Abstract
Aims: Several studies have reported that dysregulation of β catenin or k-ras mutation promotes cyclin D1 expression. This study investigated the relation between cyclin D1 expression and clinicopathological parameters in carcinoma of the ampulla of Vater (CAV), and also assessed the relation between increased cyclin D1 expression and β catenin/k-ras status in this series.
Methods: Thirty CAVs were evaluated for cyclin D1 expression by immunohistochemistry in relation to patient clinicopathological features. Aberrant β catenin expression and k-ras mutation were also investigated by immunostaining and direct sequencing, and related to cyclin D1 expression.
Results: Increased cyclin D1 expression was seen in 17 of 30 CAVs and was significantly correlated with tumour cell proliferation and disease free survival time (p = 0.018, p = 0.018, respectively). Nuclear accumulation of β catenin was found in nine of 30 cases, including four cases with missense mutations in exon 3 of CTNNB-1, and was significantly correlated with increased cyclin D1 expression (p = 0.003). k-ras gene mutation was detected in 12 of 30 cases, and was also significantly correlated with increased cyclin D1 expression (p = 0.026). Overall, 14 of 17 CAVs with increased cyclin D1 expression showed nuclear accumulation of β catenin and/or k-ras mutation.
Conclusions: Increased cyclin D1 expression appears to be associated with tumour proliferation and poorer clinical outcome in CAV. It is also associated with both aberrant β catenin expression and k-ras mutation. These results are consistent with the in vitro data that cyclin D1 can be transactivated by activated β catenin–T cell factor/LEF and k-ras pathways.
Keywords: carcinoma of ampulla of Vater, cyclin D1, β catenin, k-ras, prognosis
Cyclin D1, the product of the bcl-1 (PRAD, CCND-1) gene located on chromosome 11q13, plays an important role in regulating cell cycle progression.1,2 This protein phosphorylates retinoblastoma protein (pRB), which is thought to be a major repressor of G1 phase progression, thereby regulating progression of the cell cycle from G1 into S phase.3,4 Therefore, it is plausible that overexpression of cyclin D1 promotes cellular proliferation by suppressing pRB activity, suggesting that its upregulation during oncogenesis accelerates cell cycle progression in human carcinomas. Recently acquired data on the cell cycle provide reasonable support for this hypothesis, and previous studies on cyclin D1 have found it to be overexpressed in various human tumours, and have suggested a link between its overexpression and oncogenesis.5–9
Two more recently described regulation mechanisms for this protein involve β catenin and ras related pathways. The β catenin protein, originally identified as a submembrane component of cadherin mediated cell–cell adhesion systems,10 functions as a downstream transcriptional activator of the Wnt signalling pathway.11 When not associated with cell–cell junctions, β catenin is incorporated into a large complex, which also includes the adenomatous coli polyposis (APC) protein, the glycogen synthase kinase 3β (GSK-3β) serine/threonine protein kinase, and the axin/conduction protein, and results in phosphorylation of serine/threonine residues encoded on exon 3 of the CTNNB-1 gene.12–14 This phosphorylation results in subsequent proteolytic degradation of β catenin by the ubiquitin–proteasome system.15 Many APC mutations lead to loss of functional APC protein, resulting in the nuclear accumulation of β catenin.15 Loss of functional APC protein results in the accumulation of β catenin.16 In addition, β catenin can be stabilised by missense mutations affecting Ser33, Ser37, Thr41, and Ser45 in many series of human cancers.16–18 Stabilised β catenin translocated into the nucleus forms a complex with members of the T cell factor (TCF)/lymphoid enhancer factor (LEF) family, leading to increased transactivation of several target genes, including those encoding c-myc and cyclin D1, which were recently identified as key transcriptional targets of this pathway.11
“Only limited studies on cyclin D1 expression have been carried out with surgically removed cancers of the ampulla of Vater”
Mutations of k-ras, which are almost always confined to codon 12 or 13, and which have been described in a variety of human carcinomas, result in constitutive activation of the ras signalling pathway. Recent studies in both rat and human cell lines have shown that k-ras mutations can increase cyclin D1 expression through the mitogen activated protein kinase (MAPK) pathway.19,20 Therefore, both Wnt and MAPK pathways independently regulate the expression of cyclin D1 at the transcriptional level.16
Several recent reports have described dysregulation of cell cycle regulators at the G1 to S phase checkpoint in carcinomas of the ampulla of Vater (CAVs),21–28 and β catenin and ras related pathways have been shown to be abrogated relatively frequently. Nuclear β catenin accumulation can be seen in about 25% of CAVs,21 and k-ras mutation in about 40%.22,28
However, only limited studies on cyclin D1 expression have been carried out with surgically removed CAVs.23 Thus, we examined how dysregulated β catenin expression and/or k-ras mutation are associated with increased cyclin D1 expression in CAVs in vivo. We also investigated whether increased expression of cyclin D1 protein is associated with cell cycle progression and adverse clinical outcomes in CAVs.
METHODS
Patients and clinicopathological information
Tissue samples from CAVs were obtained from 30 patients who underwent curative pancreato-duodenectomy between 1992 and 2001 from the archival tissue files of the department of surgery, Ichihara Hospital, Teikyo University (Ichihara, Japan). Our study was approved by the human subjects committee of the Teikyo University and all the patients’ information was dealt with anonymously. The patients comprised nine women and 21 men, and their median age was 63.9 years (range, 49–77). None of the patients had previously received chemoradiotherapeutic agents. The disease free survival time was observed from September 1992 to August 2002. Histological sections, cut from 10% buffered, formalin fixed, paraffin wax embedded blocks, were routinely stained with haematoxylin and eosin. The tumours were classified histologically by the system outlined in the World Health Organisation monograph.29 We also classified the tumours using TNM staging system.29
Immunohistochemistry
Paraffin wax embedded sections (4 μm) were adhered to silanised slides, dewaxed, and hydrated by passage through xylene (three times for five minutes each), a graded series of ethanol (once each at 100%, 80%, 70%, and 50%) for five minutes, and distilled water for 10 minutes. For each stain, antigen retrieval was carried out using pressure cooking in citrate buffer (pH 7.0) for 10 minutes. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in methanol (30 minutes) between the first two steps of washing in methanol. After each following step, sections were washed with 0.01M phosphate buffered saline (pH 7.4), three times for 10 minutes. The tissue sections were then covered with protein block serum (Dako, Carpenteria, California, USA) for 20 minutes and were incubated overnight at 4°C with the anti-cyclin D1 monoclonal antibody (Zymed, San Francisco, California, USA; clone AM29; 1/2 dilution) or anti-β catenin monoclonal antibody (Transduction Laboratories, Lexington, Kentucky, USA; clone 14; 1/200 dilution). For each case, a corresponding section was incubated in phosphate buffered saline without the primary antibody as a control for non-specific staining. Biotinylated rabbit antimouse secondary antibody was added for 30 minutes, followed by the avidin–biotin–peroxidase complex for an additional 40 minutes. After washing, the sections were stained with diaminobenzidine liquid system (Dako). The sections were then counterstained with Mayer’s haematoxylin and mounted. Staining for Ki-67 (MIB-1 monoclonal antibody; 1/200 dilution; Immunotech, Marseilles, France) was carried out as described previously.30
Interpretation of immunohistochemical staining
We interpreted the cyclin D1 immunostaining as described by Arber et al.20 Only the presence of nuclear staining was regarded as positive immunoreactivity, and occasional cytoplasmic staining was not regarded as positive. Because of the heterogeneity of the tissue, the positivity and intensity were estimated by counting more than 500 tumour cells in fields with the highest intensity of staining (in some cases at the invasive edge of the carcinoma). Nuclear staining was only considered positive if the chromogen was detected in at least 5% of the nuclei. Staining intensity was graded as follows: no staining (grade 0); weak staining, comparable to adjacent non-neoplastic epithelium (grade 1); moderately positive (grade 2); and strongly positive (grade 3). Grades 0 and 1 were regarded as negative, and grades 2 and 3 were regarded as positive. We defined increased cyclin D1 expression as cases where both positivity and intensity were graded as positive. Immunohistochemical β catenin staining was interpreted as described previously.31 Staining was graded as positive if moderate to strong staining was seen in the tumour cell nuclei and/or cytoplasm. The slides were interpreted by one of the investigators (KY), who was unaware of the results of the other analyses. All slides were independently scored two or three times in the same batch, and all batches were coded and scored blind at least twice. All slides were interpreted similarly (r = 1.0 and 0.935 for the assessment of cyclin D1 positivity and intensity, respectively; and r = 0.926 for the assessment of β catenin positivity). Finally, a Ki-67 positivity index (MIB-1 PI) was calculated for each case by counting more than 500 carcinoma cells and recording the percentage of nuclei showing positive staining in fields that showed the highest intensity of cyclin D1 staining in the serial sections.
CTNNB-1 mutation analysis
All 30 CAVs were evaluated for mutations in exon 3 of CTNNB-1 by the polymerase chain reaction (PCR), followed by direct sequencing. Tumour cell clusters without contaminating stromal cells were carefully microdissected under microscopic inspection. Tissues were digested with proteinase K, and genomic DNA was purified with phenol/chloroform and precipitated with ethanol. The primer sequences used were: Fwd, 5′-ATG GAA CCA GAC AGA AAA G-3′ and Rev, 5′-TAC AGG ACT TGG GAG GTA TC-3′, generating a 152 bp fragment of exon 3, including the sequence for GSK-3β phosphorylation. DNA was extracted from the remaining tissue using sodium dodecyl sulfate lysis and proteinase K digestion.28 As a template, 100 ng of DNA was amplified by PCR in a 40 μl volume containing 50mM KCl, 10mM Tris (pH 8.3), 1.5mM MgCl2, 200μM dNTPs, 10 pmol of each primer, and 0.2 U of Taq-Gold DNA polymerase (Applied Biosystems, Tokyo, Japan). The PCR conditions were as follows: initial denaturation at 94°C for 10 minutes; 40 cycles of 94°, 55°C, and 72°C for 30 seconds each; and a final extension step at 72°C for seven minutes. Samples were sequenced directly using an ABI Prism 310 DNA sequencer (Applied Biosystems), according to the manufacture’s instructions.
K-ras mutation analysis
k-ras mutation is thought to occur mainly during the early stages of tumorigenesis in the gastrointestinal tract, and is mostly found in codon 12 or 13 in CAVs.28,32 A search for mutations of codon 12 or 13 was carried out using direct sequencing after PCR. The DNA extraction, PCR, and direct sequencing were performed as described above, except the primer sequences were as follows: Fwd, 5′-GAC TGA ATA TAA ACT TGT GG-3′ and Rev: 5′-CTA TTG TTG GAT CAT ATT CG-3′.
Statistical analysis
The data were analysed with StatView 5.0 for Windows computer software package (SAS Institute Inc, Tokyo, Japan). Intraobserver reproducibility was evaluated by Spearman’s test. Statistical analyses were performed with the two tailed Fisher’s exact test. Correlations between increased cyclin D1 expression and MIB-1 PI were analysed by the Mann-Whitney U test. The correlation between increased cyclin D1 expression and clinical outcome was calculated by the Kaplan–Meier method and analysed by log rank tests. Differences at p < 0.05 were considered to be significant.
RESULTS
Clinicopathological findings
Four of the cases were of an intramural protruding form, whereas 26 cases were of an exposed protruding type or an ulcerating form. The tumour sizes ranged from 10 to 56 mm (mean, 24.2). The cases were classified as three papillary adenocarcinomas and 27 adenocarcinomas; 12 well differentiated, 13 moderately differentiated, and two poorly differentiated adenocarcinomas (table 1 ▶). Sixteen of 30 cases showed lymph node metastasis. We also classified the tumours using the TNM staging as follows: two cases were classified as stage I, 14 as stage II, 14 as stage III, and none as stage IV (table 1 ▶). All cases were resected with grossly/microscopically negative margins (R0).
Table 1.
Relation of increased cyclin D1 expression to clinicopathological features of 30 carcinomas of the ampulla of Vater
| Cyclin D1 expression | |||
| Increased | Normal or negative | p Value | |
| Number | 17 | 13 | |
| Age (years) | 66.2 (8.2) | 62.9 (7.2) | 0.2575* |
| Sex (female/male) | 6/11 | 3/10 | 0.6908† |
| Histological grade (pap+well/mod+poor) | 6/11 | 9/4 | 0.0824† |
| Size | 25.5 (13.3) | 22.5 (5.4) | 0.9831* |
| Lymph node metastasis | 11 (64.7%) | 5 (38.5%) | 0.2685† |
| Clinical stage (stage I+II/III+IV) | 9/8 | 7/6 | 1† |
| MIB-1 LI | 50.1 (12.8) | 33.8 (20.6) | 0.018* |
Values are mean (SD).
Histological grade: pap, papillary adenocarcinoma; well, well differentiated; mod, moderately differentiated; poor, poorly differentiated.
*Mann-Whitney U test; †two tailed Fischer’s exact test.
LI, labelling index.
Cyclin D1 immunohistochemistry and relation to clinicopathological features
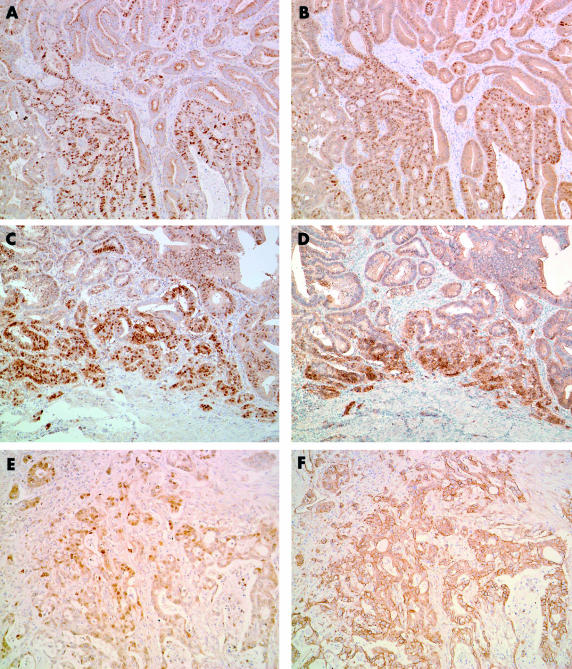
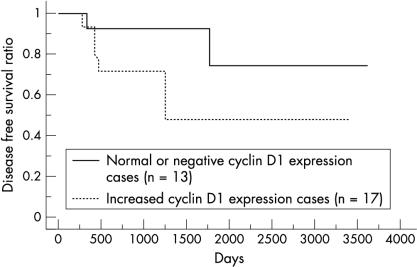
As described in a previous study,23 the non-neoplastic duodenal epithelium showed scarce and at most weak nuclear staining for cyclin D1. In CAVs, immunostaining for cyclin D1 was seen mainly within tumour cell nuclei, although positive staining was sometimes seen in the cytoplasm. The nuclear staining patterns varied from diffuse but heterogeneous to focal. The distribution of positive nuclei was particularly prominent at the front of invasion. Overall, 17 cases were deemed to have increased cyclin D1 expression (fig 1 ▶). The relation between increased cyclin D1 expression and clinicopathological features is shown in table 1 ▶. There was a marginal correlation between increased cyclin D1 expression and poorer histological grade, but this was not significant. We found a significant correlation between increased cyclin D1 expression and tumour cell proliferation—that is, the MIB-1 labelling index (LI) was higher in cases with increased cyclin D1 expression (p = 0.018) (table 1 ▶). The overall median disease free survival of patients was 888 days (range, 37–3620). The median disease free survival was 1497 days in the negative or normal cyclin D1 expression group and 548 days in the overexpression group. Five of 17 patients with increased cyclin D1 expression had distant metastases—for example, liver metastasis or peritoneal dissemination—whereas only two of 13 patients with negative or normal cyclin D1 expression showed distant metastasis at the latest period of follow up. Kaplan–Meier regression analysis showed that patients with increased cyclin D1 expression exhibited a significantly shorter disease free survival time compared with those with negative or normal cyclin D1 expression (p = 0.018; fig 2 ▶). We also performed Cox’s multivariate analysis to test the prognostic value of cyclin D1 overexpression with the other factors investigated in our study; however, we could find no differences between these factors and the patients’ disease free survival times.
Figure 1.
Cyclin D1 and β catenin expression as assessed by immunohistochemistry in carcinoma of ampulla of Vater. Moderately differentiated adenocarcinoma showing heterogeneous (A) nuclear cyclin D1 expression and (B) β catenin expression. This case shows a similar distribution pattern of cyclin D1 and β catenin staining (original magnification, ×100). This patient had a mutated CTNNB-1 gene and a wild-type k-ras gene. Well differentiated adenocarcinoma showing a similar distribution pattern for (C) cyclin D1 and (D) β catenin staining at the invasive edge of the tumour (original magnification, ×100). This patient had a mutated CTNNB-1 gene and a mutated k-ras gene. Moderately differentiated adenocarcinoma cells showing (E) diffuse nuclear cyclin D1 expression and (F) membranous β catenin expression (original magnification, ×100). This patient had a wild-type CTNNB-1 gene and a mutated k-ras gene.
Figure 2.
Kaplan–Meier regression analysis showing that patients with increased cyclin D1 expression have significantly shorter disease free survival time compared with those with normal or negative cyclin D1 expression.
β Catenin immunostaining and mutation analysis
Strong membranous β catenin staining was found in normal duodenal epithelial cells, whereas faint staining was present in their cytoplasm; nuclear staining was absent. In contrast, greatly reduced membranous expression, with nuclear or both nuclear and cytoplasmic accumulation of β catenin, was found in nine of 30 CAV tissues (fig 1 ▶). Similar to cyclin D1 immunostaining, the nuclear staining patterns were diffuse but heterogeneous to focal. Positive nuclei were particularly prominent at the front of invasion. Consistent with an earlier report, we found a significant correlation between nuclear β catenin accumulation and increased cyclin D1 expression (p = 0.0033; table 2 ▶). Four of nine CAVs with nuclear β catenin accumulation showed point mutation of the CTNNB-1 gene. The mutation types were TCT to TTT in codon 37 (Ser to Phe, one case), TCT to TGT in codon 37 (Ser to Cys, one case), ACC to GCC in codon 41 (Thr to Ala, one case), and TCT to CCT in codon 45 (Ser to Pro, one case) (table 3 ▶). These cases with mutations exhibited intense nuclear β catenin staining and increased cyclin D1 expression. Interestingly, all CTNNB-1 mutated cases and an additional two cases showed close similarity between the two staining patterns of the cyclin D1 and β catenin (fig 1 ▶).
Table 2.
Relation of increased cyclin D1 expression to β catenin and k-ras features of 30 carcinomas of ampulla of Vater
| Cyclin D1 expression | |||
| Increased | Normal or negative | p Value | |
| Number | 17 | 13 | |
| Intracellular β catenin accumulation | 0.0033* | ||
| Yes | 9 | 0 | |
| No | 8 | 13 | |
| K-ras mutation | 0.0256* | ||
| Yes | 10 | 2 | |
| No | 7 | 11 | |
| Intracellular β catenin accumulation and/or k-ras mutation | 0.0006* | ||
| Yes | 14 | 2 | |
| No | 3 | 11 | |
*Two tailed Fischer’s exact test.
Table 3.
Summary of various mutations seen in 30 carcinomas of the ampulla of Vater
| Patient | Increased cyclin D1 expression | Intracellular β catenin expression | CTNNB-1 mutation | k-ras mutation (codon 12) |
| 1 | − | − | WT | GGT to GAT (Gly to Asp) |
| 2 | − | − | WT | GGT to GTT (Gly to Val) |
| 3 | − | − | WT | WT |
| 4 | − | − | WT | WT |
| 5 | − | − | WT | WT |
| 6 | − | − | WT | WT |
| 7 | − | − | WT | WT |
| 8 | − | − | WT | WT |
| 9 | − | − | WT | WT |
| 10 | − | − | WT | WT |
| 11 | − | − | WT | WT |
| 12 | − | − | WT | WT |
| 13 | − | − | WT | WT |
| 14 | + | − | WT | GGT to GAT (Gly to Asp) |
| 15 | + | − | WT | GGT to GAT (Gly to Asp) |
| 16 | + | − | WT | GGT to GAT (Gly to Asp) |
| 17 | + | − | WT | GGT to GAT (Gly to Asp) |
| 18 | + | − | WT | GGT to GTT (Gly to Val) |
| 19 | + | − | WT | WT |
| 20 | + | − | WT | WT |
| 21 | + | − | WT | WT |
| 22 | + | + | Codon 37 TCT to TTT (Ser to Phe) | GGT to GTT (Gly to Val) |
| 23 | + | + | Codon 37 TCT to TGT (Ser to Cys) | WT |
| 24 | + | + | Codon 41 ACC to GCC (Thr to Ala) | WT |
| 25 | + | + | Codon 45 TCT to CCT (Ser to Pro) | GGT to GAT (Gly to Asp) |
| 26 | + | + | WT | GGT to GAT (Gly to Asp) |
| 27 | + | + | WT | GGT to GAT (Gly to Asp) |
| 28 | + | + | WT | GGT to GTT (Gly to Val) |
| 29 | + | + | WT | WT |
| 30 | + | + | WT | WT |
WT, wild-type gene.
K-ras mutation analysis
Twelve of the 30 cases of CAV showed k-ras mutation. The mutation types were GGT to GTT in codon 12 (Gly to Val, four cases) and GGT to GAT in codon 12 (Gly to Asp, eight cases) (table 3 ▶). In two of these 12 cases, CTNNB-1 mutation was also present. There was a significant correlation between increased cyclin D1 expression and k-ras mutation (p = 0.0256) (table 2 ▶). Overall, of the 17 carcinomas showing increased cyclin D1 expression, 14 showed nuclear accumulation of β catenin and/or k-ras mutation (p = 0.0006) (table 2 ▶). There was no significant correlation between increased cyclin D1 expression and the type of k-ras mutation. In addition, we found no correlation between nuclear β catenin accumulation and k-ras mutation.
DISCUSSION
To our knowledge, no previous reports have examined whether or not increased cyclin D1 expression in CAVs can be explained by nuclear β catenin signalling and/or k-ras mutation. We have demonstrated—using immunohistochemistry—increased expression of the cyclin D1 protein in 56.7% of CAVs in vivo. Although there were no significant correlations between increased cyclin D1 expression and various parameters such as age, sex, histological grade, tumour size, lymph node metastasis, and clinical stage, those tumours with increased cyclin D1 expression showed significantly higher MIB-1 LI values (table 1 ▶). We also found that the disease free survival time was significantly shorter in patients with increased cyclin D1 expression than in those with negative or normal expression (fig 2 ▶). In our study, increased cyclin D1 expression seemed to be associated with tumour cell proliferation and a worse prognosis in CAVs.
Many of the previous studies on cyclin D1 gene amplification, degradation, and genotype have suggested that it is associated with oncogenesis.33 Knowledge of the cell cycle provides a reasonable explanation to support these findings. The Rb tumour suppressor gene encodes pRB, which is thought to be the major repressor of G1 phase progression.34 pRB is active in early G1 in its hypophosphorylated form; in mid/late G1, the protein becomes inactivated by phosphorylation. Cyclins D1, in association with their catalytic partners the cyclin dependent kinases, are responsible for this phosphorylation of pRB and regulate progression of the cell cycle from G1 into S phase.35 Increased cyclin D1 expression maintains pRB phosphorylation, releasing additional E2Fs that continue to induce the transcription of cyclin D1. Therefore, it is plausible that overexpression of cyclin D1 increases the phosphorylation of pRB and promotes cellular proliferation by suppressing pRB activity.
“The disease free survival time was significantly shorter in patients with increased cyclin D1 expression than in those with negative or normal expression”
Aberrant nuclear accumulation of β catenin—mediating increased cyclin D1 expression and tumour cell proliferation—has been extensively documented in colorectal cancer, both in human cell lines16,17 and in vivo.36 Oncogenic degradation of β catenin in not limited to colorectal carcinoma. Similar observations have been made in several other types of cancer.6,37 Recently, it has also been shown that cyclin D1 is one of the targets of β catenin in pancreaticobiliary tumours, in which overexpression of cyclin D1 is implicated in tumour development and progression.9,38–40 In our study, we demonstrated a significant relation between increased cyclin D1 expression and nuclear β catenin accumulation in CAVs. Four of the patients had mutations in the GSK-3β phosphorylation site of CTNNB-1, and showed intense nuclear β catenin accumulation and increased cyclin D1 expression. All mutated cases and an additional two cases exhibited close similarity between the immunohistochemical staining patterns for cyclin D1 and β catenin. From these results, it appears that activated β catenin–TCF/LEF signalling, which might be partially activated by CTNNB-1 alteration, exhibits its effects via transactivation of cyclin D1 in CAVs. However, increased cyclin D1 expression was seen in six of the tumours without nuclear β catenin accumulation, thereby suggesting that other stimuli might be required to cause increased cyclin D1 expression in CAVs in vivo.
Take home messages.
Increased cyclin D1 expression appears to be associated with tumour cell proliferation and worse clinical outcome in cancer of the ampulla of Vater (CAV)
Increased cyclin D1 expression is also associated with both aberrant β catenin expression and k-ras mutation
These results support the hypothesis that activated β catenin–T cell factor/lymphoid enhancer factor and k-ras pathways might exhibit their effects via the transactivation of cyclin D1 in CAVs
Activated ras genes are known to alter the control of cell proliferation. In our study, the incidence of K-ras mutation was 40%, which is consistent with previous reports on CAVs.22,28 The k-ras protooncogene encodes p21ras, a small monomeric GDP/GTP binding protein, and regulates several important cellular functions, including proliferation, differentiation, and apoptosis.41,42 Mutations of k-ras lead to dysregulated expression of its product p21ras, so that several of its downstream effectors, such as cyclin D1, are continuously stimulated, resulting in malignant transformation.43,44 We demonstrated a significant correlation between k-ras mutation and increased cyclin D1 expression in CAVs. In addition, we found that four of six tumours with increased cyclin D1 expression but not β catenin accumulation had mutated k-ras genes. Of 17 carcinomas showing increased cyclin D1 expression, 14 showed nuclear accumulation of β catenin and/or k-ras mutation. These findings support the in vitro data that cyclin D1 can be transactivated by an activated β catenin–TCF/LEF and/or ras signalling pathway.
In summary, we have confirmed that increased cyclin D1 expression correlates with tumour cell proliferation and a poorer clinical outcome in CAVs. We have also shown an association between this overexpression and nuclear β catenin accumulation and k-ras mutation. These results support the hypothesis that activated β catenin–TCF/LEF and k-ras pathways might exhibit their effects via the transactivation of cyclin D1 in CAVs.
Abbreviations
APC, adenomatous coli polyposis
CAV, carcinoma of the ampulla of Vater
GSK-3β, glycogen synthase kinase 3β
LEF, lymphoid enhancer factor
LI, labelling index
MAPK, mitogen activated protein kinase
PCR, polymerase chain reaction
pRb, retinoblastoma protein
TCF, T cell factor
REFERENCES
- 1.Sherr CJ. Mammalian G1 cyclins. Cell 1993;73:1059–65. [DOI] [PubMed] [Google Scholar]
- 2.Draetta G, Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell 1988;54:17–26. [DOI] [PubMed] [Google Scholar]
- 3.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev 1998;12:2245–62. [DOI] [PubMed] [Google Scholar]
- 4.Sherr CJ. Cancer cell cycles. Science 1996;274:1672–7. [DOI] [PubMed] [Google Scholar]
- 5.Jiang W, Kahn SM, Tomita N, et al. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res 1992;52:2980–3. [PubMed] [Google Scholar]
- 6.Bartkova J, Lukas J, Muller H, et al. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer 1994;57:353–61. [DOI] [PubMed] [Google Scholar]
- 7.Gramlich TL, Fritsch CR, Maurer D, et al. Differential polymerase chain reaction assay of cyclin D1 gene amplification in esophageal carcinoma. Diagn Mol Pathol 1994;3:255–9. [DOI] [PubMed] [Google Scholar]
- 8.Gillett C, Fantl V, Smith R, et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res 1994;54:1812–17. [PubMed] [Google Scholar]
- 9.Gansauge S, Gansauge F, Ramadani M, et al. Overexpression of cyclin D1 in human pancreatic carcinoma is associated with poor prognosis. Cancer Res 1997;57:1634–7. [PubMed] [Google Scholar]
- 10.Ben-Ze’ev A, Geiger B. Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signalling and cancer. Curr Opin Cell Biol 1998;10:629–39. [DOI] [PubMed] [Google Scholar]
- 11.Morin PJ. Beta-catenin signalling and cancer. Bioessays 1999;21:1021–30. [DOI] [PubMed] [Google Scholar]
- 12.Behrens J, Jerchow BA, Wurtele M, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 1998;280:596–9. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda S, Kishida S, Yamamoto H, et al. Axin, a negative regulator of the Wnt signalling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J 1998;17:1371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart MJ, de los Santos R, Albert IN, et al. Downregulation of beta-catenin by human axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol 1998;8:573–81. [DOI] [PubMed] [Google Scholar]
- 15.Aberle H, Bauer A, Stappert J, et al. Beta-catenin is a target for the ubiquitin–proteasome pathway. EMBO J 1997;16:3797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999;398:422–6. [DOI] [PubMed] [Google Scholar]
- 17.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A 1999;96:5522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin SY, Xia W, Wang JC, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A 2000;97:4262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albanese C, Johnson J, Watanabe G, et al. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem 1995;270:23589–97. [DOI] [PubMed] [Google Scholar]
- 20.Arber N, Hibshoosh H, Moss SF, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology 1996;110:669–74. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami M, Kimura Y, Furuhata T, et al. Beta-catenin alteration in cancer of the ampulla of Vater. J Exp Clin Cancer Res 2002;21:23–7. [PubMed] [Google Scholar]
- 22.Zhao B, Kimura W, Futakawa N, et al. p53 and p21/Waf1 protein expression and K-ras codon 12 mutation in carcinoma of the papilla of Vater. Am J Gastroenterol 1999;94:2128–34. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Hui AM, Shi YZ, et al. Deregulation of G1/S transition is a common event in carcinoma of the ampulla of vater. Hepatogastroenterology 2002;49:1239–44. [PubMed] [Google Scholar]
- 24.Takashima M, Ueki T, Nagai E, et al. Carcinoma of the ampulla of Vater associated with or without adenoma: a clinicopathologic analysis of 198 cases with reference to p53 and Ki-67 immunohistochemical expressions. Mod Pathol 2000;13:1300–7. [DOI] [PubMed] [Google Scholar]
- 25.Imai Y, Tsurutani N, Oda H, et al. p16INK4 gene mutations are relatively frequent in ampullary carcinomas. Jpn J Cancer Res 1997;88:941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai Y, Oda H, Tsurutani N, et al. Frequent somatic mutations of the APC and p53 genes in sporadic ampullary carcinomas. Jpn J Cancer Res 1997;88:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarpa A, Capelli P, Zamboni G, et al. Neoplasia of the ampulla of Vater. Ki-ras and p53 mutations. Am J Pathol 1993;142:1163–72. [PMC free article] [PubMed] [Google Scholar]
- 28.Matsubayashi H, Watanabe H, Yamaguchi T, et al. Differences in mucus and K-ras mutation in relation to phenotypes of tumors of the papilla of vater. Cancer 1999;86:596–607. [PubMed] [Google Scholar]
- 29.Albores-Saavedra J, Henson DE, Sobin LH. The WHO histological classification of tumors of the gallbladder and extrahepatic bile ducts. A commentary on the second edition. Cancer 1992;70:410–14. [DOI] [PubMed] [Google Scholar]
- 30.Lundin J, Nordling S, von Boguslawsky K, et al. Prognostic value of Ki-67 expression, ploidy and S-phase fraction in patients with pancreatic cancer. Anticancer Res 1995;15:2659–68. [PubMed] [Google Scholar]
- 31.Qiao Q, Ramadani M, Gansauge S, et al. Reduced membranous and ectopic cytoplasmic expression of beta-catenin correlate with cyclin D1 overexpression and poor prognosis in pancreatic cancer. Int J Cancer 2001;95:194–7. [DOI] [PubMed] [Google Scholar]
- 32.Younes N, Fulton N, Tanaka R, et al. The presence of K-12 ras mutations in duodenal adenocarcinomas and the absence of ras mutations in other small bowel adenocarcinomas and carcinoid tumors. Cancer 1997;79:1804–8. [PubMed] [Google Scholar]
- 33.Izzo JG, Papadimitrakopoulou VA, Liu DD, et al. Cyclin D1 genotype, response to biochemoprevention, and progression rate to upper aerodigestive tract cancer. J Natl Cancer Inst 2003;95:198–205. [DOI] [PubMed] [Google Scholar]
- 34.Bartek J, Lukas L, Strauss M. [Control mechanisms of cell transition from the G1 phase to the S phase—the R point]. Cas Lek Cesk 1996;135:634–5. [PubMed] [Google Scholar]
- 35.Quelle DE, Ashmun RA, Shurtleff SA, et al. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev 1993;7:1559–71. [DOI] [PubMed] [Google Scholar]
- 36.Wong NA, Morris RG, McCondochie A, et al. Cyclin D1 overexpression in colorectal carcinoma in vivo is dependent on beta-catenin protein dysregulation, but not k-ras mutation. J Pathol 2002;197:128–35. [DOI] [PubMed] [Google Scholar]
- 37.Ueta T, Ikeguchi M, Hirooka Y, et al. Beta-catenin and cyclin D1 expression in human hepatocellular carcinoma. Oncol Rep 2002;9:1197–203. [PubMed] [Google Scholar]
- 38.Sugimachi K, Aishima S, Taguchi K, et al. The role of overexpression and gene amplification of cyclin D1 in intrahepatic cholangiocarcinoma. J Hepatol 2001;35:74–9. [DOI] [PubMed] [Google Scholar]
- 39.Ito Y, Takeda T, Sasaki Y, et al. Expression and clinical significance of the G1–S modulators in intrahepatic cholangiocellular carcinoma. Oncology 2001;60:242–51. [DOI] [PubMed] [Google Scholar]
- 40.Ito Y, Takeda T, Sasaki Y, et al. Expression of p57/Kip2 protein in extrahepatic bile duct carcinoma and intrahepatic cholangiocellular carcinoma. Liver 2002;22:145–9. [DOI] [PubMed] [Google Scholar]
- 41.Maruta H, Burgess AW. Regulation of the Ras signalling network. Bioessays 1994;16:489–96. [DOI] [PubMed] [Google Scholar]
- 42.Chen CY, Liou J, Forman LW, et al. Differential regulation of discrete apoptotic pathways by Ras. J Biol Chem 1998;273:16700–9. [DOI] [PubMed] [Google Scholar]
- 43.Rozakis-Adcock M, Fernley R, Wade J, et al. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature 1993;363:83–5. [DOI] [PubMed] [Google Scholar]
- 44.Janes PW, Daly RJ, deFazio A, et al. Activation of the Ras signalling pathway in human breast cancer cells overexpressing erbB-2. Oncogene 1994;9:3601–8. [PubMed] [Google Scholar]