Abstract
Background: The E-cadherin–catenin adhesion complex is crucial for intercellular adhesiveness and maintenance of tissue architecture. Its impairment is associated with poorly differentiated phenotype and increased invasiveness of carcinomas.
Aims: To evaluate E-cadherin, β catenin, γ catenin, and ezrin expression and its relation to histopathological features of primary and metastatic Wilms’s tumours.
Methods: Immunohistochemistry was used to determine the expression and cellular distribution of E-cadherin, β catenin, γ catenin, and ezrin in primary and metastatic Wilms’s tumours. Western blotting was used to determine polypeptide size and expression of E-cadherin and β catenin in Wilms’s tumours compared with normal kidney.
Results: Moderate expression of E-cadherin was found mainly in cytoplasm and occasionally cell membranes of dysplastic tubules, whereas low expression was seen in cytoplasm of blastemal cells. Primary and metastatic tumours showed moderate to high β catenin expression in blastemal and epithelial cells, with predominantly membranous and cytoplasmic staining. Occasional nuclear staining was noted in metastatic tumours. Low to high γ catenin and ezrin expression was seen in cytoplasm of blastemal and epithelial cells of primary and metastatic tumours. Higher amounts of 92 kDa β catenin were detected in tumours than in normal kidney. Low expression of 120 kDa E-cadherin was seen in moderately differentiated tumours, whereas expression was lacking in poorly differentiated tumours.
Conclusions: Compared with primary tumours, metastatic tumours showed lower expression of E-cadherin and γ catenin, with nuclear staining for β catenin. Low E-cadherin was associated with poorly differentiated tumours. These results suggest that abnormal expression of adhesion proteins correlates with the invasive and metastatic phenotype in Wilms’s tumours.
Keywords: Wilms’s tumour, E-cadherin (CDH1), β catenin (CTNNB1), γ catenin (CTNNG/CTNNBIP1), ezrin
Wilms’s tumour, or nephroblastoma, is the most common paediatric kidney cancer, and it arises in 1/10 000 children, usually below the age of 5, and accounts for approximately 8% of all childhood tumours.1 Approximately 10–15% of patients with Wilms’s tumour present with metastasis, with 30% of these patients eventually succumbing to their disease.2 Loss of wild-type p53 or aberrant expression of mutant p53 are indicative of progression to a metastatic phenotype in several human cancers.3 In Wilms’s tumour, p53 has been postulated to interact physically with the Wilms’s tumour suppressor gene, WT1, modulating the transcriptional activity of its target genes and contributing to the tumour aetiology and malignant progression of Wilms’s tumour.3,4 We have reported along with others that deregulated p53 is an important feature of Wilms’s tumours that recur or metastasise.5–7 Other biological factors governing the pathobiology of invasive and metastatic Wilms’s tumours remain unknown.
WT1, the Wilms tumour suppressor gene,8 has been shown to regulate the transcription of several cell surface proteins important for kidney differentiation, such as syndecan-I, podocalyxin, and most recently E-cadherin.9,10 During normal nephrogenesis, E-cadherin expression is induced in the condensing metanephric mesenchyme during the mesenchymal–epithelial transition, parallelling the expression pattern of WT1 proteins.1 In normal human kidney development, the expression of both E-cadherin and β catenin is relatively high, coinciding with the establishment of a polarised epithelium in nephrons.11 Several different studies have revealed loss of hetereozygosity on chromosome 16q in between 17% and 25% of Wilms’s tumours; however, recent findings indicate that E-cadherin probably does not play a role as a tumour suppressor gene in Wilms’s tumour.12 β Catenin has been shown to contain mutations associated with WT1 mutations in Wilms’s tumours.11,13,14
Cell–cell adhesion junctions composed of E-cadherin and its associated intracellular catenins (β, γ, α) play an important role in the maintenance of cell integrity and cell morphology of epithelial cells. Ezrin, a member of the ERM (ezrin, radixin, and moesin) family of proteins, is concentrated at sites of cell–cell contact and acts as a crosslinker between the actin cytoskeleton and proteins of the cell surface membrane.15 Co-precipitation studies have revealed an association of ezrin with E-cadherin and β catenin through hepatocyte growth factor (HGF) induced phosphorylation of ezrin, resulting in reduced cell–cell adhesiveness and an increase in cell motility and invasiveness.16 Therefore, ezrin regulates cell–cell and cell–matrix adhesion by interacting with E-cadherin and β catenin, and may thus play an important role in the control of adhesion and invasiveness of cancer cells.16 It is believed that dysfunction or disruption of these cell adhesion molecules, specifically E-cadherin, is associated with invasiveness and with metastatic behaviour and poor clinical outcome in several epithelium derived malignancies.17
“E-cadherin, ezrin, and catenins have been shown to be downstream substrates of the met signal transduction pathway linked to the process of tumour invasion and metastasis”
We have recently established coexpression of HGF and its cognate receptor, met, in Wilms’s tumours with higher levels of expression being seen in homotypic metastatic tumours.18 HGF and met have been shown to be involved in invasion and metastasis in different cancers and tumour cell lines.19,20 Activation of met by HGF has been shown to amplify several downstream signalling pathways, including cell motility.21 The ability of HGF to disperse epithelial cells has focused attention on adhesion molecules of the cadherin–catenin complex.22,23 E-cadherin, ezrin, and catenins have been shown to be downstream substrates of the met signal transduction pathway linked to the process of tumour invasion and metastasis.24,25
We now report the differential expression of E-cadherin, β catenin, γ catenin, and ezrin proteins in a series of primary and metastatic Wilms’s tumours and abnormal expression patterns associated with the invasive and metastatic phenotype in Wilms’s tumour.
MATERIALS AND METHODS
Tissue samples
Our study was based on tissue specimens from untreated patients with Wilms’s tumour obtained from the division of pathology at The Hospital for Sick Children, Toronto, Canada. A series comprising 37 Wilms’s tumours was accrued for our study. Table 1 ▶ shows the clinicopathological parameters (tumour stage, histopathology, metastases, and percentage of differentiated elements) of group A, which comprised nine paired cases of primary tumour and the corresponding metastasis; group B, which comprised 10 primary (non-metastatic) tumours; and group C, which comprised eight metastatic tumours. The cases from group C were from patients who initially presented with metastases and therefore no primary was available. All tumours were assessed by staff pathologists and histological stage classifications were determined according to the National Wilms’s study tumour group.
Table 1.
Clinicopathological features of patients with Wilms’s tumour
| Case | Stage | Age | Sex | Histopathology | Metastases | Histological features | |||
| % EP | % BL | % STR | M | ||||||
| Paired primary and metastatic tumour samples | |||||||||
| WiT 49p | II | 3 years | F | UF | – | 30 | 60 | 10 | Yes |
| WiT 49m | IV | 4 years | UF (diffuse) | Lung | 30 | 60 | 10 | Yes | |
| WiT 135 | III | 5 years | F | FH | – | 15 | 80 | 5 | No |
| WiT 135x | III | 5 years | FH | Lymph | 15 | 80 | 5 | No | |
| WiT 100 | II | 3 years | M | UF (diffuse) | – | 5 | 60–70 | 20–25 | Yes |
| WiT 108 | IV | 4 years | FH | Liver | 5 | 95 | 0 | No | |
| WiT 156* | II | 6 years | M | FH | – | 20 | 70 | 10 | No |
| WiT 157v | IV | 6 years | FH | Lymph | 20 | 70 | 10 | No | |
| WiT 168v | III | 8 years | F | FH | Lymph | 5 | 95 | 0 | No |
| WiT 167v | IV | 8 years | FH | Lung | 5 | 95 | 0 | No | |
| WiT 131v | II | N/A | M | UF (diffuse) | – | 10 | 60 | 30 | Yes |
| WiT 151v | III | N/A | UF (diffuse) | Pleural mass | 30 | 60 | 10 | Yes+adipose | |
| WiT 149v | III | 4 years | F | FH | Hilar lymph | 5 | 80 | 15 | No |
| WiT 149w | IV | 4 years | FH | Lung | 5 | 80 | 15 | No | |
| WiT 140 | II | 4 years | F | FH | – | 5 | 75 | 20 | No |
| WiT 140v | IV | 4 years | FH | Lung | 5 | 75 | 20 | No | |
| WiT 146* | I | 6 years | F | FH | – | 5 | 85 | 10 | No |
| WiT153 | II | 7 years | UF (focal) | Omentum | 5 | 85 | 10 | No | |
| Unpaired primary tumour samples | |||||||||
| WiT 180 | II | 5 years | F | UF (diffuse) | – | 30 | 40 | 30 | Yes+cartilage |
| WiT 173 | II | 5 years | F | FH | – | 10 | 85 | 5 | No |
| WiT 178 | II | 3 years | M | FH | – | 10 | 80 | 10 | Yes |
| WiT 176 | II | 5 years | F | FH | – | 20 | 70 | 10 | No |
| WiT 179 | II | 5 years | F | UF (diffuse) | – | 60 | 30 | 10 | No |
| WiT 175 | I | 1 year | M | FH | – | 60 | 0 | 40 | Yes |
| WiT 183 | II | 3 year | F | FH | – | 25 | 65 | 10 | No |
| WiT 158* | I | 2 year | F | FH | – | 65 | 30 | 5 | Yes |
| WiT 148 | II | 9 months | M | FH | – | 15 | 70 | 15 | No |
| WiT 141* | II | 6 years | M | FH | – | 60 | 10 | 30 | Yes |
| WiT 154* | I | 3 years | M | FH | – | 25 | 45 | 30 | No |
| Unpaired metastatic samples | |||||||||
| WiT 168 | IV | 5 years | M | FH | Adrenal | 2 | 95 | 3 | Yes |
| Hilar lymph | 95 | 2 | 3 | No | |||||
| Inter-aortocaval lymph | 95 | 2 | 3 | No | |||||
| WiT 144v | III | 1 years | F | FH | Peri-aortic lymph | 60 | 20 | 20 | Yes |
| WiT 183v | IV | 12 years | M | FH | Neck | 10 | 50 | 40 | No+cartilage |
| WiT 172 | III | 5 years | F | FH | Hilar lymph | 10 | 60 | 30 | No |
| WiT 123 | IV | 6 years | F | UF (focal) | Lung | 30 | 70 | 0 | No |
| WiT 157* | III | 8 years | M | FH | Pericaval lymph | 20 | 60 | 20 | Yes |
| WiT 181v | IV | 1 years | M | FH | Lung | 10 | 20 | 70 | No |
| WiT 190 | III | 3 years | M | FH | Mass below spleen | 30 | 60 | 5 | No |
Tumours exhibiting muscle are designated “heterotypic Wilms’s tumours”, whereas tumours with no evidence of muscle are designated “homotypic Wilms’s tumours”.
*Tumours included for western blot analysis.
BL, blastemal; EP, epithelial; FH, favourable histology; M, muscle; N/A, not available; STR, stromal; UF, unfavourable histology (anaplasia).
Western blot analysis
Protein was extracted from snap frozen tissue with NP40 lysis buffer (150mM NaCl, 1% NP-40 (vol/vol), 0.5% sodium deoxycholate (wt/vol), 50mM Hepes, pH 7.5, and complete protease tablet (Roche-Boehringer Mannheim, Quebec, Canada), and western blot analysis was performed as described previously.26 Aliquots (50 μg) of tumour protein extracts were analysed for six tumours (WiT 146, WiT 156, WiT 141, WiT 154, WiT 158, and WiT 157). Protein was quantified spectrofluorometrically using the Bradford assay (Bio-Rad, Hercules, California, USA). Blots were incubated with primary antibodies, anti-E-cadherin (1/50 dilution; mouse monoclonal (4A2C7); Zymed, San Francisco, California, USA), anti-β catenin (1/100 dilution; mouse monoclonal (C19220); Transduction Laboratories, Mississauga, Ontario, Canada) for one hour at room temperature, followed by several washes at room temperature in 1% skimmed milk in Tris buffered saline and 0.1% Tween 20. The secondary antibody, goat antimouse IgG alkaline phosphatase Fab fragments (1/1000 dilution; Roche-Boehringer Mannheim) was applied for one hour at room temperature. The membranes were incubated in CDPStar (Roche-Boehringer Mannheim) and exposed to Kodak X-omatic film for five minutes to one hour.
Immunohistochemistry
Immunohistochemistry was performed on paraffin wax embedded tumours after antigen retrieval, as described previously.18 The primary antibodies against E-cadherin (1/50 dilution; mouse monoclonal (4A2C7); Zymed), β catenin (1/100 dilution; mouse monoclonal (C19220); Transduction Laboratories), γ catenin (1/100 dilution; goat polyclonal (C-20); Santa Cruz), and ezrin (1/50 dilution; goat polyclonal (C-15); Santa Cruz) were incubated overnight at 4°C in antibody diluting buffer (Dimensions Laboratories, Mississauga, Canada). The secondary antibodies used were biotinylated goat antimouse IgG (1/200 dilution; Molecular Probes, Eugene, Oregon, USA) and biotin SP conjugated Affinipure donkey antigoat IgG (1/200 dilution; Jackson Laboratories, West Grove, Pennsylvania, USA), and were applied for one hour at room temperature. Finally, avidin horseradish peroxidase conjugate (1/1000 dilution; Neutravidin; Molecular Probes) was applied for one hour at room temperature. The bound antibody complex was visualised by reaction in 3,3‘diaminobenzidine substrate (Stable DAB; Research Genetics, Huntsville, Alabama, USA), and sections were counterstained with Meyer’s haematoxylin.
Evaluation of immunohistochemical staining
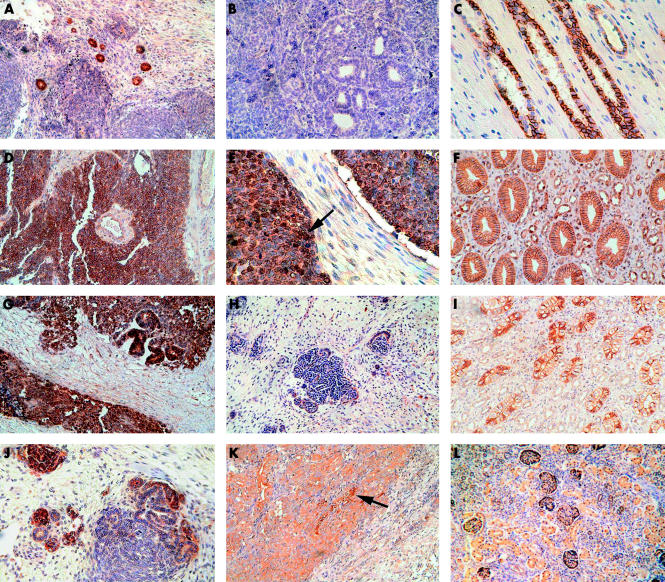
The staining intensity was scored using a semiquantitative scale: −, negligible; +, weak; ++, moderate; +++, strong; and ++++, very strong. The staining was evaluated by two independent observers without knowledge of the clinical outcomes. Tumour samples that revealed membranous (that is, intercellular staining at the cell membrane) patterns for E-cadherin, β catenin, γ catenin, and ezrin were designated as expected (that is, normal). Expression were considered to be abnormal when the proteins displayed positive cytoplasmic (with the exception of β catenin) or nuclear staining. The staining intensities of E-cadherin and β catenin in tumour cells were evaluated using the adjacent normal kidney epithelium as an internal positive control, thus allowing direct comparison within the same tissue specimen. In normal kidney epithelium, E-cadherin and β catenin proteins were strongly expressed at the intercellular borders of the proximal tubules and cell–cell boundaries of the collecting ducts, respectively (fig 1 C,F ▶). Distal tubules showed weak expression of E-cadherin and β catenin. There was no staining at the lumenal surface or in the basal aspects of the cell membrane of tubules. γ Catenin was expressed at the cell membrane in collecting ducts and distal tubules, and ezrin in the distal tubules and glomerular epithelial cells of normal kidney (fig 1 I,L ▶). No nuclear immunoreactivity was found for β catenin, E-cadherin, γ catenin, or ezrin in normal kidney. A negative control slide was prepared by omission of the primary antibody.
Figure 1.
Expression of cadherin–catenin complex proteins in primary and metastatic Wilms’s tumours and normal adjacent kidney using immunohistochemistry. (A) E-cadherin expression in primary Wilms’s tumour showing a moderate to high degree of staining in the cytoplasm of dysplastic tubules, weaker staining in the blastema, and negligible staining in stroma (peroxidase; brown colour); (B) metastatic Wilms’s tumours showing a low degree of staining in dysplastic tubules and adjacent blastema; and (C) normal kidney control showing strong cell membrane staining in proximal tubules. (D) β Catenin expression in primary Wilms’s tumour shows strong staining in the cytoplasm of blastemal cells; (E) metastatic Wilms’s tumour reveals nuclear (arrow) and cytoplasmic staining in blastemal cells, with negligible staining in stroma; and (F) normal kidney control showing strong staining at the cell membrane in the collecting ducts. (G) γ Catenin expression in primary Wilms’s tumour showing moderate staining in the blastemal cells, with strong staining in the cytoplasm of dysplastic tubules and negligible staining in surrounding stroma; (H) metastatic Wilms’s tumours showing a low degree of staining in the cytoplasm of blastemal and epithelial cells; and (I) normal kidney control showing strong cell membrane staining in the distal tubules. (J) Ezrin expression in primary Wilms’s tumour showing moderate and high degrees of staining in some of the dysplastic tubules, with weaker staining in blastemal cells, and negligible staining in the stroma; (K) metastatic Wilms’s tumours showing moderate staining in the cytoplasm of blastemal cells and strong staining in the cytoplasm of dysplastic tubules (arrow) near the invasive margin; and (L) normal kidney control showing weak staining in the cytoplasm of tubules and in epithelial cells of the glomeruli.
Statistical analysis
A nine point ordinal scale was used to analyse the data, with 1 representing the lowest staining category (− or negative) and 9 the highest (++++ or very strong). All other possible combinations in between were scored to represent the ordered nature of the staining categories. Tumours were nested into three groups: primary, metastatic, and primary (non-metastatic). Four measurements representing the crossed effects between proteins (E-cadherin and β catenin) and cell types (blastemal and epithelial) were taken for each tumour. Because these measurements were taken in the same tumour some correlation was assumed, so that analysis of variance for a correlated cross nested design was used to investigate the main effects of protein, cell type, and group, together with their two way and three way interactions. The mixed model theory was used to analyse the data. The PROC MIXED statistical program (SAS, Cary, North Carolina, USA) was used to perform the ANOVA. Table 2 ▶ shows the significance of the main effects and two and three way interactions. Individual contrasts were calculated to assess a priori differences of specific combinations of the three main effects (table 3 ▶). A p value of 0.05 or less was considered significantly different.
Table 2.
A repeated measure analysis of variance for a crossed nested design (using mean scores and p values)
| Effect | Mean scores | p Value |
| Protein | ||
| E-cadherin | 3.3235 | <0.0001 |
| β catenin | 6.2769 | |
| Cell type | ||
| Blastemal | 4.5217 | 0.0700 |
| Epithelial | 5.0312 | |
| Protein v cell type | ||
| E-cadherin v blastemal | 2.7714 | 0.0068 |
| E-cadherin v epithelial | 3.9090 | |
| β Catenin v blastemal | 6.3235 | |
| β catenin v epithelial | 6.2258 | |
| Group v protein v cell type | 0.0817 |
p<0.05 is significant.
Table 3.
Post hoc comparisons to test three way interaction effects
| Group/protein/cell type | SEM | p Value |
| Metastatic/E-cad/Bl v primary/E-cad/Bl | 0.7866 | 0.3510 |
| Metastatic/E-cad/Ep v primary/E-cad/Ep | 0.8135 | 0.4950 |
| Metastatic/β cat/Bl v primary/β cat/Bl | 0.7866 | 0.0686 |
p<0.05 is significant.
β cat, β catenin; Bl, blastemal; E-cad, E-cadherin; Ep, epithelial.
RESULTS
Expression of E-cadherin in primary and metastatic Wilms’s tumours
E-cadherin expression in primary and metastatic tumours was evaluated using immunohistochemistry (summarised in table 4 ▶). The expression of E-cadherin was relatively low in the cytoplasm of blastemal cells in seven of 19 of the primary tumours and nine of 16 of the metastatic tumours. Low to moderate E-cadherin expression was seen predominantly in the cytoplasm of epithelial cells and at the apical (lumenal end) of dysplastic tubular cells in 10 of 19 of the primary tumours and 11 of 16 of the metastatic tumours (fig 1 A,B ▶). E-cadherin was not expressed in five metastatic tumours and three primary tumours that were representative of more undifferentiated phenotypes, comprising 95% and 70–85% blastemal cells, respectively. One primary tumour with features of 30% epithelial cells, 60% blastemal cells, and diffuse anaplasia was also negative for E-cadherin.
Table 4.
Expression patterns of E-cadherin, β catenin, γ catenin, and ezrin in Wilms’s tumours and staining intensity using immunohistochemistry
| E-cadherin | β Catenin | γ Catenin | Ezrin | |||||||||
| Tumour group | Ep | Bl | Str | Ep | Bl | Str | Ep | Bl | Str | Ep | Bl | Str |
| Primary tumours | –/++ | –/++ | – | –/+++ | +++ | – | + | –/++ | – | + | –/++ | – |
| Paired metastatic tumours | –/+++ | –/++ | – | +/++++ | +/++++ | – | +/++ | –/+ | – | +/++ | –/++ | – |
| Primary (non-metastatic) tumours | –/+++ | –/+ | – | –/++++ | ++/++++ | – | +/+++ | –/++ | – | ++++ | ++/++++ | – |
| Unpaired metastatic tumours | –/+++ | –/++ | – | ++/++++ | +/++++ | – | +/+++ | –/+ | – | –/+++ | ++/+++ | – |
Each column shows the average range for all the tumours. Results are shown for all the tumours listed in table 1 ▶. Semiquantitative scale (relative to normal human kidney staining): –, negligible; +, weak; ++, moderate; +++, strong; ++++, very strong.
Bl, blastemal; Ep, epithelial; Str, stromal.
Most of the E-cadherin staining was found at the cell membrane in primary tumours not undergoing metastasis (group B), and in the cytoplasm of cells in metastatic tumours (groups A and C). Only two metastatic tumours showed nuclear staining (not shown). Although E-cadherin staining patterns varied within the tumours, probably because of variations in cellular differentiation, expression did correlate with increasing histological differentiation.
Expression of β catenin in primary and metastatic Wilms’s tumours
A high degree of expression of the β catenin protein was seen in blastemal and epithelial cells of primary tumours, with only two cases expressing low to moderate amounts. β Catenin was immunolocalised to both the cytoplasm and membrane of blastemal and epithelial cells (fig 1 D,E ▶). Moderate to high β catenin expression was seen in all metastatic tumours, except for one case, which showed weak staining. In the metastatic cases, β catenin was immunolocalised to the cytoplasm and membrane, with seven of 16 cases showing nuclear staining in both blastemal and epithelial cells. In cases of metastatic tumour with distant metastases, an increase in staining intensity was seen in both cell types near the tumour invasive front (not shown).
One particular case (WiT 100), a recurrent metastatic tumour, demonstrated strong expression of both E-cadherin and β catenin in the nuclei of both blastemal and epithelial cells, with no apparent membranous or cytoplasmic staining. The liver metastasis (WiT 108) of WiT 100 showed an absence of E-cadherin and β catenin expression in both cell types. In all tumour cases examined, the stroma was consistently negative for E-cadherin and β catenin in both primary and metastatic Wilms’s tumours.
Both the degree of expression of E-cadherin and β catenin and the pattern of expression showed no apparent association with tumour stage. There were no apparent differences between E-cadherin and β catenin expression between primary tumours from group A and primary tumours from group B (table 4 ▶). However, the expression of β catenin was higher than that of E-cadherin in all the tumour groups studied (p < 0.0001; table 2 ▶). There was a significant difference between the degree of staining for both the proteins (E-cadherin and β catenin) in the two cell types, with stronger staining in epithelial cells than in blastemal cells (p = 0.0700). A two way interaction between the protein and cell type was also tested (p = 0.0068; table 2 ▶). There was no significant interaction on three way analysis noted between group–protein–cell type (p = 0.0817; table 2 ▶), and no significant difference between the degree of expression of E-cadherin and β catenin in primary and metastatic tumours for the two cell types (table 3 ▶).
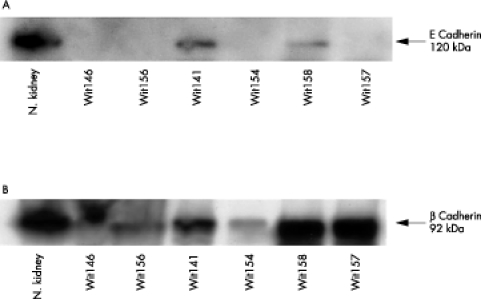
Western blot analysis of E-cadherin and β catenin expression in Wilms’s tumours
Classic Wilms’s tumours are designated as homotypic Wilms’s tumours, whereas tumours exhibiting muscle differentiation are designated as heterotypic Wilms’s tumours (table 1 ▶). Tumour tissues from six homotypic and heterotypic Wilms’s tumour cases were used to examine E-cadherin and β catenin expression by western blot analysis (fig 2 A,B ▶). Poorly differentiated primary homotypic Wilms’s tumours and one case of metastatic Wilms’s tumour (WiT 157) showed no evidence of the 120 kDa E-cadherin polypeptide by western blot analysis. Follow up of the primary homotypic tumours (WiT 146 and WiT 156) showed progression to a metastatic phenotype. Differentiated primary heterotypic Wilms’s tumours expressed the 120 kDa E-cadherin polypeptide and did not progress to a metastatic state. In general, E-cadherin expression in the tumours was lower than that seen in the normal kidney control.
Figure 2.
Western blot analysis of E-cadherin and β catenin in Wilms’s tumours. (A) The 120 kDa E-cadherin protein was expressed in primary heterotypic Wilms’s tumours (WiT 141 and WiT 158), whereas primary homotypic (WiT 146, WiT 156, and WiT 154) tumours showed a lack of expression. The metastatic Wilms’s tumours exhibiting heterotypic differentiation showed loss of E-cadherin expression (WiT 157). (B) The 92 kDa β catenin protein was expressed in both primary heterotypic and homotypic tumours and metastatic heterotypic Wilms’s tumours. The positive control was normal (N.) kidney.
As measured by western blot analysis, high amounts of the 92 kDa β catenin polypeptide were found in both primary (homotypic and heterotypic) tumours and in a single case of metastatic Wilms’s tumour, with increased amounts being found in primary heterotypic tumours that did not metastasise. No association between the differentiation state of the tumours and the degree of β catenin staining was seen.
In normal kidney, similar amounts of β catenin and E-cadherin were detected by western blot. In Wilms’s tumours, the expression of the β catenin protein was higher than that for E-cadherin using western blot analysis, and this correlated with the immunohistochemical findings (table 5 ▶).
Table 5.
Comparison between western blot analysis and immunohistochemistry for E-cadherin and β catenin in Wilms’s tumours
| Western blot analysis | Immunohistochemistry | |||
| Wilms’s tumours | E-cadherin expression | β Catenin expression | E-cadherin expression | β Catenin expression |
| 120 kDa | 92 kDa | |||
| Wit 146 | – | ++ | – | ++/+++ |
| Wit 156 | – | ++ | ++ | +++ |
| Wit 141 | ++ | ++ | + | ++ |
| Wit 154 | – | + | – | + |
| Wit 158 | ++ | ++++ | – | ++++ |
| Wit 157 | +++ | ++++ | +/+++ | +++ |
Semiquantitative scale (relative to normal human kidney): –, negligible; +, weak; ++, moderate; +++, strong; ++++, very strong.
Expression of γ catenin and ezrin proteins in primary and metastatic Wilms’s tumours
The expression of γ catenin was high in the cytoplasm of blastemal and epithelial cells in primary tumours from group A (fig 1G ▶). However, the expression of γ catenin was consistently low in both cell types in primary tumours from group B. In metastatic tumours, moderate expression of γ catenin was seen mainly in the epithelial cells of local metastatic tumours and low expression was seen in blastemal cells of distant metastatic tumours (fig 1H ▶). Nuclear staining for γ catenin was seen in some of the tumours from group B, near to the invasive front of tumours undergoing distant metastasis (not shown). This nuclear staining pattern was similar to that noted in colorectal and lung carcinomas.27
Immunostaining was strong and homogeneous for ezrin in blastemal and epithelial cells of primary tumours from group B. Areas of differentiated blastema appearing as pseudoglomeruli showed positive staining for ezrin (fig 1J ▶). In general, the degree of staining was moderate and low in local and distant metastases, respectively (fig 1K ▶). Occasional regions of blastema showed a positive focus of cytoplasmic staining surrounded by negatively stained blastema. In dysplastic tubules situated within regions of blastema, ezrin staining was localised to the cytoplasm and at the lumenal portion of tubular cells residing within areas of stroma. Metastatic tumours showed an increase in staining at the tumour invasive front (not shown). Similar patterns of γ catenin and ezrin expression were found in the primary and metastatic tumours from group A (table 4 ▶). Stromal cells were negative for both γ catenin and ezrin.
DISCUSSION
Tumour cell invasion is a multistep process requiring complex alterations in adhesive interactions. One of the molecules implicated in the progress of tumour invasion is E-cadherin, a transmembrane glycoprotein that mediates calcium dependent cell adhesion between epithelial cells.28,29 Through its extracellular calcium binding domains, E-cadherin forms a molecular zipper with other E-cadherin proteins that are located in the adherens junctions between adjacent cells. The intracellular domain of E-cadherin interacts with other cytosolic proteins such as catenins, ezrin, and actin filaments.15 Specifically, β catenin and γ catenin are membrane undercoat proteins that bind directly to E-cadherin and link the cytoplasmic terminal tail of E-cadherin to the actin cytoskeleton mediating the function of E-cadherin.30 Met activation by HGF has also been shown to modulate the phosphorylation of catenins, leading to changes in the cellular distribution of adhesion molecules.31 These findings demonstrate the importance of the association between HGF/met, the cadherin/catenin complex, and ezrin proteins in the acquisition of a tumour invasive phenotype.
In our present study, E-cadherin showed a loss of membrane-type expression, with a moderate to low degree of staining in the cytoplasm of blastemal and epithelial cells in primary and metastatic tumours. Recently published studies on E-cadherin in Wilms’s tumour support our observations.12 The loss of expression of E-cadherin in cell membranes, often with relocation to the cytoplasm, has been observed in many malignancies, and is an indicator of abnormal cadherin function.17,27 Cellular redistribution of E-cadherin from the membrane to the cytoplasm and suppression of its function in vitro have been associated with the ability of HGF to induce tyrosine phosphorylation of β catenin and γ catenin.31
“E-cadherin showed a loss of membrane-type expression, with a moderate to low degree of staining in the cytoplasm of blastemal and epithelial cells in primary and metastatic tumours”
The 120 kDa E-cadherin polypeptide was expressed in differentiated heterotypic Wilms’s tumours that did not undergo metastasis, but was absent in poorly differentiated homotypic Wilms’s tumours that progressed to a more metastatic phenotype. Similar findings correlating the loss of E-cadherin expression and either a high metastatic probability or advanced stages of tumour were identified in renal cell carcinomas and in a few cases of Wilms’s tumour by immunohistochemistry only and not by western blot analysis.12,32 Our study showed an overall decrease in the expression of E-cadherin, which was inversely proportional to the degree of tumour differentiation. Reduced expression of E-cadherin has been shown to induce dedifferentiation and invasiveness in tumour cells.31 The mechanisms underlying the reduced or absent expression of E-cadherin in tumours are not fully understood; however, CpG methylation in the E-cadherin promoter region has been proposed as a common mechanism of its inactivation in human tumours.33 Mutations in E-cadherin have also been found in a variety of carcinomas such as gastric, breast, endometrium, and ovary.12
In Wilms’s tumour, we found high amounts of β catenin protein in the cytoplasm and membrane of tumour cells of primary and metastatic tumours, with some metastatic cases demonstrating nuclear expression. Moderate to high amounts of the 92 kDa β catenin polypeptide were found in primary (homotypic and heterotypic) tumours and metastatic tumours relative to normal kidney. Normally, β catenin is localised to the cell membrane and is absent or present in very low amounts within the cytoplasm. Mutations within APC, GSK3B, or axin genes can cause β catenin to accumulate within the cytoplasm, predisposing towards its transfer to the nucleus.34 Nuclear staining for β catenin not only correlates with the possible transcriptional regulation of genes involved in proliferation and invasion, but also with mutations in the β catenin gene as seen in hepatocellular carcinomas, Wilms’s tumours, and prostate carcinomas.11,17 Our present studies on E-cadherin and β catenin expression patterns confirm recently reported findings in Wilms’s tumour.12,35
We have recently reported high levels of HGF and met expression in Wilms’s tumour.18 We therefore postulate that an extracellular signal such as HGF may alter the phosphorylation status of the catenins and release them from the cadherin–catenin complex. This in turn would contribute to tumour metastases not only by disrupting the E-cadherin linkage to the actin cytoskeleton, but also by releasing β catenin into the cytosol, where it could function as a signal transducer to promote cell growth and migration.
Take home messages.
Metastatic Wilms’s tumours showed lower expression of E-cadherin and γ catenin, and reduced nuclear staining for β catenin compared with primary tumours
Low E-cadherin expression was associated with poorly differentiated tumours
Abnormal expression of adhesion molecules may reflect the increased expression and potential activity of hepatocyte growth factor/met in these tumours
These proteins may play an important role in the progression of Wilms’s tumour towards the acquisition of an invasive phenotype and may contribute to the development of metastases
In general, the expression of γ catenin was high in the cytoplasm of blastemal and epithelial cells in primary tumours with metastasis and lower in primary tumours not undergoing metastasis. Metastatic tumours showed lower expression of γ catenin expression, with some tumours demonstrating nuclear expression. Higher expression of γ catenin was noted at the tumour invasive front, similar to β catenin, although the degree of expression of γ catenin differed from that of β catenin. It has been suggested that γ catenin may play a different role in adhesion and metastasis and may be regulated by a different mechanism to that controlling β catenin.36
Our studies showed very high expression of ezrin in the cytoplasm of dysplastic tubules and pseudoglomeruli in primary non-metastatic tumours compared with primary tumours undergoing metastasis, and moderate expression in metastatic tumours. In general, ezrin showed a similar degree and pattern of expression to that seen for γ catenin. Occasional foci of blastema showed strong cytoplasmic staining for ezrin, suggesting possible pseudoglomeruli differentiation. In cells with polarised structures, ezrin may function as a membrane–cytoskeletal linker that regulates cell surface morphology and thus is expressed along the cell membrane.16 However, in cells responding to extracellular stimuli, such as HGF, ezrin may facilitate migration and morphogenetic changes because of its involvement in cytoskeletal reorganisation, thereby changing its localisation to the cytoplasm.25
In summary, metastatic Wilms’s tumours showed lower expression of E-cadherin and γ catenin, with nuclear staining for β catenin, when compared with primary tumours. Low expression of E-cadherin was also associated with a poorly differentiated tumour phenotype. We have obtained preliminary evidence of increased expression of β catenin, γ catenin, and ezrin near the tumour invasive front in metastatic Wilms’s tumours. Several studies in various carcinomas have shown a relation between E-cadherin/catenin expression and clinicopathological variables, implicating these proteins as potential prognostic indicators of tumour progression.37–39 Further studies would be needed to establish a similar association in Wilms’s tumour. However, we postulate that the changes seen in the expression and subcellular distribution of E-cadherin, β catenin, γ catenin, and ezrin in Wilms’s tumour may reflect the increased expression and potential activity of HGF/met in these tumours. Thus, these proteins may play an important role in the progression of Wilms’s tumour towards the acquisition of an invasive phenotype and may contribute to the development of metastases.
Acknowledgments
The authors thank D Tang for technical assistance with immunohistochemistry, L Morikawa for tissue sectioning, and D Aguilar for assistance with image processing. The statistical analysis was performed by D Stephens (Research Institute, Hospital for Sick Children). This work was supported by funds provided by the Canadian Cancer Society to the National Cancer Institute of Canada (NCIC) Fund 3443 to HY and by the National Institute of Health (NIH) Fund R01 CA89279 to BRGW. The Hospital for Sick Children Foundation Student Scholarships at the University of Toronto provided funds for JA.
REFERENCES
- 1.Scharnhorst V, van der Eb AJ, Jochemsen AG. WT1 proteins: functions in growth and differentiation. Gene 2001;273:141–61. [DOI] [PubMed] [Google Scholar]
- 2.Jones KP, Grundy PE, Coppes MJ. Recent advances in the genetics of childhood renal cancers: a report of the 3rd international conference on the molecular and clinical genetics of childhood renal tumors, together with the Mitchell Ross symposium on anaplastic and other high risk embryonal tumors of childhood, 8–10th April 1999, Wistar Institute, Philadelphia, PA. Med Pediatr Oncol 2000;35:126–30. [DOI] [PubMed] [Google Scholar]
- 3.Malkin D, Sexsmith E, Yeger H, et al. Mutations of the p53 tumour suppressor gene occur infrequently in Wilms’ tumour. Cancer Res 1994;54:2077–9. [PubMed] [Google Scholar]
- 4.Maheswaran S, Park S, Bernard A, et al. Physical and functional interaction between WT1 and p53 proteins. Proc Natl Acad Sci U S A 1993;90:5100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahoti C, Thorner P, Malkin D, et al. Immunohistochemical detection of p53 in Wilms’ tumors correlates with unfavorable outcome. Am J Pathol 1996;148:1577–89. [PMC free article] [PubMed] [Google Scholar]
- 6.Govender D, Harilal P, Hadley GP, et al. p53 protein expression in nephroblastomas: a predictor of poor prognosis. Br J Cancer 1998;77:314–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardeesy N, Falkoff D, Petruzzi MJ, et al. Anaplastic Wilms’ tumour, a subtype displaying poor prognosis, harbours p53 gene mutations. Nat Genet 1994;7:91–7. [DOI] [PubMed] [Google Scholar]
- 8.Coppes MJ, Campbell CE, Williams BR. The role of WT1 in Wilms tumorigenesis. FASEB J 1993;7:886–95. [DOI] [PubMed] [Google Scholar]
- 9.Ellisen LW. Regulation of gene expression by WT1 in development and tumorigenesis. Int J Hematol 2002;76:110–16. [DOI] [PubMed] [Google Scholar]
- 10.Hosono S, Gross I, English MA, et al. E-cadherin is a WT1 target gene. J Biol Chem 2000;275:10943–53. [DOI] [PubMed] [Google Scholar]
- 11.Maiti S, Alam R, Amos CI, et al. Frequent association of beta-catenin and WT1 mutations in Wilms tumors. Cancer Res 2000;60:6288–92. [PubMed] [Google Scholar]
- 12.Schulz S, Becker KF, Braungart E, et al. Molecular analysis of E-cadherin and cadherin-11 in Wilms’ tumours. J Pathol 2000;191:162–9. [DOI] [PubMed] [Google Scholar]
- 13.Koesters R, Ridder R, Kopp-Schneider A, et al. Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms’ tumors. Cancer Res 1999;59:3880–2. [PubMed] [Google Scholar]
- 14.Kusafuka T, Miao J, Kuroda S, et al. Codon 45 of the beta-catenin gene, a specific mutational target site of Wilms’ tumour. Int J Mol Med 2002;10:395–9. [PubMed] [Google Scholar]
- 15.Louvet-Vallee S. ERM proteins: from cellular architecture to cell signalling. Biol Cell 2000;92:305–16. [DOI] [PubMed] [Google Scholar]
- 16.Hiscox S, Jiang WG. Ezrin regulates cell–cell and cell–matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci 1999;112(Pt 18):3081–90. [DOI] [PubMed] [Google Scholar]
- 17.Campbell RJ, Pignatelli M. Molecular histology in the study of solid tumours. Mol Pathol 2002;55:80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alami J, Williams BR, Yeger H. Expression and localization of HGF and met in Wilms’ tumours. J Pathol 2002;196:76–84. [DOI] [PubMed] [Google Scholar]
- 19.Ferracini R, Di Renzo MF, Scotlandi K, et al. The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene 1995;10:739–49. [PubMed] [Google Scholar]
- 20.Rong S, Segal S, Anver M, et al. Invasiveness and metastasis of NIH 3T3 cells induced by Met–hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci U S A 1994;91:4731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weidner KM, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol 1993;121:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen EM, Knesel J, Goldberg ID, et al. Scatter factor modulates the metastatic phenotype of the EMT6 mouse mammary tumour. Int J Cancer 1994;57:706–14. [DOI] [PubMed] [Google Scholar]
- 23.Nakopoulou L, Gakiopoulou H, Keramopoulos A, et al. c-Met tyrosine kinase receptor expression is associated with abnormal beta-catenin expression and favourable prognostic factors in invasive breast carcinoma. Histopathology 2000;36:313–25. [DOI] [PubMed] [Google Scholar]
- 24.Jiang WG, Hiscox S, Singhrao SK, et al. Induction of tyrosine phosphorylation and translocation of ezrin by hepatocyte growth factor/scatter factor. Biochem Biophys Res Commun 1995;217:1062–9. [DOI] [PubMed] [Google Scholar]
- 25.Hiscox S, Jiang WG. Association of the HGF/SF receptor, c-met, with the cell-surface adhesion molecule, E-cadherin, and catenins in human tumour cells. Biochem Biophys Res Commun 1999;261:406–11. [DOI] [PubMed] [Google Scholar]
- 26.Chevalier G, Yeger H, Martinerie C, et al. novH: differential expression in developing kidney and Wilm’s tumors. Am J Pathol 1998;152:1563–75. [PMC free article] [PubMed] [Google Scholar]
- 27.El-Bahrawy MA, Poulsom R, Jeffery R, et al. The expression of E-cadherin and catenins in sporadic colorectal carcinoma. Hum Pathol 2001;32:1216–24. [DOI] [PubMed] [Google Scholar]
- 28.Perantoni AO. Cell adhesion molecules in the kidney: from embryo to adult. Exp Nephrol 1999;7:80–102. [DOI] [PubMed] [Google Scholar]
- 29.Qian ZR, Li CC, Yamasaki H, et al. Role of E-cadherin, alpha-, beta-, and gamma-catenins, and p120 (cell adhesion molecules) in prolactinoma behavior. Mod Pathol 2002;15:1357–65. [DOI] [PubMed] [Google Scholar]
- 30.Noe V, Chastre E, Bruyneel E, et al. Extracellular regulation of cancer invasion: the E-cadherin–catenin and other pathways. Biochem Soc Symp 1999;65:43–62. [PubMed] [Google Scholar]
- 31.El-Bahrawy MA, Pignatelli M. E-cadherin and catenins: molecules with versatile roles in normal and neoplastic epithelial cell biology. Microsc Res Tech 1998;43:224–32. [DOI] [PubMed] [Google Scholar]
- 32.Shimazui T, Oosterwijk-Wakka J, Akaza H, et al. Alterations in expression of cadherin-6 and E-cadherin during kidney development and in renal cell carcinoma. Eur Urol 2000;38:331–8. [DOI] [PubMed] [Google Scholar]
- 33.Beavon IR. The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer 2000;36:1607–20. [DOI] [PubMed] [Google Scholar]
- 34.Takayama T, Shiozaki H, Shibamoto S, et al. Beta-catenin expression in human cancers. Am J Pathol 1996;148:39–46. [PMC free article] [PubMed] [Google Scholar]
- 35.Koesters R, Niggli F, Von Knebel Doeberitz M, et al. Nuclear accumulation of beta-catenin protein in Wilms’ tumours dagger. J Pathol 2003;199:68–76. [DOI] [PubMed] [Google Scholar]
- 36.Syrigos KN, Harrington K, Waxman J, et al. Altered gamma-catenin expression correlates with poor survival in patients with bladder cancer. J Urol 1998;160:1889–93. [PubMed] [Google Scholar]
- 37.Imura J, Ichikawa K, Takeda J, et al. Beta-catenin expression as a prognostic indicator in cervical adenocarcinoma. Int J Mol Med 2001;8:353–8. [PubMed] [Google Scholar]
- 38.Tanaka M, Kitajima Y, Edakuni G, et al. Abnormal expression of E-cadherin and beta-catenin may be a molecular marker of submucosal invasion and lymph node metastasis in early gastric cancer. Br J Surg. 2002;89:236–44. [DOI] [PubMed] [Google Scholar]
- 39.Parker C, Rampaul RS, Pinder SE, et al. E-cadherin as a prognostic indicator in primary breast cancer. Br J Cancer 2001;85:1958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]