient with Sjogren’s syndrome showing (A) a periductal focus and (B) an area of more extensive lymphocytic infiltration associated with a proliferating duct.
Histology and immunohistochemistry
Sections (5 μm thick) were cut for histological examination and immunocytochemical analysis. Sections were dried at 56°c before being dewaxed in xylene and rehydrated through a graded alcohol series to phosphate buffered saline (PBS).
Immunocytochemical staining was performed on trypsin treated sections (0.1% trypsin; Difco trypsin 250; Becton Dickinson, France; 30 minutes at room temperature) using affinity purified polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, California, USA) to TGF-β isoforms (sc82, sc90, and sc146) at 0.5–2.0 μg/ml, as described previously.31 Endogenous peroxidase activity was quenched using 3% hydrogen peroxide in water and non-specific antibody uptake by proteins was blocked using 20% goat serum in PBS. The primary antibodies were diluted in PBS containing 1% bovine serum albumin (Sigma, Poole, Dorset, UK). Immunoperoxidase staining was carried out using a biotin–streptavidin system (StrAviGen; Multilink; Biogenex, San Ramon, California, USA) and the site of antibody binding was visualised using diaminobenzidine reagent. When required, the brown reaction product was darkened using 0.5% CuSO4 in saline (five minutes) and the sections were counterstained with Meyer’s haematoxylin. Negative staining controls included omission of the primary layer and substitution of the primary layer with PBS. Specificity of the anti-TGF-β isoform antibodies was confirmed by checkerboard peptide blocking experiments. The working dilution of each antibody was incubated with a 10 fold excess (wt/wt) of peptide (Santa Cruz) overnight at 4°C before staining. In all cases, staining was abolished by homologous peptide but unaffected by pre-incubation with peptides corresponding to other isoforms.
Staining for Ki67 antigen was performed using monoclonal antibody MM1 (Novocastra, Newcastle upon Tyne, UK) in a similar manner to that described for the TGF-β isoforms except that microwave antigen retrieval was used instead of trypsin (citrate buffer, pH 6.0 for 50 minutes).
Evaluation of stained sections
The staining intensity (absorbance) of all ducts within each labial salivary gland section (range, 6–20 ducts) was measured at a magnification of ×200 using a Seescan Prism 512 system (Seescan Imaging Ltd, Cambridge, UK) linked to a Leitz Diaplan microscope and a black and white single chip camera. For the larger parotid specimens, absorbance measurements were made on 10 areas of ductal (dilated and collecting duct) epithelium for each specimen. Measurements were performed essentially as described previously31–33 on non-counterstained and non-CuSO4 treated sections. The results from normal labial salivary glands were compared with those from glands from patients with primary SS. In BLEL parotid glands, the staining intensity of the ductal epithelium was measured in regions associated with confluent lymphocyte infiltrates (avoiding areas of epithelium containing large numbers of intraepithelial lymphocytes), and in areas where there were only isolated foci of lymphocytes and the normal glandular architecture was maintained. The staining intensity of ductal epithelium in both these areas was compared with that of ducts in histologically normal parotid glands.
Statistics
Data were analysed using Minitab version 9.2 and the significance of differences determined using the Mann-Whitney U test.
RESULTS
All three TGF-β isoforms were detected in the cytoplasm of most lymphocytes, fibroblasts, endothelial cells, and duct cells in all labial and parotid glands. Acinar cell expression was usually absent or weak except for a small number of acini in labial glands from patients with primary SS. The cytoplasm of the few lymphocytes in normal glands expressed these growth factors, but in areas containing large periductal lymphocyte foci in primary SS glands, and where there was confluent lymphocyte infiltration in BLEL glands, staining was variable, with some cells showing strong cytoplasmic expression and others being negative. In contrast, lymphocytes within duct lumens in BLEL glands were strongly positive for all three isoforms.
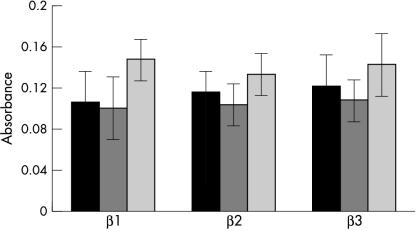
In all specimens, glandular expression of the three isoforms was more pronounced in ductal epithelium than in acinar cells and staining for TGF-β3 usually appeared darker than that seen for TGF-β1 and TGF-β2 (fig 2 ▶). Within each isoform, there were differences in glandular expression of TGF-β between primary SS and BLEL glands and their corresponding control tissues. Thus, ductal epithelium in primary SS glands appeared to stain darker than that of normal control glands (fig 3 ▶; table 2 ▶). Absorbance measurements on ductal epithelium confirmed this observation and showed that although there was no significant difference in intensity for TGF-β1 (p = 0.067), staining for both TGF-β2 and TGF-β3 was significantly darker in primary SS glands than in the control glands (p = 0.02 and 0.0012; fig 4 ▶). Acinar staining was variable, with staining being most pronounced in some acini adjacent to inflammatory cell foci. Subjectively, there was no difference in ductal staining for the TGF-β isoforms and the size of the surrounding inflammatory cell infiltrate.
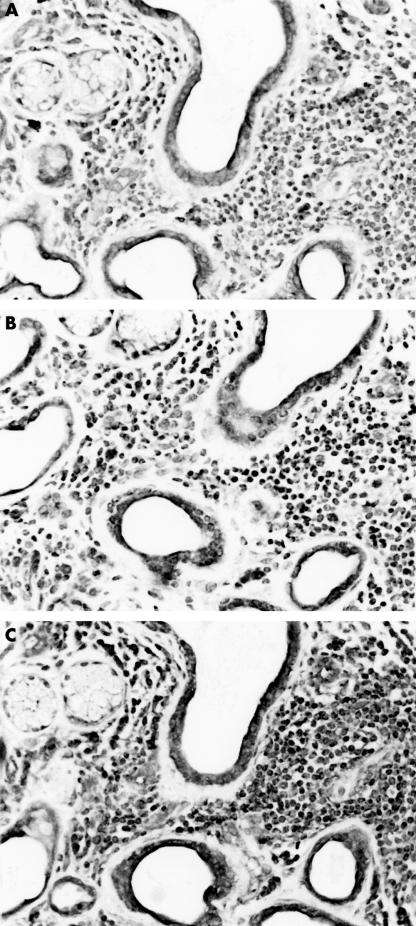
Figure 2.
Labial gland from a patient with Sjogren’s syndrome showing lymphoid infiltration and duct dilation stained for (A) transforming growth factor β1 (TGF-β1), (B) TGF-β2, and (C) TGF-β3.
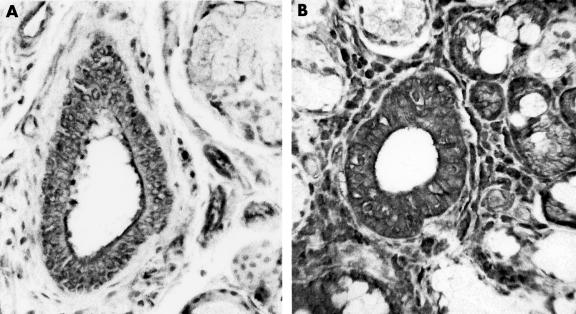
Figure 3.
Ductal staining for transforming growth factor β3 in labial glands from (A) a healthy volunteer and (B) a patient with primary Sjogren’s syndrome.
Table 2.
Summary of differences in ductal expression of the transforming growth factor β (TGF-β) isoforms
| p Value | ||||
| Comparison | Observed difference | TGF-β1 | TGF-β2 | TGF-β3 |
| pSS v control labial gland | pSS > control | NS | <0.02 | <0.0012 |
| pSS v BLEL | pSS = BLEL | NS | NS | NS |
| BLEL* v control parotid | BLEL < control | <0.04 | NS | NS |
| BLEL infiltrated v BLEL non-infiltrated | Infiltrated > non-infiltrated | NS | NS | NS |
| Control labial gland v control parotid gland | Parotid > labial | <0.015 | <0.015 | <0.015 |
*TGF-β expression by ducts with and without associated lymphoid foci.
BLEL, benign lymphoepithelial lesion; NS, not significant; pSS, primary Sjogren’s syndrome.
Figure 4.
Absorbance measurements of the ductal expression of the three transforming growth factor β isoforms in labial glands from patients with primary Sjogren’s syndrome (black bars) and controls (grey bars).
In contrast to labial glands, staining of ductal epithelium in parotid glands appeared to be darkest in histologically normal specimens, with staining in BLEL specimens varying according to the presence or absence of lymphoid infiltrates (fig 5 ▶; table 2 ▶). Although absorbance measurements confirmed that ductal staining in normal parotid glands was darker than that seen in both non-infiltrated and infiltrated areas of the BLEL glands, the results were only significant for TGF-β1 (p < 0.04 for both areas; fig 6 ▶). In a comparison of infiltrated and non-infiltrated areas in the same sections, all three isoforms showed non-significantly higher mean absorbance values in infiltrated areas (fig 6 ▶).
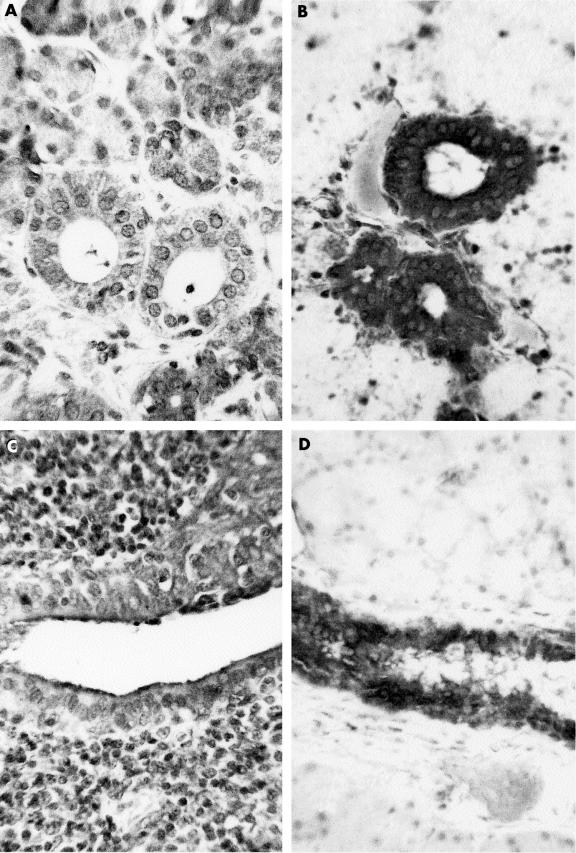
Figure 5.
Ductal staining for transforming growth factor β1 in a benign lymphoepithelial lesion ((A) non-infiltrated area; (C) area associated with lymphoid infiltrate) and histologically normal parotid (B, D) glands.
Figure 6.
Absorbance measurements of the ductal expression of the three transforming growth factor β isoforms in benign lymphoepithelial lesion parotid glands in areas with (black bars) and without (dark grey bars) associated lymphoid infiltrates and control glands (light grey bars).
Although numbers of control tissues were small (n = 5), there were obvious differences in the ductal expression of the TGF-β isoforms between labial and parotid glands (table 2 ▶). Absorbance readings confirmed that ductal staining for all isoforms was greater in histologically normal parotid glands than in labial glands from healthy control subjects (p < 0.015 for all isoforms). A similar comparison of ductal staining between primary SS and BLEL tissues revealed no significant differences.
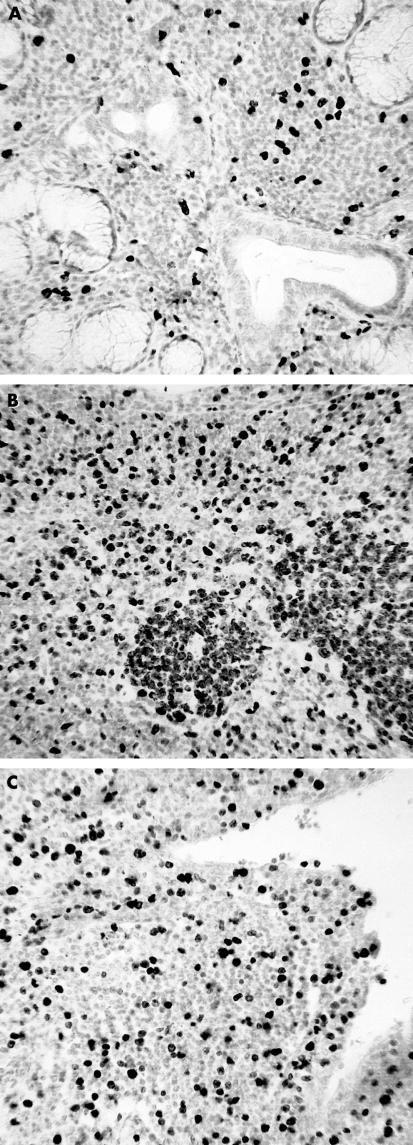
Because of the potential relation between local expression of TGF-β and lymphoid and epithelial cell proliferation, sections from all cases were stained for Ki67 antigen. Ki67 positive lymphocytes were not detected in control glands but were seen in all BLEL and primary SS glands. Positive cells within primary SS labial glands were scattered throughout the lymphoid infiltrate and formed a small proportion of the total lymphoid infiltrate (< 10%). In contrast, large foci of Ki67 positive lymphocytes within germinal centre-like structures, together with scattered positive cells, were detected in BLEL glands (fig 7 ▶).
Figure 7.
Ki67 positive cells within the lymphoid infiltrates of (A) Sjogren’s syndrome labial and (B, C) benign lymphoepithelial lesion (BLEL) parotid glands. Proliferating cells are found within germinal centre-like structures within the extensive infiltrates (B) and the duct walls of BLEL specimens (C).
Ki67 positive epithelial cells were rarely detected in control glands and were only occasionally present within the duct walls of primary SS glands. Such cells were relatively common in BLEL glands, with walls of dilated ducts containing a large proportion (20–30%) of cells staining for Ki67.
DISCUSSION
This is the first study to investigate the expression of all three TGF-β isoforms in labial salivary glands from patients with primary SS or parotid glands showing normal histology and the features of BLEL. The data indicate that alterations occur in the ductal expression of TGF-β between health and disease and that there are differences in baseline expression between labial and parotid glands in health. Thus, ductal expression of all TGF-β isoforms was increased in primary SS but decreased in BLEL compared with controls, which themselves differed, with parotid glands showing higher baseline expression than labial glands. These results suggest that salivary glands differ in their requirements for the ductal production of the TGF-β isoforms for normal physiological function. Furthermore, upregulation or downregulation of expression levels associated with lymphoid infiltration appear to be dependent upon the normal baseline for the gland. Thus, the expression of all TGF-β isoforms in ducts associated with lymphoid foci was the same in both labial glands and parotid glands.
Except for the report by Koski and co-workers, who investigated TGF-β2,23 all previous studies of the glandular expression of TGF-β protein24–26 or mRNA27–29 in SS have been limited to TGF-β1 and labial glands. TGF-β2 protein expression could not be detected in glandular epithelium in health or disease and the ductal expression of TGF-β1 protein was either decreased25,26 or increased24 in SS. Furthermore, the only study of gene expression of the required sensitivity allowing cellular localisation of TGF-β1 mRNA (that is, microdissection followed by non-quantitative PCR)29 found no difference between glands from patients with primary SS and controls.
In terms of TGF-β1 protein expression, the results of our study agree with those of Cauli and colleagues,24 who showed increased staining in both acini and ducts of labial glands from patients with primary SS, and found that ductal staining was more intense than that of acini. Furthermore, studies of TGF-β1 mRNA expression by conjunctival epithelium in SS associated keratoconjunctivitis sicca found raised concentrations when compared with healthy controls.34 It is difficult to reconcile these results with the two reports indicating reduced ductal expression of TGF-β1 in SS,25,26 particularly because one25 appears to use the same anti-TGF-β1 peptide antibody that we used, albeit on frozen sections. The other study found an inverse relation between the intensity of TGF-β1 staining and the size of the surrounding inflammatory cell infiltrate.26 The fact that most of our specimens contained small to medium sized lymphoid foci cannot explain the difference in results because the expression in control glands was lower.
The possibility that alterations of glandular expression of the TGF-β isoforms might be reflected in salivary cytokine values has not been investigated. Indeed, the only published data on salivary concentrations of TGF-β1 appear in a technical paper reporting a new quantification method,35 and indicate that pilocarpine stimulated saliva from rats contains approximately half the amount of TGF-β1 found in plasma (about 4 ng/ml saliva).
“In benign lymphoepithelial lesion glands, reduced glandular expression of tumour growth factor β1 might explain the presence of large lymphoid infiltrates and the high degree of both lymphocyte and epithelial cell proliferation”
It is now clear that TGF-β1 is important in the control of the immune system and has multiple functions, including suppression of the growth and differentiation of B and T cells, control of autoimmune reactions, and the accumulation of lymphocytes within organs.12–14,36 However, TGF-β1 is known to have multiple cellular targets and can regulate the growth and differentiation of non-immune cells, including epithelium. The relation between TGF-β1 expression and epithelial cell proliferation is clearly shown in rat intestinal crypt epithelium, where values are lowest in the proliferative crypt zone and highest in the portion of the crypt villus unit characterised by the absence of mitotic activity.19 It is likely that epithelially derived TGF-β1 is important for the normal functioning of salivary glands and the regulation of the resident/transient lymphoid population, in addition to the growth and proliferation of parenchymal cells.
In BLEL glands, reduced glandular expression of TGF-β1 might explain the presence of large lymphoid infiltrates and the high degree of both lymphocyte and epithelial cell proliferation. Such a relation in terms of ductal epithelium could be important in the formation of both dilated ducts and epithelial islands, which are characteristic features of BLEL. Interestingly, the mean TGF-β1 absorbance of epithelium in primary SS glands was higher (not significant) than that seen in control labial glands, and was similar to that seen in ducts within BLEL glands. The greatest difference in glandular TGF-β1 expression was between the two sets of control tissues, with parotid glands showing significantly higher ductal absorbance readings than labial glands. If TGF-β1 derived from salivary ducts is important in the glandular changes seen in primary SS and BLEL, the data suggest that it may play different roles in different glands and may reflect normal, baseline expression or be modulated by the activities of the other isoforms or other cytokines. Thus, the characteristic presence of epithelial islands in BLEL with extensive lymphocyte and epithelial cell proliferation is associated with a significant reduction in ductal TGF-β1 from normal high (with respect to labial glands) baseline values. In contrast, the changes within labial glands in primary SS are associated with an increase in TGF-β values to those seen in BLEL from a low normal baseline level of expression.
Although the activities of TGF-β1 have been investigated extensively, relatively little is known about the other two human isoforms, except that TGF-β3 moderates the effect of TGF-β1 in that it inhibits fibrosis and scarring.37 Glandular changes in TGF-β2 and TGF-β3 expression essentially reflected those found for TGF-β1 and were more pronounced in primary SS than in BLEL. These differential changes in isoform expression within and between glands are difficult to interpret because of our lack of knowledge about the functional activities and interactions of the different isoforms, and because comparisons between isoforms are invalid as a result of the fact that absorbance readings are not directly related to the absolute amounts of TGF-β present.
It is possible that the differences in TGF-β expression seen in our immunohistochemical study might simply reflect the local disease process within the glands rather than indicating that TGF-β has an active role in pathogenesis. However, the fact that ducts in BLEL parotid glands showed similar reductions in TGF-β1 staining, irrespective of whether they were associated with lymphoid foci, suggests that changes in glandular growth factor expression precede local inflammatory cell infiltration. This possibility would agree with the data from the TGF-β1 knockout mouse model13–15 and the concept that reduced ductal TGF-β1 expression results in lymphoid infiltration and epithelial proliferation. Unfortunately, because of the small size of labial gland biopsy specimens, the numbers of ducts not associated with inflammatory cell infiltrates were small and similar data could not be collected.
Further studies are required to confirm these results and to clarify the potential role of the TGF-β isoforms in the pathogenesis of the salivary gland changes characteristic of SS and BLEL. Cell culture studies using a recently published system for the long term culture of non-neoplastic salivary gland epithelial cells38 could provide information on baseline values of TGF-β mRNA expression and protein production in health and disease. This system has also been used for epithelial cell–lymphocyte co-culture experiments, which would offer a means of investigating a variety of functional and molecular interactions in vitro. Furthermore, with the rapid advances in laser capture microdissection, gene array technology, and proteomics it should be possible to combine the techniques to evaluate in vivo, cell specific differences in TGF-β gene and protein expression in salivary glands from patients with SS and BLEL.39–41
Take home messages.
The ductal expression of all transforming growth factor β (TGF-β) isoforms was increased in primary Sjogren’s syndrome (SS) but decreased in benign lymphoepithelial lesions (BLEL) compared with controls
In control tissue, the parotid glands showed higher baseline expression of TGF-β than did the labial glands
These alterations in the ductal expression of TGF-β may be important in the control of lymphoid infiltration and the proliferation of lymphocytes and ductal epithelium
In conclusion, our study has shown a variety of differences in ductal TGF-β expression between health and disease and between minor and major glands in health and disease. Confirmation of whether these complex changes in the ductal expression of TGF-β in primary SS and BLEL are important in the control of lymphoid infiltration and the proliferation of lymphocytes and ductal epithelium await further study.
Abbreviations
BLEL, benign lymphoepithelial lesion
MESA, myoepithelial sialadenitis
PBS, phosphate buffered saline
PCR, polymerase chain reaction
SS, Sjogren’s syndrome
TGF-β, transforming growth factor β
REFERENCES
- 1.Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjogren’s syndrome. J Clin Pathol 1968;21:656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenspan JS, Daniels TE, Talal N, et al. The histopathology of Sjogren’s syndrome in labial gland biopsies. Oral Surg Oral Med Oral Pathol 1974;37:217–29. [DOI] [PubMed] [Google Scholar]
- 3.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjogren’s syndrome: results of a prospective concerted action supported by the European Community. Arthritis Rheum 1993;36:340–7. [DOI] [PubMed] [Google Scholar]
- 4.Bloch KJ, Buchana WW, Wohl MJ, et al. Sjogren’s syndrome. A clinical, pathological and serological study of sixty-two cases. Medicine 1965;44:187–213. [PubMed] [Google Scholar]
- 5.Fox RI, Howell F, Bone R, et al. Primary Sjogren’s syndrome: clinical and immunopathologic features. Semin Arthritis Rheum 1984;14:77–105. [DOI] [PubMed] [Google Scholar]
- 6.Harris NL. Lymphoid proliferations of the salivary glands. Am J Clin Pathol 1999;111:S94–103. [PubMed] [Google Scholar]
- 7.Carbone A, Gloghini A, Ferlito A. Pathological features of lymphoid proliferations of the salivary glands: lymphoepithelial sialadenitis versus low-grade B-cell lymphomas of the MALT type. Ann Otol Rhinol Laryngol 2000;109:1170–5. [DOI] [PubMed] [Google Scholar]
- 8.Quintana PG, Kapadia SB, Bahler DW, et al. Salivary gland lymphoid infiltrates associated with lymphoepithelial lesions: a clinicopathologic, immunophenotypic and genotypic study. Hum Pathol 1997;28:850–61. [DOI] [PubMed] [Google Scholar]
- 9.Godwin JT. Benign lymphoepithelial lesion of the parotid gland (adenolymphoma, chronic inflammation, lymphoepithelioma, lymphocytic tumour, Mikulicz disease); report of eleven cases. Cancer 1952;5:1089–103. [DOI] [PubMed] [Google Scholar]
- 10.Morgan WS, Castleman B. A clinicopathological study of Mikulicz’s disease. Am J Pathol 1953;29:471–503. [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts AB, Sporn MB. The transforming growth factor-βs. In: Sporn MB, Roberts AB, eds. Peptide growth factors and their receptors, Vol. 1, Heidelberg: Springer, 1990:419–72.
- 12.Moustakas A, Pardali K, Gaal A, et al. Mechanisms of TGF-β signalling in regulation of cell growth and differentiation. Immunol Lett 2002;82:85–91. [DOI] [PubMed] [Google Scholar]
- 13.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 1992;359:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boivin GP, O’Toole BA, Orsmby IE, et al. Onset and progression of pathological lesions in transforming growth factor-β1-deficient mice. Am J Pathol 1995;146:276–88. [PMC free article] [PubMed] [Google Scholar]
- 15.McCartney-Francis NL, Mizel DE, Redman RS, et al. Autoimmune Sjogren’s-like lesions in salivary glands of TGFβ1 deficient mice are inhibited by adhesion-blocking peptides. J Immunol 1996;157:1306–12. [PubMed] [Google Scholar]
- 16.Wahl SM. Transforming growth factor β (TGF-β) in inflammation: a cause and a cure. J Clin Immunol 1992;12:61–74. [DOI] [PubMed] [Google Scholar]
- 17.McCartney-Francis NL, Wahl SM. TGFβ: a matter of life and death. J Leukoc Biol 1994;55:401–9. [DOI] [PubMed] [Google Scholar]
- 18.Border WA, Ruoslahti E. Transforming growth factor β in disease: the dark side of tissue repair. J Clin Invest 1992;90:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnard JA, Beauchamp RD, Coffey RJ, et al. Regulation of intestinal epithelial cell growth by transforming growth factor type β. Proc Natl Acad Sci U S A 1989;86:1578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor beta. Biochim Biophys Acta 1990;1032:79–87. [DOI] [PubMed] [Google Scholar]
- 21.Kim G-Y, Lee H-H, Cho S-W. Differential effects of transforming growth factor β1 and bone morphogenic proteins in cultured rat osteogenic sarcoma and mink lung epithelial cells. Biochem Mol Biol Int 1994;33:253–62. [PubMed] [Google Scholar]
- 22.Kramer IM, Patel R, Spartigo D, et al. Initiation of growth inhibition by TGFβ is unlikely to occur in G1. J Cell Sci 1994;107:3469–75. [DOI] [PubMed] [Google Scholar]
- 23.Koski H, Konttinen YT, Gu X-H, et al. Transforming growth factor β2 in labial glands in Sjogren’s syndrome. Ann Rheum Dis 1995;54:744–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cauli A, Yanni G, Pitzalis C, et al. Cytokine and adhesion molecule expression in the minor salivary glands of patients with Sjogren’s syndrome and chronic sialadenitis. Ann Rheum Dis 1995;54:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kizu Y, Sakurai S, Katagiri S, et al. Immunohistological analysis of transforming growth factor B1 expression in normal and inflamed salivary glands. J Clin Pathol 1996;49:728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa N, McGuff H, Dang H, et al. Cytokine profiles of salivary glands from patients with Sjogren’s syndrome. Arthritis Rheum 1993;36:S43. [DOI] [PubMed] [Google Scholar]
- 27.Boumba D, Skopouli FN, Moutsopoulos HM. Cytokine mRNA expression in labial salivary gland tissues from patients with primary Sjogren’s syndrome. Br J Rheumatol 1995;34:326–33. [DOI] [PubMed] [Google Scholar]
- 28.Ohyama Y, Nakamura S, Matsuzaki G, et al. Cytokine messenger RNA expression in labial salivary glands of patients with Sjogren’s syndrome. Arthritis Rheum 1996;39:1376–84. [DOI] [PubMed] [Google Scholar]
- 29.Fox PC, Brennan M, Di Sun P. Cytokine expression in human labial minor salivary gland epithelial cells in health and disease. Arch Oral Biol 1999;44:S49–52. [DOI] [PubMed] [Google Scholar]
- 30.Basu MK, Price JD, Matthews JB. Benign lymphoepithelial lesion in a patient with Sjogren’s disease and an IgG paraprotein. J Oral Pathol 1983;12:515–26. [DOI] [PubMed] [Google Scholar]
- 31.Mason GI, Hamburger J, Matthews JB. Mast cells, extracellular matrix components, TGFβ isoforms and TGFβ receptor expression in labial glands in systemic sclerosis. Ann Rheum Dis 2000;59:183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason GI, Matthews JB. In situ determination of different dehydrogenase activity profiles in the linings of odontogenic keratocysts and radicular cysts. Histochem J 1996;28:187–93. [DOI] [PubMed] [Google Scholar]
- 33.Paterson I, Matthews JB, Huntley S, et al. Decreased expression of TGFβ cell surface receptors during progression of human squamous cell carcinoma. J Pathol 2001;193:458–67. [DOI] [PubMed] [Google Scholar]
- 34.Pflugfelder SC, Jones, D, Ji ZH, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr Eye Res 1999;19:201–11. [DOI] [PubMed] [Google Scholar]
- 35.Van Waarde MAWH, van Assen AJ, Kampinga HH, et al. Quantification of TGF-β in biological material using cells transfected with plasminogen activator inhibitor-1 promoter–luciferase construct. Anal Biochem 1997;247:45–51. [DOI] [PubMed] [Google Scholar]
- 36.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol 1998;16:137–61. [DOI] [PubMed] [Google Scholar]
- 37.Shah M, Foreman DM, Ferguson MWJ. Neutralisation of TGFβ1 and TGFβ2 or exogenous addition of TGFβ3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995;108:985–1002. [DOI] [PubMed] [Google Scholar]
- 38.Dimitriou ID, Kapsogeorgou EK, Abu-Helu RF, et al. Establishment of a convenient system for the long-term culture and study of non-neoplastic human salivary gland epithelial cells. Eur J Oral Sci 2002;110:21–30. [DOI] [PubMed] [Google Scholar]
- 39.Simone NL, Bonner RF, Gillespie JW, et al. Laser capture microdissection: opening the frontier to molecular analysis. Trends Genet 1998;14:272–6. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann M, Olson K, Cavender A, et al. Gene expression in a pure population of odontoblasts isolated by laser capture microdissection. J Dent Res 2001;80:1963–7. [DOI] [PubMed] [Google Scholar]
- 41.Lawrie LC, Curran S, McLeod HL, et al. Application of laser capture microdissection and proteomics in colon cancer. Mol Pathol 2001;54:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]