Abstract
Aims: Monitoring treated patients with thyroid cancer for recurrent or metastatic disease is currently based upon the serial measurement of circulating plasma thyroglobulin (Tg) concentrations. However, the clinical usefulness of Tg immunoassays is limited by poor sensitivity and interference from anti-Tg antibodies. This study investigated whether the detection of Tg mRNA in peripheral blood, using reverse transcriptase polymerase chain reaction (RT-PCR), is of value in the biochemical surveillance of patients with thyroid cancer.
Methods: RNA was extracted from peripheral blood of five normal controls, six patients with abnormal thyroid function tests, and 28 patients who had undergone thyroidectomy for well differentiated thyroid cancer. From each, an 87 bp product from base pair 262 to 348 in the cDNA sequence of the thyroglobulin gene was amplified by RT-PCR.
Results: Tg mRNA was detected in normal individuals and patients with thyroid cancer. In the group of patients studied, identification of metastatic thyroid tissue by radioiodine scanning correlated better with Tg mRNA assay results than with serum Tg concentrations (accuracy 84% v 75%). No interference from circulating Tg antibodies was apparent. In patients studied prospectively over a 12 month period, there was a significant correlation between detectable Tg mRNA in peripheral blood and the presence or absence of metastatic disease, as demonstrated by radioiodine scanning.
Conclusions: These results suggest that detection of Tg mRNA in blood is a more sensitive marker for metastatic thyroid disease than Tg immunoassay, and appears to be unaffected by the presence of circulating anti-Tg antibodies.
Keywords: thyroglobulin mRNA, thyroid cancer
Differentiated thyroid cancer is the most common endocrine malignancy in North America and Europe, accounting for approximately 1% of all cancers, with an incidence of 3.7 to 4.7/100 000.1 Treatment usually consists of near total thyroidectomy, followed by ablative radioiodine 131I treatment, and by the long term administration of thyroid hormone to achieve pituitary thyrotrophin suppression.2 Despite its excellent prognosis, recurrent disease does occur in approximately 20–40% of patients.3
Current techniques to monitor the progress of patients with thyroid cancer include nuclear scanning and serial serum thyroglobulin (Tg) measurement. Tg is a protein synthesised exclusively by thyroid follicular cells and most differentiated thyroid cancer cells. Although the measurement of serum Tg concentrations is important in the long term management of patients, its application can be limited by the presence of circulating anti-Tg antibodies, which can be found in 10–25% of patients,4 and also the production of variant forms of Tg by some tumours, which are undetectable by standard immunoassays.5 The sensitivity of the Tg immunoassay can be as low as 40%, especially in patients receiving exogenous thyroid hormone treatment at the time of Tg measurement.6 To achieve maximal sensitivity, thyrotrophin (TSH) stimulation (effected by thyroid hormone withdrawal) is necessary,7 although this may be associated with unwanted symptomatic hypothyroidism and possible tumour growth. Recently, recombinant TSH has been suggested as an alternative method to achieve maximal sensitivity of diagnostic tests,8 and its use appears to have fewer adverse effects than the withdrawal of thyroid hormone replacement.
“The sensitivity of the thyroglobulin immunoassay can be as low as 40%, especially in patients receiving exogenous thyroid hormone treatment at the time of thyroglobulin measurement”
A novel approach has been recently developed by several laboratories for the sensitive and early detection of circulating tumour cells in patients with thyroid cancer, which may precede the detection of recurrent or metastatic disease by other currently used methods. This may have important therapeutic and prognostic implications. The approach is based upon the detection of Tg mRNA as a tumour marker, using the reverse transcriptase polymerase chain reaction (RT-PCR) in circulating thyrocytes from patients with residual or metastatic thyroid disease.6,9–13 Most studies suggest that this assay is more sensitive than standard immunoassays for Tg. It enables the detection of circulating thyrocytes from patients without the need for thyroid hormone withdrawal, and can also be of use in those with circulating anti-Tg antibodies. The purpose of our present study was to evaluate the usefulness of the Tg mRNA assay in routinely monitoring patients with thyroid cancer on TSH suppressive treatment, by determining its sensitivity and specificity and comparing it with the results using an established Tg radioimmunoassay.
MATERIALS AND METHODS
Patients and specimens
Our study included 28 patients (table 1 ▶) with well differentiated thyroid carcinoma on follow up, who had previously undergone thyroidectomy (either total or partial) and received 131I ablation according to the current protocols used at the University Hospitals of Coventry and Warwickshire NHS Trust, UK. Twenty patients had papillary adenocarcinoma and eight had follicular adenocarcinoma. The period from the original diagnosis ranged from four to 17 years (mean, 7.5 years). According to the clinical data available, 13 patients showed no evidence of metastases, whereas the remainder were found to be positive for locoregional or distant metastases by radioiodine imaging. Furthermore, bimonthly assays for Tg mRNA and immunoreactive Tg were carried out in five additional patients who were negative for distant metastases at the beginning of our study. All patients with thyroid malignancies were routinely maintained on 100–150 μg L-thyroxine each day to suppress circulating TSH. Six patients with clinical and biochemical evidence of non-malignant thyroid disease and five normal individuals with no history or clinical evidence of thyroid disease were also evaluated.
Table 1.
Characteristics of patients and controls
| Patients | Clinical status/evidence of metastasis | Time after diagnosis (years) | Tg Ab status | TSH value (mU/l) |
| 1–14 | Thyroid cancer/yes | 4/5/6/6/7/12/9/13/17/13/5/6/4/7 | Negative | TSH <0.1 |
| 15–24 | Thyroid cancer/no | 6/5/6/7/12/14/4/6/8/9 | Negative | TSH <0.1 |
| 25–27 | Thyroid cancer/no | 5/4/10 | Positive | TSH <0.1 |
| 28 | Thyroid cancer/yes | 6 | Positive | TSH <0.1 |
| 29–34 | Hypothyroidism | Negative | TSH >15 | |
| 35–39 | Normal: euthyroid | Negative | 3> TSH >0.4 |
Ab, antibody; Tg, thyroglobulin; TSH, thyrotrophin.
Blood samples for Tg mRNA measurement by RT-PCR were obtained in addition to samples for measurement of serum Tg (assay sensitivity, 5 μg/litre). The Tg immunoassay was carried out at the regional endocrine laboratory, University Hospital Birmingham NHS Trust, UK using an in house radioimmunoassay.14 All serum samples were screened for the identification of anti-Tg antibodies using a commercial turkey erythrocyte haemagglutination kit (Thymune-M; Murex Biotech Ltd, Dartford, Kent, UK).15 Radioiodine scanning and relevant radiographic (x ray, computed tomography, magnetic resonance imaging) studies were used to image metastases. Laboratory investigators were blinded to the clinical status of the patients.
Our study was approved by the Coventry ethics committee and the research and development board of the University Hospitals Coventry and Warwickshire NHS Trust, Coventry, UK. Written informed consent was obtained from all patients who participated in the study.
RNA extraction and RT-PCR
RNA was prepared from 4 ml EDTA blood. Whole blood samples were separated into 0.3 ml aliquots. Total RNA was extracted using the PUREscript kit (Gentra Systems, Minneapolis, Minnesota, USA), according to the manufacturer’s protocol for blood samples. RNA yields ranged from 10 to 20 μg/sample.
Total RNA (1 μg) was heated for 10 minutes at 70°C and then incubated for 50 minutes at 42°C in a reaction volume containing 200 U reverse transcriptase (Life Technologies; Invitrogen Ltd, Paisley, UK), 75 pmol random hexamer primers (Pd (n)6; Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK), 10nM dithiothreitol, 1mM of each dNTP, and 10 U RNase inhibitor. RT negative or template negative samples were prepared for each individual sample and served as controls for the detection of assay contamination. The reaction was heat inactivated at 90°C for five minutes; after cooling to 37°C, 1 μl RNase H (Life Technologies) was added, and samples were then incubated for 20 minutes at 37°C.
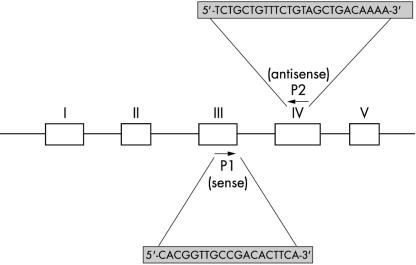
Ten per cent of the synthesised cDNA served as substrate for PCR amplification. Each sample was run in triplicate. The final reaction mixture contained 1× Taq polymerase buffer, 1 mmol/litre of each primer (Tg or β actin), 0.1 mmol/litre of each dNTP, and 2.5 U Taq polymerase (Life Technologies). To avoid false positive results caused by contamination of genomic DNA, primers specific for exons 3 and 4 of the thyroglobulin gene that spanned a 1.5 kb intron were designed to amplify an 87 bp product from base pairs 262 to 348 in the cDNA sequence of the thyroglobulin gene (fig 1 ▶).7 After initial denaturation at 94°C for four minutes, 42 amplification cycles consisting of denaturation at 94°C for one minute, annealing for one minute at 60°C, and extension for one minute at 94°C were performed. Final extension was for four minutes at 72°C.
Figure 1.
Schematic representation of the thyroglobulin 5′ region gene map and the relative positions of the specific polymerase chain reaction primers.
The success of all the RT reactions was confirmed in PCR reactions that amplified human β actin cDNA simultaneously. The primers used for the β actin PCR reactions were: 5′-GTGGGGCGCCCCAGGCACCA-3′ (sense) and 5′-CTCCTTAATGTCACGCACGAT TTC-3′ (antisense). All RT-PCR products were analysed by electrophoresis through 3% agarose gels, followed by ethidium bromide staining to ensure amplification of the appropriate size product. Samples omitting template (distilled water) were included to identify contamination. The PCR products were sequenced using an automated DNA sequencer. Sequence data were analysed using Blast Nucleic Acid Database Searchers from the National Centre for Biotechnology Information, USA.
Assay performance characteristics
The overall performance of the Tg RT-PCR and Tg immunoassay results was defined by calculating the sensitivity (proportion of patients with the disease who were correctly identified by the test), specificity (proportion of patients without the disease who were correctly identified by the test), and accuracy (proportion of patients who were correctly categorised) of each test at the time of blood sampling.
Statistical analysis
Statistical analysis was performed using the McNemar’s exact test to compare Tg RT-PCR and Tg immunoassay results in the patient groups with metastatic and non-metastatic disease.
RESULTS
Detection of Tg mRNA in peripheral blood
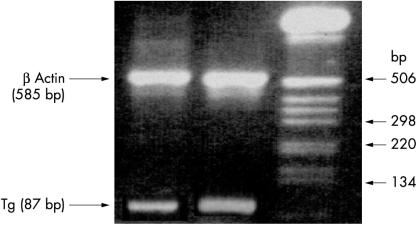
To detect low amounts of circulating Tg mRNA, we used a sensitive RT-PCR assay capable of recognising Tg mRNA transcripts in as few as 10 thyroid cells/ml of blood.6 The assay was optimised for both the synthesis of cDNA and the amplification of Tg cDNA by RT-PCR, whereas the selection of Tg PCR primers prevented the generation of PCR products arising from the amplification of residual genomic DNA. Direct nucleotide sequencing analysis indicated that 585 bp and 87 bp products were amplified from β actin and Tg mRNAs, respectively (fig 2 ▶). The successful amplification of β actin in each sample served to confirm the synthesis of cDNA. Tg mRNA was also detected after PCR amplification of cDNAs prepared from all six patients with evidence of non-malignant thyroid disease and the five normal individuals.
Figure 2.
Identification of thyroglobulin (Tg; 87 bp band) and β actin (585 bp band) mRNA in peripheral blood by simultaneous reverse transcription polymerase chain reaction amplification in an agarose gel stained with ethidium bromide.
Comparison of Tg mRNA assay and immunoassay
Patients were classified according to thyroid tumour histology: 20 patients had papillary adenocarcinoma and eight had follicular adenocarcinoma. The efficacy of the Tg mRNA amplification was not influenced by tumour histology, and successful amplification of Tg mRNA transcripts was achieved from both types of adenocarcinomas (data not shown).
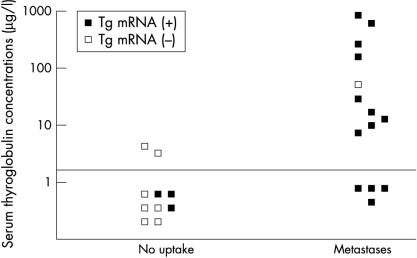
The measurement of Tg concentrations was possible in 24 of the 28 patients with thyroid cancer, although the accurate measurement of circulating Tg values was prohibited because of high amounts of circulating anti-TG antibodies. To validate the Tg mRNA assay clinically, patients were grouped according to the absence (n = 10) or presence (n = 14) of regional/distant metastases, based upon their most recent radioiodine scan. The left hand section of fig 3 ▶ illustrates the data from those patients with no radioiodine uptake: eight had undetectable Tg values, although three were positive for Tg mRNA. In the two patients who had raised Tg concentrations, Tg mRNA was not detectable. One of the 10 patients in this group had detectable lesions based on computed tomography scans but no evidence of radioiodine uptake, raising the possibility that the tumour may have lost its ability to take up radioiodine, which may reflect the functioning of the sodium iodide symporter.16 This patient was negative for both Tg mRNA and Tg protein.
Figure 3.
Comparison of serum thyroglobulin (Tg) concentrations, radioiodine scanning, and Tg mRNA assay results in 24 patients during treatment with thyroxine. Patients are classified into two groups, according to the most recent radioiodine scan. Serum Tg concentrations below 5 μg/litre were considered undetectable.
The right hand part of fig 3 ▶ shows the results from 14 patients with locoregional and distant metastases (five with pulmonary and three with osseous metastases). Thirteen of these patients had detectable Tg mRNA. However, only 10 (p < 0.01) had measurable Tg concentrations. Importantly, all four patients with Tg values below the assay sensitivity of 5 μg/litre were found to be positive for Tg mRNA. Somewhat paradoxically, one patient with a high serum Tg concentration (57 μg/litre) was found to be negative for circulating Tg mRNA.
Table 2 ▶ summarises the comparison of the overall performance characteristics of the Tg mRNA assay with the serum Tg immunoassay for all 24 patients. The Tg mRNA assay appeared to have greater sensitivity and overall accuracy than the serum Tg immunoassay (93% v 71% and 84% v 75%, respectively) using 131I scanning as the “gold standard” for the identification of recurrent thyroid disease. The Tg mRNA results were found to correlate better with the radioiodine scanning results. However, the Tg immunoassay appeared to have a higher specificity than the Tg mRNA assay when compared with the gold standard.
Table 2.
Comparison of the thyroglobulin (Tg) mRNA assay with the Tg immunoassay
| Tg mRNA | Tg immunoassay | |
| Sensitivity | 93 | 71 |
| Specificity | 70 | 80 |
| Accuracy | 84 | 75 |
Performance was assessed by comparing assay results with radioiodine whole body scan findings.
Tg mRNA assay in patients with anti-Tg antibodies
Of the four patients with anti-Tg antibodies, one had evidence of metastatic disease. Representative results are shown in fig 4 ▶, lanes 6–8. The presence of anti-Tg antibodies did not interfere with the PCR amplification of the 87 bp fragment of circulating Tg mRNA in the patient with metastases (fig 4 ▶; lane 6), whereas the others were negative for Tg mRNA (fig 4 ▶; lanes 7–8). Several samples from patients with and without metastases with positive or negative Tg mRNA results were also run simultaneously to ensure successful PCR amplification (fig 4 ▶; lanes 1–5).
Figure 4.
Amplification of thyroglobulin (Tg) mRNA fragments, serum Tg concentrations, and clinical details from eight patients with thyroid cancer.
Long term follow up of patients using serial estimations of Tg mRNA
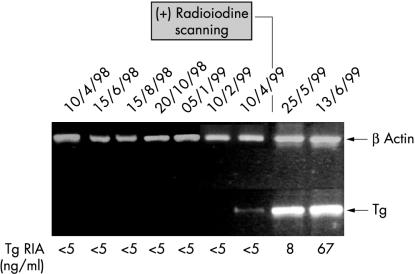
Five patients were studied prospectively for a period of 11 months, two to four years after the initial surgical and medical treatment for thyroid cancer. Blood samples from these patients were obtained every two months. Extracted RNA was stored at −80°C until RT-PCR was performed; all samples were run simultaneously to eliminate interassay variations. In four patients, no Tg mRNA transcripts or serum Tg were detected in peripheral blood, suggesting the absence of local or distant metastases (data not shown), a finding subsequently confirmed by follow up radioiodine scans. Interestingly, one patient was found to be positive for Tg mRNA transcripts, although the corresponding Tg immunoassay result was < 5 μg/litre, which suggested the absence of metastatic disease (fig 5 ▶). The presence of metastases was demonstrated by radioiodine imaging performed 20 days later. Results from subsequent Tg mRNA and Tg assays confirmed recurrent thyroid disease; serum Tg concentrations of 8 and 67 μg/litre were obtained at 45 and 63 days, respectively.
Figure 5.
Use of thyroglobulin (Tg) mRNA reverse transcription polymerase chain reaction assay to monitor for disease progression and metastasis over a 12 month period in a patient with thyroid cancer. RIA, radioimmunoassay.
DISCUSSION
The use of molecular diagnostic techniques, such as RT-PCR, which enable the specific amplification of small numbers of mRNA molecules, has led to the development of several methods for the specific amplification of small foci of malignant tissue, either primary or metastatic, in addition to circulating cancer cells in patients with metastatic prostate cancer,17,18 colorectal cancer,19 malignant melanoma,20 and breast cancer.21,22 Several groups have used RT-PCR based methods with variable sensitivity, RNA extraction, and PCR amplification protocols for the early detection of circulating thyroid tumour cells in patients with thyroid cancer.6,10–12 Most but not all investigators13 agree that the Tg mRNA assay is more sensitive than the Tg immunoassay. In our study, we used an assay with well defined characteristics.6,9,10
Take home messages.
The detection of thyroglobulin (Tg) mRNA in blood using the reverse transcription polymerase chain reaction is a more sensitive marker for metastatic thyroid disease than immunoassay for Tg protein
The Tg mRNA assay appears to be unaffected by the presence of circulating anti-Tg antibodies, which is particularly important for patients on thyrotrophin suppressive treatment who have positive anti-Tg antibodies
“One of the most valuable applications of this assay may be in the management of patients with thyroid cancer who are positive for antithyroglobulin antibodies, which interfere with most conventional thyroglobulin immunoassays”
Here, the Tg mRNA assay proved to be more sensitive and, although less specific, had greater overall accuracy than the Tg immunoassay in residual malignant disease. Metastatic thyroid cancer was correctly identified in all but one patient studied. In contrast, the serum Tg immunoassay had a lower sensitivity. These results are similar to those published previously for this assay in a different thyroid cancer population.6,9 The negative Tg mRNA result in the remaining patient with raised serum Tg concentrations and a positive radioiodine scan was notable. This patient may have had a different splice variant or polymorphism of the Tg gene,23 which could not be amplified by the PCR primers used in our laboratory. Interestingly, some patients without detectable metastatic disease on radioiodine imaging were positive for Tg mRNA, which resulted in the apparent lower specificity of the Tg mRNA assay. This might partly be the result of the lower sensitivity of the diagnostic scanning techniques or indeed may reflect changes in the expression of the genes encoding thyroglobulin and the sodium iodide transporter.24,25 There are several reports of patients who were positive for Tg mRNA but consistently had negative radioiodine scans, in whom metastases were eventually detected.10 Interestingly, the results from two patients negative for Tg mRNA with raised Tg concentrations may suggest a complementary role for these assays in the elucidation of unusual tumour properties, such as their ability to synthesise and secrete proteins. Nevertheless, the greater sensitivity of the Tg mRNA assay was clearly shown in one patient, who was part of a prospective group of patients, and who initially had no evidence of metastatic disease, was negative for Tg mRNA trancripts, and had undetectable serum Tg protein concentrations. In this patient, the presence of circulating Tg mRNA was detected much earlier than the rise in serum Tg concentrations; consequent radioiodine scanning confirmed metastatic disease.
One of the most valuable applications of this assay may be in the management of patients with thyroid cancer who are positive for Tg antibodies, which interfere with most conventional Tg immunoassays. In the small patient group investigated, the presence of anti-Tg antibodies did not compromise the efficacy of the Tg mRNA assay. However, larger trials of patients expressing anti-Tg antibodies would be required to confirm these results.
In conclusion, Tg mRNA is detectable in normal subjects and patients with non-malignant thyroid disease, in addition to most patients with residual thyroid tumour who are treated with exogenous thyroid hormone replacement. In agreement with previous reports, our results have confirmed the greater sensitivity of the Tg mRNA assay compared with the Tg immunoassay, particularly for patients on TSH suppressive treatment who have positive anti-Tg antibodies. The development of quantitative methods9,26 for assaying Tg mRNA will probably be of value in measuring disease progression and its response to treatment. Long term prospective studies are warranted to evaluate the potential clinical usefulness of this assay.
Acknowledgments
Supported by the R&D committee of the University Hospitals Coventry and Warwickshire NHS Trust UK, Robert Gaddie Memorial Fund, Peel Medical Research Trust, and the Mercia Health Benefits.
Abbreviations
RT-PCR, reverse transcriptase polymerase chain reaction
Tg, thyroglobulin
TSH, thyrotrophin
REFERENCES
- 1.Wheeler MH. Management of thyroid carcinoma. Surgery 1999;17:11–17. [Google Scholar]
- 2.Singer PA, Cooper DS, Daniels GH, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. Arch Intern Med 1996;156:2165–72. [PubMed] [Google Scholar]
- 3.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994;97:418–28. [DOI] [PubMed] [Google Scholar]
- 4.Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 1998;83:1121–7. [DOI] [PubMed] [Google Scholar]
- 5.Pacini F, Pinchera A, Giani C, et al. Serum thyroglobulin concentrations and 131I whole body scans in the diagnosis of metastases from differentiated thyroid carcinoma (after thyroidectomy). Clin Endocrinol 1980;13:107–10. [DOI] [PubMed] [Google Scholar]
- 6.Ringel MD, Ladenson PW, Levine MA. Molecular diagnosis of residual and recurrent thyroid cancer by amplification of thyroglobulin messenger ribonucleic acid in peripheral blood. J Clin Endocrinol Metab 1998;83:4435–42. [DOI] [PubMed] [Google Scholar]
- 7.Ozata M, Suzuki S, Miyamoto T, et al. Serum thyroglobulin in the follow-up of patients with treated differentiated thyroid cancer. J Clin Endocrinol Metab 1994;79:98–105. [DOI] [PubMed] [Google Scholar]
- 8.Basaria M, Graf H, Cooper DS. The use of recombinant thyrotropin in the follow-up of patients with differentiated thyroid cancer. Am J Med 2002;112:721–5. [DOI] [PubMed] [Google Scholar]
- 9.Ringel MD, Balducci-Silano PL, Anderson JS, et al. Quantitative reverse transcription-polymerase chain reaction of circulating thyroglobulin messenger ribonucleic acid for monitoring patients with thyroid carcinoma. J Clin Endocrinol Metab 1999;84:4037–42. [DOI] [PubMed] [Google Scholar]
- 10.Ditkoff BA, Marvin MR, Yemul S, et al. Detection of circulating thyroid cells in peripheral blood. Surgery 1996;120:959–64. [DOI] [PubMed] [Google Scholar]
- 11.Biscolla RPM, Cerutti JM, Maciel RMB. Detection of recurrent thyroid cancer by sensitive nested reverse transcription-polymerase chain reaction of thyroglobulin and sodium iodide symporter messenger ribonucleic acid transcripts in peripheral blood. J Clin Endocrinol Metab 2000;85:3623–7. [DOI] [PubMed] [Google Scholar]
- 12.Tallini G, Ghossein RA, Emanuel J, et al. Detection of thyroglobulin, thyroid peroxidase, and RET/PTC1 mRNA transcripts in the peripheral blood of patients with thyroid disease. J Clin Oncol 1998;16:11158–66. [DOI] [PubMed] [Google Scholar]
- 13.Bojunga J, Roddiger S, Stanisch M, et al. Molecular detection of thyroglobulin mRNA transcripts in peripheral blood of patients with thyroid disease by RT-PCR. Br J Cancer 2000;82:1650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black EG, Cassoni A, Gimlette TM, et al. Serum thyroglobulin in thyroid cancer. Lancet 1981;8244:443–5. [DOI] [PubMed] [Google Scholar]
- 15.Cayzer I, Chalmers SR, Doniach D, et al. An evaluation of two new haemagglutination tests for the rapid diagnosis of autoimmune thyroid disease. J Clin Pathol 1978;31:1147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arturi F, Russo D, Giuffrida D, et al. Sodium iodide symporter (NIS) gene expression in lymph-node metastases of papillary thyroid carcinomas. Eur J Endocrinol 2000;143:623–7. [DOI] [PubMed] [Google Scholar]
- 17.Katz AE, Olsson CA, Raffo AJ, et al. Molecular staging of prostate cancer with the use of an enhanced reverse transcriptase-PCR assay. Urology 1994;43:765–75. [DOI] [PubMed] [Google Scholar]
- 18.Corey E, Arfman EW, Liu AY, et al. Improved reverse transcriptase-polymerase chain reaction protocol with exogenous internal competitive control for prostate-specific antigen mRNA in blood and bone marrow. Clin Chem 1997;43:443–52. [PubMed] [Google Scholar]
- 19.Smith B, Selby P, Southgate J, et al. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet 1991;338:1227–9. [DOI] [PubMed] [Google Scholar]
- 20.Wong LS, Cantrill JE, Odogwu S, et al. Detection of circulating tumour cells by reverse transcriptase-polymerase chain reaction technique. Br J Surg 1997;84:834–9. [PubMed] [Google Scholar]
- 21.Kruger W, Krzizanowski C, Holweg M, et al. Reverse transcriptase/polymerase chain reaction detection of cytokeratin-19 mRNA in bone marrow and blood of breast cancer patients. J Cancer Res Clin Oncol 1999;122:679–86. [DOI] [PubMed] [Google Scholar]
- 22.Luppi M, Morselli M, Bandieri E, et al. Sensitive detection of circulating breast cancer cells by reverse-transcriptase polymerase chain reaction of maspin gene. Ann Oncol 1996;7:619–24. [DOI] [PubMed] [Google Scholar]
- 23.Olivieri A, Marzullo A, Salabe GB, et al. Isoelectric focusing and immunoblotting analysis of thyroglobulin from different thyroid diseases. Thyroidology 1991;3:13–16. [PubMed] [Google Scholar]
- 24.Black EG, Sheppard MC. Serum thyroglobulin measurements in thyroid cancer: evaluation of “false” positive results. Clin Endocrinol 1991;35:519–20. [DOI] [PubMed] [Google Scholar]
- 25.Pineda JD, Lee T, Ain K, et al. Iodine-131 therapy for thyroid cancer patients with elevated thyroglobulin and negative diagnostic scan. J Clin Endocrinol Metab 1995;80:1488–92. [DOI] [PubMed] [Google Scholar]
- 26.Wingo ST, Ringel MD, Anderson JS, et al. Quantitative reverse transcription-PCR measurement of thyroglobulin mRNA in peripheral blood of healthy subjects. Clin Chem 1999;45:785–9. [PubMed] [Google Scholar]