Abstract
Homo- or heterodimeric compounds that affect dimeric protein function through interaction between monomeric moieties and protein subunits can serve as valuable sources of potent and selective drug candidates. Here, we screened an in-house dimeric natural product collection, and panepocyclinol A (PecA) emerged as a selective and potent STAT3 inhibitor with profound anti-tumor efficacy. Through cross-linking C712/C718 residues in separate STAT3 monomers with two distinct Michael receptors, PecA inhibits STAT3 DNA binding affinity and transcription activity. Molecular dynamics simulation reveals the key conformation changes of STAT3 dimers upon the di-covalent binding with PecA that abolishes its DNA interactions. Furthermore, PecA exhibits high efficacy against anaplastic large T cell lymphoma in vitro and in vivo, especially those with constitutively activated STAT3 or STAT3Y640F. In summary, our study describes a distinct and effective di-covalent modification for the dimeric compound PecA to disrupt STAT3 function.
Key words: Signal transducer and activator of transcription 3, STAT3 inhibitor, Anaplastic large T cell lymphoma, Dimerization, Dimeric compounds, Natural product, Panepocyclinol A, Di-covalent modification
Graphical abstract
Dimeric natural product panepocyclinol A inhibits STAT3–DNA binding via a unique di-covalent modification on STAT3 dimer, thereby exhibiting profound anti-tumor efficacy against ALCL neoplasms harboring constitutively activated STAT3 mutants.

1. Introduction
Dimerization of proteins often serves as an essential mechanism for their functional activation, most notably in disease-associated receptors or transcription factors1. For example, proteins of the Signal transducer and activator of transcription (STAT) family form either homo- or hetero-dimers following tyrosine phosphorylation, thus initiating nuclear translocation of STAT proteins for the regulation of target gene transcription that modulates cell proliferation, survival, migration and so on2. STAT3, one of seven STAT family members, has been found aberrantly activated in a broad range of cancer types. In particular, abnormal dimerization caused by STAT3 mutations has been linked to poor prognosis of hematopoietic malignancies3, 4, 5, 6. Distinct strategies have been employed to develop inhibitors to block STAT3 signaling and activity in the past two decades. Among them, disrupting STAT3 dimerization by targeting the SH2 domain yields effective STAT3 inhibitors such as stattic7 and S3I-2018. However, despite a large amount of effort that has been put into seeking selective and potent STAT3 inhibitors, there has been no STAT3-targeted drug approved for clinical application9.
Diverse homo- or heterodimeric compounds arising from identical or similar monomers can potentially affect the function of dimeric proteins via binding between individual monomers and protein subunits10. Such dimeric compounds have been shown to exhibit high potency and selectivity towards their respective dimerized protein targets, sometimes through new mechanisms of action11, 12, 13, 14. For example, FK1012 and its derivatives have been developed as a tool kit for precise control of biological processes such as signaling transduction or transcriptional regulation by bringing chimeric proteins containing the FKBP12 domain into proximity15, 16, 17. MK-1454, a heterodimer of two nucleotides that binds to the interface of dimeric STING to activate the STING signaling pathway, is now in clinical trials as an immuno-oncology therapeutic18,19. Therefore, structurally complex and diverse dimeric compounds that modulate protein dimerization can play a prominent role in the discovery of chemical probes and drug candidates20,21.
In this study, we screened an in-house collection of dimeric compounds and revealed a selective STAT3 inhibitor, panepocyclinol A, that targets a pocket formed at the STAT3 dimer interface, resulting in an irreversible di-covalent modification that blocks STAT3 dimer transcription activity. Additionally, panepocyclinol A exhibits profound anti-proliferative activity against ALCL neoplasms with constitutively activated STAT3. Taken together, our study provides a mechanistic framework for STAT3 targeting that is markedly different from that of other STAT3 inhibitors.
2. Materials and methods
2.1. Reagents
Panepocyclinol series compounds were isolated from the fungus Panus rudis F0131522. In brief, the fungus Panus rudis F01315 was cultured on potato-dextrose agar (PDA) medium at 28 °C for 18 days. The fermented agar cakes were diced and extracted with EtOAc−MeOH−AcOH (v/v/v, 80/15/5). After the removal of solvents under vacuum, the crude extract was suspended in EtOAc and washed with H2O. The EtOAc layer was then concentrated and resuspended in MeOH and petroleum ether. The MeOH layer was concentrated to give the defatted extract. A series of chromatographic methods, including medium-pressure liquid chromatography (MPLC), Sephadex LH-20, HPLC XDB C18 were used to afford panepoxyquinols. STAT3 inhibitors Stattic (Cat.# HY13818), C188-9 (Cat.# HY112288), BP-1-102 (Cat.# HY-100493), S3I-201 (Cat.# HY-15146) were purchased from MedChemExpress, Shanghai, China.
2.2. Cell culture
Anaplastic Large Cell Lymphoma cell lines Karpas299 and SU-DHL-1 were purchased from Cobioer Biosciences Co., Ltd., Nanjing, China. Cell lines were cultured in RPMI-1640 medium (BasalMedia, Shanghai, China) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. All cell lines were tested for mycoplasma contamination and were found to be negative.
2.3. Animals
BALB/c nu/nu mice and NOD SCID mice at 4–6 weeks of age were purchased from Charles River, Beijing, China. Experiments were performed in accordance with the guidelines from the Institutional Animal Care and Use Committee at the Experimental Animal Centre (approval no. XMULAC20190032). Mice were maintained in the 12-h light/12-h dark cycles with free access to food and water.
2.4. Cell viability assay
Cells were seeded at a density of 5 × 103 cells (adherent cells) or 2 × 104 cells (suspension cells) per well in 96-well plates. Compounds were serially diluted in the growth medium, and then added in triplicate to 96-well plates and incubated for 48 h. Cell viability was determined using MTS (Promega, WI, USA), according to the manufacturer's instructions. Results were analyzed by GraphPad Prism 5 (GraphPad Software lnc., Boston, MA, USA).
2.5. Transcription factors (TF) activation profiling
Karpas299 cells were treated with DMSO or 10 μmol/L PecA for 8 h. Cells were then harvested and suspended in 100 μL Buffer A (10 mmol/L HEPES (pH 7.9), 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 0.15% NP-40) containing 1% protease inhibitor, followed by incubation for 15 min. Homogenates were centrifuged at 12,000×g for 30 min at 4 °C (Eppendorf, 5417R, Germany). The pellet was washed 3 times with 1 mL Buffer A, resuspended in 150 μL Buffer B (20 mmol/L HEPES (pH 7.9), 0.4 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 0.5% NP-40) containing 1% protease inhibitor, and sonicated. The supernatant was used as the nuclear fraction. The nuclear extracts were then incubated with biotin-labeled probes and the TF activities were determined according to the manufacturer's instructions of TF Activation Profiling Plate Array I (FA-1001, Signosis, CA, USA).
2.6. Protein expression and purification
Full-length of mouse STAT3 and truncated STAT3 (127–688 aa) were cloned into pFastBac HTA vector fusion with a 6∗His tag and TEV site (Invitrogen). Transfection, virus generation, and amplification were carried out in sf9 insect cells according to the official protocol of the Bac-to-Bac Baculovirus expression system (Invitrogen). Sf9 insect cells expressing indicated proteins were suspended and lysed by sonication in lysis buffer (20 mmol/L Tris-HCl, 150 mmol/L NaCl, 5 mmol/L KCl, 20 mmol/L imidazole, 1 mmol/L tris(2-carboxyethyl) phosphine hydrochloride, pH 8.0) supplemented with protease inhibitor mixture. After centrifuging at 20,000 rpm (Beckman, Avanti JXN-30, IN, USA) for 1 h at 4 °C, the supernatant was incubated with Ni-NTA Sepharose beads (GE Healthcare, USA). The beads were washed with wash buffer (20 mmol/L Tris-HCl, 500 mmol/L NaCl, 1% Glycerol, 1 mmol/L TCEP, 20 mmol/L imidazole, pH 8.0) and then the protein was eluted with the same buffer supplemented with 300 mmol/L imidazole. The proteins were then dialyzed in stocking buffer (20 mmol/L Tris-HCl, 500 mmol/L NaCl, 1% Glycerol, 1 mmol/L TCEP, pH 8.0) and then frozen in −80 °C before use.
2.7. Fluorescence polarization assay
Proteins (1 μmol/L), fluorescent-labeled peptides (25 nmol/L) and series concentrations of compounds diluted in FP buffer (50 mmol/L NaCl, 10 mmol/L Hepes (pH 7.5), 1 mmol/L EDTA, 0.1% Nonidet P-40, and 1 mmol/L dithiothreitol) were incubated in black 96-well microtiter plates for 1 h at RT. Millipolarization units (mP) were determined using an Ultra Evolution 96-well plate reader Tecan Spark. 5ʹ-FAM-GpYLPQTV-NH2 was used for STAT3 and 5ʹ-FAM-GpYDKPHVL-NH2 was for STAT1 detection. Inhibition curves were fitted using GraphPad Prism 5 (GraphPad Software lnc., Boston, MA, USA).
2.8. Chromatin immunoprecipitation-sequencing (ChIP-seq)
Chromatin IP was performed using a Chromatin IP Kit (Cat# 9003, Cell Signaling Technology). Briefly, cells were fixed with formaldehyde and lysed, and chromatin was fragmented by partial digestion with micrococcal nuclease. Chromatin immunoprecipitations were then performed using anti-STAT3 antibody (Cat# 9139, Cell Signaling Technology) and ChIP-Grade Protein G Magnetic Beads. After the reversal of protein and DNA cross-links, the DNA was purified using DNA purification spin columns. Sequencing was performed by Novogene Co., Ltd. (Beijing, China).
2.9. Western blot
Cells were harvested and lysed in lysis buffer (50 mmol/L Tris-HCL, 150 mmol/L NaCl, 1% Triton X-100, 5% glycerol, 1 mmol/L phenylmethylsulfonyl fluoride, pH 7.5). Total protein concentrations were measured by BCA analysis (Beyotime, Shanghai, China). 4×LDS sample buffer with DTT (final concentration of 100 mmol/L) was added into each sample and then heated for 10 min. For immunoblotting, equal amounts of protein were resolved by SDS-PAGE, and then electrophoretically transferred to the Immobilon-P PVDF membrane (PALL, MI, USA). Proteins were detected with appropriate primary antibodies and peroxidase-conjugated secondary antibodies. Immunoreactive protein bands were detected by the enhanced chemiluminescence reagent, according to the manufacturer's instructions (Advansta, CA, USA). Antibody against STAT3 (Cat# 9139), phospho-STAT3 (Tyr705) (Cat# 9131), STAT1 (Cat# 14994), STAT2(Cat# 72604), STAT4 (Cat# 2653), STAT5 (Cat# 9363S), STAT6 (Cat# 5397) were purchased from Cell Signaling Technology (CST) and used in a dilution of 1:1000, β-actin (Cat# A5316) was from Sigma‒Aldrich and used in a dilution of 1:5000.
2.10. Electrophoretic mobility shift assay (EMSA)
STAT3 consensus oligonucleotides were synthesized and labeled with biotin at both ends. EMSA was carried out with a LightShift Chemiluminescent EMSA Kit (Cat# 20148, Thermo Scientific) according to the manufacturer's protocols.
STAT3 consensus oligo (5′‒3′), GAT CCT TCT GGG AAT TCC TAG ATC; STAT3 mutant oligo (5′‒3′), GAT CCT TCT GGG CCG TCC TAG ATC.
2.11. Realtime-PCR
RNA was isolated by an RNA isolation kit (Cat# DP419, Tiangen, Beijing, China). cDNA was synthesized by using Hifair III 1st Strand cDNA Synthesis kit (Cat# 11141ES60, Yeasen, Shanghai, China) according to the manufacturer's protocol. Real-time PCR was performed using Sybr Green-based detection in the Rotor-Gene according to the manufacturer's instructions. ACTB levels were used as normalization controls.
The primers were as bellow (5′‒3′): ACTB-F, CAGCCTTCCTTCCTGGGCATG; ACTB-R, ATTGTGCTGGGTGCCAGGGCAG; TLR6-F, TAGTGAACATGATTCTGCCTGG; TLR6-R, TCGTAATGGCACCACTCACT; IL4R-F, CATTGTCATCCTGGCCGTCT; IL4R-R, TGCTCCAGAAAACAGGGCAA; CDK2-F, CCCTGGATGAAGATGGACGG; CDK2-R, GATGGGGTACTGGCTTGGTC; MYC-F, GTCAAGAGGCGAACACACAAC; MYC-R, TTGGACGGACAGGATGTATGC; MCL1-F, GTGCCTTTGTGGCTAAACACT; MCL1-R, AGTCCCGTTTTGTCCTTACGA; BCL2L1-F, GACTGAATCGGAGATGGAGACC; BCL2L1-R, GCAGTTCAAACTCGTCGCCT; IRF4-F, GCGGTGCGCTTTGAACAAG; IRF4-R, ACACTTTGTACGGGTCTGAGA.
2.12. Analysis of cross-linking spectra
Purified full-length STAT3 protein was incubated with PecA at room temperature for 1 h. The mixture was then applied to SDS-PAGE gel followed by commassie blue staining. The dimer STAT3 bands and untreated STAT3 bands were subjected to LC‒MS/MS. The raw data were analyzed by pLink2.023. Potential cross-linking spectra presented in dimer samples but not in untreated control samples were shown.
2.13. Xenograft models
Cells were filtered through 70 μm cell strainers (BD, NJ, USA) and then suspended in media without serum. Xenografts were initiated by subcutaneous injection of cancer cells into the right flank near the axillary fossa of mice. For Karpas299 cells, each NOD SCID mice were subcutaneously injected with 5 × 106 cells. For Jeko-1 cells, BALB/c nu/nu mice were subcutaneously injected with 1 × 107 cells. The tumor volume was monitored once per day using the standard Eq. (1):
| V = Length × Width2 × 0.5 | (1) |
When tumors were grown to approximately 100–150 mm3, mice (half males and half females) were randomly assigned into each experimental group and treated by tail vein injection with vehicle [10% (w/v) Kolliphor HS 15 (Sigma) in normal saline] or PecA formulated in vehicle at indicated doses daily for a week. The injection volume was 0.1 mL per 10 g. Mouse body weights were monitored daily. At 6 h post the final dose, mice were euthanized and tumor tissues were separated, weighed, and subjected to immunoblotting, immunohistochemistry, TUNEL staining, and routine histopathological examination.
2.14. Hematoxylin and eosin (H&E) staining
Tumor tissues were fixed in 4% paraformaldehyde, embedded in paraffin, cut into 4 μm thick sections, and applied to slides. Standard staining with hematoxylin and eosin using an H&E kit (Cat# AR1180, Boster, Wuhan, China) was performed on 4 μm thick sections from each slide.
2.15. TUNEL staining
TUNEL staining was performed using the DeadEnd Fluorometric TUNEL System (Promega, WI, USA) following manufacturer instructions. Briefly, 4 μm thick paraformaldehyde-fixed, paraffin-embedded tissue sections were deparaffinized and rehydrated. The tissue was fixed with 4% paraformaldehyde and permeabilized with proteinase K solution. Then, the tissue was refixed with paraformaldehyde and equilibrated with an equilibration buffer. Following these, sections were incubated with TdT reaction mixture at 37 °C for 60 min in a humidified chamber, immersed in 2 × SSC for 15 min to stop the reaction, mounted and counterstained with VECTASHIELD + DAPI (Vector Lab, Burlingame, CA, USA) for 15 min at room temperature. Negative controls: sections incubated with rTdT incubation buffer (without rTdT enzyme). Positive controls: sections treated with DNase I to cause DNA fragmentation.
2.16. Genome-scale CRISPR-Cas9 knockout (GeCKO)
The methodology employed was largely in accordance with the established GeCKO screening protocol24. Human CRISPR KO Library (GeCKO v2), containing 123411 sgRNAs targeting 19050 genes, was purchased from Addgene (Cat.# 1000000049). Anaplastic Large Cell Lymphoma cell lines Karpas299 and SU-DHL-1 were transduced with the GeCKO library at a MOI of 0.3, followed by puromycin selection (500 ng/mL) for 7 days. On Day 7, 3 × 107 cells were frozen down for genomic DNA analysis as a baseline. The remaining cells were split into six replicates, three replicates were cultured in supplemented with 1 μmol/L PecA, while an equivalent volume of DMSO was used in the other three replicates as a control. After screening for 14 days, a minimum of 3 × 107 cells were harvested for genomic DNA sequencing. NGS data were subsequently processed and analyzed using Python scripts.
2.17. MD simulation details
The two STAT3 protein-DNA complexes without and with covalent inhibitors, denoted as the STAT3 and STAT3_ligand models, were constructed from the crystal structure (PDB ID:1BG1) using the PyMOL software (Schrödinger, LLC, New York, NY, USA)25. The missing residues in the STAT3 complexes were added using the protein preparation module26 of the Schödinger software (Schrödinger, LLC, New York, NY, USA)27. In the STAT3_ligand model, the covalent molecule PecA was manually docked to the binding site located at the interface of the chains A and B of STAT3. The two active C atoms of PecA were covalently connected with the two S atoms of the CYS712 residues on the chains A and B, respectively.
In order to perform MD simulation of the STAT3 complex, we have developed the force fields of the PecA molecule by calculating its RESP charge28 using the B3LYP29,30/6-311G(d,p) method in the Gaussian 09 software (Gaussian Inc., Wallingford, CT, USA)31. Its bonded and van der Waals parameters were generated from the GAFF force fields32. The AMBER ff19SB force fields33 were used to describe the proteins, while the OL15 force fields were used for the DNA fragments in the complexes. The PecA molecule was treated as a nonstandard residue in the AMBER ff19SB force fields. The STAT3 and STAT3_ligand models were further immersed in cubic water boxes full of the TIP3P34,35 water molecules The Na+ ions were added to neutralize the whole systems. The STAT3 and STAT3_ligand protein-DNA systems contain a total number of 266,572 and 266,620 atoms, respectively.
The MD simulations of the two complex systems were carried out in the AMBER20 software (University of California, San Francisco, CA, USA)36. To start MD simulations, we first conducted energy minimizations of whole systems with 10,000 steps and then heated them to the temperature of 300 K for 1 ns. The SHAKE algorithm37 was applied to constrain the bonds involving H atoms. The cutoff for van der Waals interactions was set to 10 Å, and the long-range electrostatic interaction was estimated using the Particle Mesh Ewald method38. The Langevin thermostat39 and Berendsen barostat40 were used to keep the systems at a temperature of 300 K and a standard atmosphere pressure, respectively. Finally, each system was equilibrated for 500 ns and the simulated trajectories were used for data analysis later. The binding energies of the proteins and DNAs in the complexes with and without covalent inhibitors were evaluated with the Molecular Mechanics Poisson-Boltzmann Surface Area (MM-PBSA) method41,42 based on the simulated 500 ns trajectories.
2.18. KINOMEscan
PecA was profiled against a panel of 468 kinases using KINOMEscan technology43 an active site-dependent competition binding assay at 10 μmol/L (Supporting Information Table S3). The results were reported as percent of control DMSO (ctrl%), in which lower numbers represent higher affinity binding as shown in Eq. (2):
| Ctrl (%) = (Test compound signal − Positive control signal)/(Negative control signal − Positive control signal) × 100 | (2) |
where the negative control was set at 100%, and the positive control compound was set at 0%.
2.19. Statistical analysis
Data were expressed as mean ± standard error of mean (SEM) or mean ± standard deviation (SD). Differences between the two groups were assessed by a two-tailed unpaired Student's t-test and multiple group comparisons were assessed by a one-way or two-way ANOVA followed by Tukey-Kramer test with GraphPad Prism 5 (GraphPad Software lnc., Boston, MA, USA).
3. Results
3.1. Panepocyclinol A inhibits STAT3 signaling
To discover dimeric compounds that have potential anti-tumor efficacy, we screened an in-house collection of 83 dimeric natural products against six cancer cell lines, including melanoma A375, anaplastic large cell lymphoma Karpas299, gastric cancer BGC-823, hepatoma HepG2, osteosarcoma U2OS, and breast cancer MCF7. All compounds were tested at 10 μmol/L, with cell viability determined using an MTS assay (Fig. 1A). This screen of cancer cell lines indicated that panepocyclinol A (PecA) (Fig. 1B) had promising anti-proliferative activity, with a survival rate of about 1.22% and 7.69% in A375 cells and Karpas299 cells, respectively (Supporting Information Table S1). The panepocyclinol-type compounds (structures in Table S1), which feature a densely functionalized core scaffold consisting of a 6/6/6/5-fused tetracyclic carbon skeleton, were dimers originated from monomeric epocyquinol22. Structure-activity relationship (SAR) analysis showed that the anti-proliferative potency decreased in the order PecA/PecH/PecB and PecA/PecE/PecD, suggesting that the α-chloro unsaturated ketone and the α,β-epoxide ketone functioned as the pharmacophoric elements of the epoxyquinol dimers (Table S1). We also found that PecH was less stable than PecA, which may partially account for its lower bioactivity.
Figure 1.
PecA inhibits the transcription activity of STAT3. (A) Cell viability screen of an in-house dimer compound collection. After treatment with 10 μmol/L compounds for 48 h, cell viabilities were triplicate tested by MTS and then normalized to the value of the DMSO-treated group. (B) Structure of PecA. (C) Transcription factors activation profiling was used to analyze the activities of indicated transcription factors in Karpas299 cells upon DMSO or 10 μmol/L PecA treatment. Data are presented as mean ± SD (n = 3). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. (D) Fluorescence polarization assay measured the affinity of diverse compounds for purified full-length STAT3. (E) GSEA analysis for the RNA-seq results of Karpas299 and SU-DHL-1 cells upon DMSO or 10 μmol/L PecA treatment. (F) The genome-wide profiles of STAT3 ChIP-seq peaks in Karpas299 cells upon DMSO or 10 μmol/L PecA treatment. Gene ontology analysis for the significantly reduced genes upon PecA treatment was shown (fold change <0.5). (G) ChIP-seq tracks in DMSO or PecA-treated Karpas299 cells along the indicated gene loci.
In light of its best inhibitory effects on cancer cell proliferation, we then selected PecA for further detailed analyses of its biological effects. Assays with an expanded panel of 31 diverse cancer cell lines indicated that PecA exerted potent anti-proliferative activity, with IC50 values below 10 μmol/L against most of the tested cell lines, and lower than 1 μmol/L for several lymphoma and melanoma cell lines in particular (Supporting Information Table S2). We then applied a Genome-scale CRISPR-Cas9 knockout (GeCKO)24 in Anaplastic Large Cell Lymphoma (ALCL) cell lines Karpas299 and SU-DHL-1 to screen for genes whose depletion correlated to PecA resistance. High throughput sequencing revealed ∼1200 depleted genes that contributed to PecA resistance to varying extents (Supporting Information Fig. S1A). Further gene ontology (GO) analysis suggested that the greatest enrichment was for genes involved in transcription and intracellular signal transduction (Fig. S1B).
We then performed transcription factor (TF) activation profiling to identify the regulatory networks that participated in response to PecA (Fig. 1C). Among the differentially regulated TFs, we noticed signal transducer and activator of transcription (STAT) family proteins exhibited widely varying transcriptional activity under PecA treatment in Karpas299 cells. In particular, STAT3 activity was the most significantly suppressed, whereas STAT1 was also slightly down-regulated, and other members were not significantly affected (Fig. 1C). Moreover, PecA suppressed STAT3 activation of the luciferase reporter in a dose-dependent manner (Supporting Information Fig. S2A). STAT family TFs, especially STAT3, are known to play important regulatory functions in immunity, inflammation, and tumorigenesis, and they are activated by cytokines and growth factors through kinase-mediated tyrosine phosphorylation and dimerization2,44. Thus, we next tested whether PecA suppressed STAT3 through a canonical upstream kinase, such as JAK1/2, Src, or others. Kinome scan assays indicated that none of the 466 human kinases were targeted by PecA at 10 μmol/L concentration (Supporting Information Table S3), indicating that PecA did not affect STAT3 transcription activity through its upstream kinases.
Subsequent in vitro fluorescence polarization assays using a fluorescent probe specific to STAT3 suggested the occurrence of robust interactions between PecA and purified STAT3 protein, with an EC50 value of ∼1 μmol/L. However, PecB and PecC, with carried modifications on the α-chloro unsaturated ketone, showed diminished binding affinity with STAT3, with EC50 > 100 μmol/L (Fig. 1D). Moreover, PecA exhibited negligible interaction with purified STAT1 protein, with EC50 > 10 μmol/L (Fig. S2B). STAT3 knockout in Karpas299 and SU-DHL-1 cells, in which endogenous STAT3 is constitutively active45, led to significant resistance to PecA, while STAT1 knockout had no obvious influence on sensitivity (Figs. S2C and S2D). Together, these data indicated that PecA directly interacts with STAT3 but not STAT1, and that the inhibitory effects of Panepocyclinol analogs towards STAT3 are positively correlated with their anti-cancer activity.
We next investigated the impacts of PecA on STAT3 signaling by RNA-seq in Karpas299 cells treated with or without PecA. Gene set enrichment analysis (GSEA) utilizing the GSE21670_STAT3_KO_VS_WT_CD4_TCELL_UP gene set, comprising 197 genes up-regulated in STAT3 knockout CD4 T cells versus wild-type CD4 T cells, indicated concordant differences between PecA treatment and STAT3 knockout in CD4 T cells. Consistently, GSEA analysis employing the DAUER_STAT3_TARGETS_UP gene set also suggested that the top 50 genes up-regulated in STAT3 overexpressing cells were down-regulated upon PecA treatment (Fig. 1E). Overall, the expression levels of STAT3 downstream target genes were negatively correlated with PecA treatment. Further chromatin immunoprecipitation sequencing (ChIP-seq) using antibody targeting STAT3 showed that differentially expressed genes (DEGs) were predominantly enriched with genes involved in immune system, inflammatory response, and cell fate determination in PecA-treated cells compared with untreated ALCL control cells (Fig. 1F). For example, TLR6, IL4R, and IRF4, which play essential roles in innate immunity, and MCL1, CDK2, and RIPK1, which participate in cell fate control, are all STAT3 downstream target genes that were differentially suppressed under PecA treatment (Fig. 1G). Collectively, these data indicated that PecA specifically targets STAT3 and down-regulates its signaling activity.
3.2. PecA selectively induces the formation of a STAT3 covalent homodimer
To investigate the mechanism by which PecA acts on STAT3, we first detected STAT3 expression in ALCL cell lines treated with PecA. Immunoblotting for STAT3 by standard SDS-PAGE revealed a STAT3-related band with molecular weight (MW) of ∼200 kD, nearly double that of STAT3 monomer (∼100 kD, exact MW 88 kD), which increased in a dose-dependent manner with PecA concentration in both Karpas299 and SU-DHL-1 cells (Fig. 2A). STAT3 phosphorylation at Y705 is a hallmark of its activated state. We found that Y705 phosphorylation was decreased in the ∼100 kD STAT3 band, but increased in the ∼200 kD, also in a PecA dose-dependent manner (Fig. 2A). This ∼200 kD STAT3 band was unique in PecA-treated Karpas299 cells, and couldn't be observed in other well-known STAT3 inhibitors such as stattic, S3I-201, BP-1-102 and C188-9 (Fig. 2B). Moreover, this putative 200 kD STAT3 complex was also observed in vitro under purified STAT3 incubation with a concentration gradient of PecA (Fig. 2C).
Figure 2.
PecA selectively induces covalent homodimer of STAT3. (A) Karpas299 (upper) and SU-DHL-1 (lower) cells were treated for different concentrations of PecA for 2 h. Cell lysates were subjected to SDS-PAGE gel for Western blot. (B) Karpas299 cells were treated with various STAT3 inhibitors for 4 h. STAT3 and p-STAT3 were analyzed by Western blotting. (C) Purified full-length STAT3 protein (20 nmol/sample) was incubated with a series concentration of PecA at room temperature for 1 h. The mixture was then applied to SDS-PAGE gel followed by commassie blue staining. (D) Karpas299 cells were treated with 30 μmol/L of PecA or its analogs for 2 h. Cell lysates were then subjected to SDS-PAGE for Western blot. (E) Karpas299 cells upon PecA treatment were lysed for Western blot. Appropriate antibodies were used as indicated. (F) Karpas 299 cells were treated with 20 μmol/L of PecA for a time course. (G) Full-length STAT3 or truncated STAT3 (127–688 aa) proteins were incubated with series concentration of PecA for 1 h. A biotin-labeled DNA probe was used to detect the DNA binding affinity for STAT3 by EMSA. Mutant probe to STAT3 (mut) was used as a negative control.
Since proteins are generally denatured and reduced by SDS-PAGE, the appearance of this specific ∼200 kD band at approximately double the MW of STAT3 indicated that PecA induced the formation of a STAT3 homodimer. The addition of extra DTT into cell samples prior to SDS-PAGE could not abolish ∼200 kD band comprised of either STAT3 or p-STAT3Y705 homodimers, suggesting that the formation of the larger STAT3 complex was not mediated by reducible disulfide bonds, but in an irreversible manner (Supporting Information Fig. S3A). Moreover, PecA specifically induced dimer formation by STAT3, but no other STAT family members (Fig. 2D), although all seven STAT members dimerized under disuccinimidyl suberate (DSS) treatment, a bifunctional amide cross-linker46 (Fig. S3B). Similarly, no dimer was observed in the in vitro incubation of PecA with purified full-length STAT1 protein (Fig. S3C). Taken together, these data indicated that PecA specifically triggers irreversible crosslinking, resulting in STAT3 homodimerization.
Given that PecA bears multiple electrophilic groups, we hypothesized that it could induce crosslinking between two STAT3 monomers through di-covalent attachment. Since the α-chloro unsaturated ketone (A ring) and α,β-epoxide ketone (C ring) are key structural features for cytotoxicity (Table S1), it was reasonable to speculate that these two potentially reactive sites were responsible for PecA-STAT3 conjugation. To verify this assumption, we tested STAT3 dimer formation under treatment with PecA, α-methoxy-substituted PecB, the C ring epoxide-opened panepocyclinol D (PecD), or C ring α-chloro, β-hydroxyl-substituted panepocyclinol E (PecE). The results indicated that PecB could not induce STAT3 dimerization, suggesting that the α-chloro unsaturated ketone in the A ring served as the reactive site. Additionally, PecE treatment led to a weakened STAT3 dimer band, STAT3 failed to dimerize under PecD treatment, indicating that the α,β-epoxide ketone in the C ring served as another conjugative site (Fig. 2E). These data thus revealed that PecA forced STAT3 dimerization through its A ring α-chloro unsaturated ketone and the C ring α, β-epoxide ketone.
Canonical STAT3 activation involves Y705 tyrosine phosphorylation, followed by dimerization, and then nuclear translocation to activate target gene transcription. Thus, dimerization plays a vital role in STAT3 biological function. Historically, blocking STAT3 dimerization was an effective strategy for the discovery of STAT3 inhibitors47,48. Thus, we sought to determine how PecA could induce STAT3 dimerization while also repressing its transcriptional regulatory activity. To this end, we treated Karpas299 cells with 20 μmol/L PecA for different time periods, which revealed that both the dimer and monomer p-STAT3Y705 bands decreased with time of exposure to PecA until undetectable at 4 h of treatment (Fig. 2F). In addition, EMSA assays showed that the affinity between the purified full-length STAT3 protein (STAT3 FL) and a biotin-conjugated DNA probe was dramatically decreased by pre-incubation with PecA (Fig. 2G). However, a truncated STAT3 variant (127-688 amino acids) with abolished dimerization under PecA treatment (Fig. S3D) could interact with the DNA probe and was largely insensitive to PecA (Fig. 2G). These data demonstrated that PecA-mediated STAT3 dimerization blocked its phosphorylation level and also reduced its DNA binding affinity, ultimately impairing its transcriptional activity.
3.3. PecA crosslinks STAT3 monomers via Cys712 and Cys718 residues
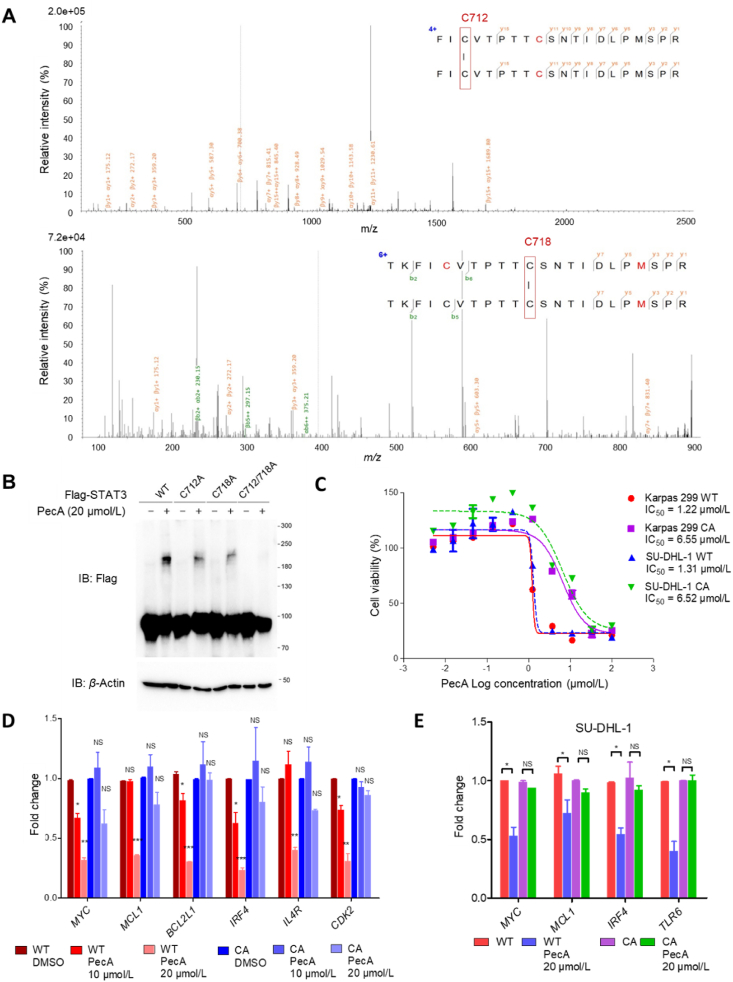
To identify the specific amino acids in STAT3 cross-linked by PecA, full-length STAT3 treated with PecA was subjected to SDS-PAGE, and the band containing the STAT3 dimer complex was excised for LC‒MS/MS analysis. Using pLink2.0, a search engine for cross-linked peptides23, two potential cross-link spectra were identified, formed via cross-linked C712-C712 and C718-C718. This data indicated that the C712 and C718 residues likely mediated cross-linking between PecA and STAT3 monomers (Fig. 3A), and the PecA-induced STAT3 dimerization was completely abolished in a C712A/C718A STAT3 double mutant (Fig. 3B). ALCL cells harboring this STAT3 C712A/C718A (CA) double mutant had approximately six-fold higher IC50 values to PecA than that of cells expressing wild-type (WT) STAT3 (Fig. 3C). Furthermore, the expression of STAT3 target genes were largely unaffected upon PecA treatment in Karpas299 (Fig. 3D) and SU-DHL-1 cells (Fig. 3E) carrying the STAT3 C712A/C718A double mutant. In addition, the C712 and C718 residues positioned in the bottom of the pocket are unique to STAT3 and absent in all other STATs (Supporting Information Fig. S4), further explaining PecA selectivity for STAT3. These cumulative results demonstrated that C712 and C718 of STAT3 were required for direct covalent modification by PecA.
Figure 3.
Cysteins 712 and 718 of STAT3 are responsible for the covalent modifications of PecA. (A) The dimer bands and untreated STAT3 bands were analyzed by LC‒MS/MS. The raw data were analyzed by pLink2.0. Potential cross-linking spectra presented in dimer samples but not in untreated control samples were listed. (B) Karpas299 cells harboring different STAT3 mutations were treated with 20 μmol/L PecA for 2 h. Cell lysates were then subjected to SDS-PAGE. (C) STAT3 wildtype or a C712A/C718A mutant (CA) was stably introduced into Karpas299 or SU-DHL-1 cells by Crispr-Cas9. IC50 values of PecA were measured by MTS assay. (D, E) Karpas299 (D) or SU-DHL-1 cells (E) harboring wildtype or C712A/C718A mutant STAT3 were treated as indicated concentrations of PecA for 4 h. The mRNA levels of STAT3 target genes were quantified by real-time PCR. Data are presented as mean ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, NS, not significant.
3.4. Molecular dynamics simulation of STAT3 dimer
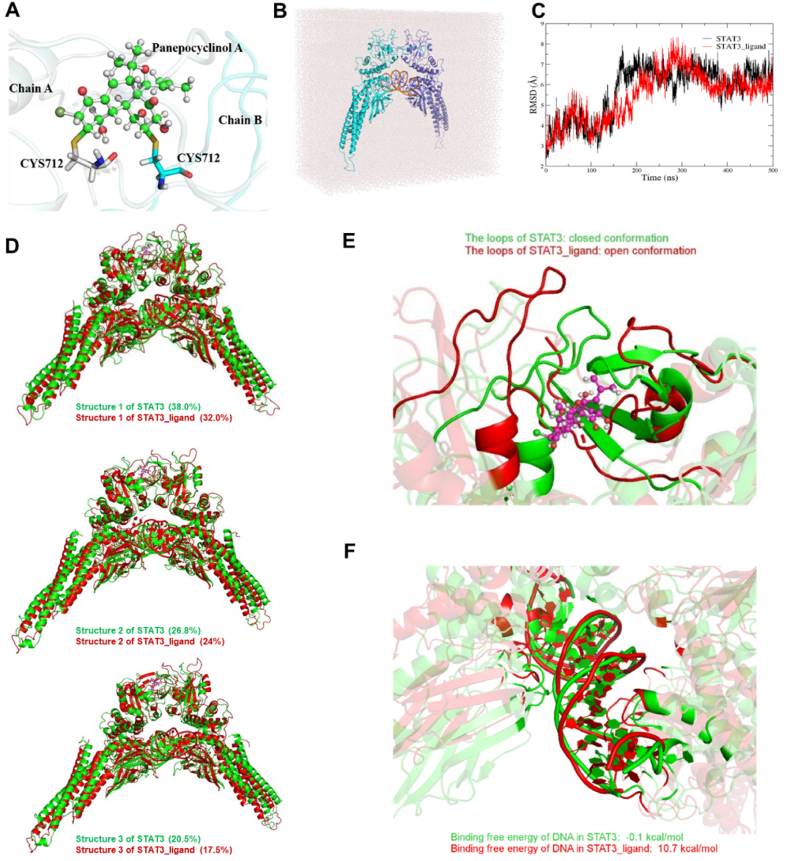
Since our attempts to obtain the co-crystal structure of STAT3-PecA haven't succeeded, we used molecular dynamics (MD) simulation to discriminate the conformational difference of the covalently inhibited STAT3_ligand and STAT3 systems using a published STAT3 dimer crystal structure comprising residues 136–716 of STAT3 (PDB: 1BG1). In the STAT3_ligand model, the covalent molecule PecA was manually docked to the binding site located at the interface of the chains A and B of STAT3, as shown in Fig. 4A. The two active carbon atoms of PecA were covalently connected with the two sulfur atoms of the C712 residues on the chains A and B, respectively. Cys718 is located in the disordered region of C terminus which is absent in this model. We then developed the force fields of the PecA molecule. The STAT3 and STAT3_ligand models were further immersed in cubic water boxes full of the TIP3P34,35 water molecules (Fig. 4B).
Figure 4.
MD simulation of the STAT3-PecA complex. (A) A scheme illustrates the covalent connection of the two carbon atoms of the PecA to the two sulfur atoms of the C712 residues on the chains A and B of the STAT3_ligand complex. The covalently connected PecA is shown in the ball-and-stick representation. (B) An illustration of the STAT model immersed in a cubic water box. (C) Calculated RMSD curves of the STAT3 and STAT3_ligand complexes from 500 MD simulation trajectories. (D) A comparison of top 3 representative structures of the STAT3 and STAT3_ligand model derived from clustering analysis of simulated trajectories. (E) Comparison of the major conformations of the complexes shows that the loops at the binding sites of the STAT3 and STAT3_ligand complexes maintain closed and open conformations, respectively. The PecA bound at the binding site of the STAT3_ligand complex was colored in purple. (F) A comparison of the conformations of the DNAs in the STAT3 and STAT3_ligand complexes. The binding free energies of the two complexes were calculated.
The MD simulations of the two complex systems were then carried out in the AMBER20 software36. To start MD simulations, we first conducted energy minimizations of whole systems with 10,000 steps and then heated them to the temperature of 300 K for 1 ns. Finally, each system was equilibrated for 500 ns (Fig. 4C) and the simulated trajectories were used for data analysis later. The binding energies of the proteins and DNAs in the complexes with and without covalent inhibitors were also evaluated based on the simulated 500 ns trajectories.
We conducted clustering analysis on the 500 ns trajectories and obtained their representative structures. The percentages of the top 3 representative structures of the STAT3 system are 38.0%, 26.8%, and 20.5%, and these of the STAT3_ligand system are 32.0%, 24.0%, and 17.5%, respectively. Fig. 4D presents a comparison of the secondary structures of the first representative structures of the STAT3 and STAT3_ligand. It can be seen that the conformations of the proteins and DNA have changed remarkably after the STAT3 was covalently connected with the PecA molecule. It seems that the leg-like α-helices of the protein dimer in the structure of the STAT3_ligand complex clamped a little tighter than the green counterparts of the STAT3 complex. Similar conformational changes in the α-helices of the complexes have also been observed in other clustered representative structures (Fig. 4D).
We also speculate that the conformation change of the α-helices of the protein dimer of STAT3_ligand is caused by the interaction of PecA and STAT3 dimer. Fig. 4E presents a comparison of the binding sites with and without PecA. In the STAT3 complex, it was found that the two loops of the chains A and B stayed in the closed conformations. In contrast, the red loops at the binding site of the STAT3_ligand complex remain open since PecA has occupied the site due to covalent inhibition. Our results of MD simulations indicate that the covalent inhibition might cause the conformational change of the binding site of the STAT3_ligand complex, and lead to correlated conformational changes of the α-helices of the protein dimer, as well as the DNA fragment clamped between two monomers (Fig. 4F). The calculated binding free energies of the DNAs and proteins of the STAT3 and STAT3_ligand complexes using the MM-PBSA methods are −0.1 and 10.7 kcal/mol, respectively. Comparison of the relative binding free energies of two complexes indicates that the covalent binding of PecA has made the STAT3-DNA complex less stable than the PecA-free state by 10.8 kcal/mol, resulting in significant repression of STAT3 functions, which is consistent with that observed in experiments.
3.5. PecA suppresses ALCL cells with STAT3 Y640F mutant in vitro and in vivo
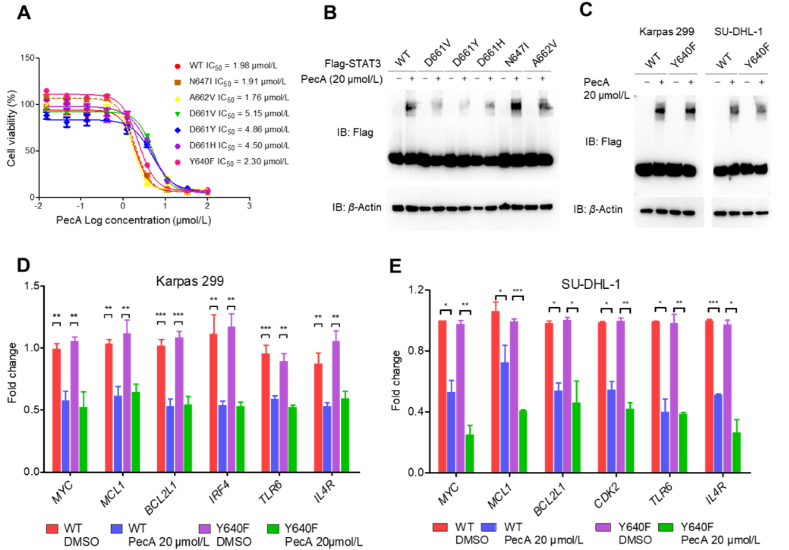
STAT3 is reportedly found in a predominantly activated state and/or carrying mutations in hematopoietic malignancies such as anaplastic large T cell lymphoma (ALCL), T-cell large granular lymphocytic leukemia (T-LGL), diffuse large B cell lymphoma (DLBCL), and others4,5,49. We noticed that recurrent mutations in STAT3 are mainly located in the SH2 domain, which is responsible for dimerization50. We therefore hypothesized that PecA exhibits its inhibitory effects by mediating covalent dimerization through cysteines outside of the SH2 domain, suggesting that PecA binding could potentially override the effects of recurrent STAT3 mutations associated with hematopoietic malignancies. To test this possibility, we individually introduced a set of STAT3 mutants previously found in patients with hematopoietic malignancies into Karpas299 cells (Supporting Information Fig. S5A). Cell viability assays showed that PecA exhibited a range of potencies against cells harboring Y640F, N647I, and A662V mutations since they exhibited comparable sensitivity to that of WT STAT3 (Fig. 5A and Fig. S5B). Consistent with this result, PecA induced dimerization of STAT3Y640F and STAT3N647I similar to its effect in WT STAT3 (Fig. 5B and C), whereas STAT3 D661 mutants (including D661V, D661Y, and D661H) showed nearly 2-fold higher IC50 to PecA compared to WT control cells (Fig. 5A), as well as decreased dimerization upon PecA treatment (Fig. 5B).
Figure 5.
PecA is efficacious against STAT3wt and STAT3Y640F ALCL. (A) Karpas299 cells with STAT3 mutations were treated with series concentrations of PecA for 48 h. Cell viability was tested by MTS. (B) Karpas299 cells harboring different STAT3 mutations were treated with PecA. STAT3 dimerization was analyzed by Western blot. (C) Karpas299 cells harboring wildtype STAT3 or STAT3 Y640F mutations were treated with 20 μmol/L PecA for 2 h. Cell lysates were then subjected to SDS-PAGE. (D, E) Wildtype STAT3 or STAT3 Y640F mutations engineered Karpas299 (D) or SU-DHL-1 cells (E) were treated with 20 μmol/L PecA for 4 h. The mRNA levels of STAT3 target genes were quantified by real-time PCR. Data are presented as mean ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Since Y640F, which increases STAT3 transcriptional activity, is reportedly the most frequently recurring STAT3 mutation, accounting for 40%–70% of total mutations6,3, we performed qPCR assays to quantify the expression of STAT3 target genes in Karpas299 and SU-DHL-1 cells harboring STAT3WT or STAT3Y640F treated with PecA. The results indicated that STAT3 target gene expression was reduced in both WT and Y640F-expressing cells, thus confirming that PecA suppressed STAT3 transcription regulatory activity (Fig. 5D and E). These data suggested that PecA inhibition of STAT3 could override the effects of WT STAT3 or STAT3Y640F hyperactivity.
We next evaluated the in vivo anti-tumor efficacy of PecA in lymphoma Jeko-1 xenograft mouse models. Administration of 20 mg/kg PecA once daily or twice daily for 7 days resulted in significant tumor growth inhibition compared to controls as shown by the tumor volumes and tumor weights (Fig. 6A‒C). No obvious body weight loss or gross signs of toxicity in PecA-treated groups were observed (Supporting Information Fig. S6A). We further conducted xenograft mice harboring tumors with either WT STAT3 overexpression or Y640F mutant Karpas299 cells and then administered vehicle or 20 mg/kg of PecA twice daily. We found that tumor growth was substantially lower in mice treated with PecA compared to controls (Fig. 6D), without significant loss of body weight in either the WT or Y640F groups (Fig. S6B). After 7 days treatment of PecA, tumors were significantly smaller in PecA-treated groups than of vehicle groups (Fig. 6E and F). Furthermore, forced dimerization of STAT3 was also observed in PecA-treated tumor samples. STAT3 signaling was abrogated by PecA as indicated by reduced Y705 phosphorylation levels in STAT3 (Fig. 6G). We also observed significantly higher cancer cell death under PecA treatment through TUNEL staining (Figs. S6C and S6D) and Hematoxylin and eosin staining (Fig. 6H). Taken together, these results demonstrated that PecA exhibits profound anti-tumor efficacy in vivo against ALCL neoplasms with constitutively activated STAT3 or STAT3Y640F.
Figure 6.
PecA suppresses tumor growth in xenograft models of STAT3wt and STAT3Y640F lymphoma. (A‒C) Lymphoma Jeko-1 xenograft tumor models were administrated with the vehicle, 20 mg/kg PecA once daily (qd) or twice daily (bid) by tail vein injection. Data are presented as mean ± SEM (n = 6). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, NS, not significant. The tumor volumes were monitored daily (A). Tumors were separated. Weights of tumors (B) and representative photos (C) for each experimental group are shown. (D‒H) xenograft mouse models harboring wildtype or Y640F STAT3 transfected Karpas299 cells were treated with vehicle or 20 mg/kg PecA twice a day by tail vein injection. The tumor volumes and body weights were measured every day (D). The tumors were harvested 7 days after drug treatment. Weights of tumors (E) and representative photos (F) for each experimental group are shown. The levels of STAT3 and p-STAT3 were analyzed by Western blot (G). H&E staining was shown (H), scale bar = 50 μm.
4. Discussion
Novel chemotypes may result in unexpected, and possibly unprecedented bioactivity or can identify previously unrecognized mechanisms of action. The panepocyclinol-type compounds are characterized by a densely functionalized core scaffold consisting of a 6/6/6/5-fused tetracyclic carbon skeleton. This scaffold originates from the syn-[4+2] Diels‒Alder cyclo-addition of monomeric epoxyquinols, and could then be decorated through chlorination, methylation, acetylation, or hydration reactions, thereby diversifying the panepocyclinol scaffold into panepocyclinols A‒H. In our previous work, we revealed the biosynthetic route of epoxyquinol-based dimeric compounds and demonstrated their diverse biological activities, such as promoting embryonic stem cell (ESCs) differentiation, suppressing inflammatory effect, and so on22. In the present study, we delved into the molecular mechanism of PecA's anti-tumor activity and revealed the di-covalent modification of STAT3 by PecA. Having reactive moieties of α-chloro unsaturated ketone and α, β-epoxide ketone, PecA covalently interacts with separate STAT3 monomers in the pocket formed by the SH2 domain of STAT3 dimers. This unique di-covalent modification on STAT3 dimers stems from the inherent dimeric nature of PecA, which further illustrates that structurally complex and diverse dimeric compounds can play a prominent role in the discovery of chemical probes and drug candidates.
Many disease-associated proteins require higher-order assembly to activate their function. Previous strategies to disrupt protein-protein interaction (PPI) have long been applied in drug discovery. In the last ten years, the field of PPI modulation has rapidly expanded to a new direction of molecular glues or PPI stabilization. For example, Wojtaszek et al.51 demonstrated JH-RE-06 acted as a dimerization inducer of REV1, thus disrupting the REV1–REV7 interaction and DNA polymerase zeta recruitment to sensitize the tumor to cisplatin. Li et al.52 reported that NVS-STG2 elicited potent STING-mediated immune responses in cells and antitumor activities by inducing high-order oligomerization of STING. Together, molecular glue-like small molecules have the ability to target dynamic and seeming featureless protein interfaces, especially those “difficult to drug” or “yet to be drugged” targets via traditional approaches.
PecA induced STAT3 dimers formation via di-covalent modification, exerting a molecular glue-like feature. This mechanism of action by PecA is distinct from that of known SH2 domain-targeting STAT3 inhibitors which disrupt STAT3 dimerization, such as Stattic7, STA-2153, and S3I-2018. Our data also showed that PecA exhibited the most potent inhibition among the tested STAT3 inhibitors. These findings suggest the potential thermodynamical advantage of molecular glues, especially covalent molecular glues, since they usually induce the most stable ternary complex.
Targeting unique PPI interfaces also provides the possibility to improve drug selectivity. In our case, the unique binding pocket within STAT3 dimer results in high selectivity for PecA, but not other STAT family proteins. Numerous strategies have emerged for the identification of selective and potent STAT3 inhibitors for clinical use, including STAT3 degraders54 and so on. However, no STAT3-targeted drugs have been approved to date. Our work thus contributes a distinct approach to disrupting STAT3 function. Since PecA has shown profound in vivo anti-tumor efficacy against STAT3-driven ALCL neoplasms, our results indicate that PecA can serve as a promising starting point for de novo design of STAT3-targeted small molecule drugs.
By MD simulation, we found that di-covalent modification of STAT3 dimer by PecA changed the conformations of the α-helices of STAT3, thus impeding interaction between STAT3 and DNA. However, the precise binding mode was not yet defined, particularly since C718 is situated within a highly flexible region that is not represented in the available crystal structure of STAT3. Our attempts to obtain the co-crystal structure of STAT3 and PecA have also failed. Therefore, the exact binding mode of STAT3-PecA remains further investigation.
5. Conclusions
In summary, our work reveals that dimeric natural product panepocyclinol A (PecA) emerged as a selective STAT3 inhibitor. PecA inhibits binding between STAT3 and DNA by cross-linking C712/C718 residues in separate monomers with two distinct Michael receptors, resulting in a unique di-covalent modification of the STAT3 dimer. PecA exhibits profound anti-tumor efficacy in vitro and in vivo against ALCL neoplasms with constitutively activated STAT3 mutants discovered in clinic. Our work thus contributes a distinct approach for disrupting STAT3 function and indicates PecA can serve as a promising starting point for de novo design of STAT3-targeted small molecule drugs.
Author contributions
Xianming Deng, Fei Xia, and Li Li conceived the project. Li Li, Qihong Deng, and Yanling Wei performed the biological experiments, acquired and analyzed data. Yuezhou Wang and Xiaoyang Li determined the structures of natural compounds. Fei Gao, Wenhua Lian, Yunzhan Li, and Fu Gui evaluated drug efficacy in vivo. Su-Jie Zhu and Cai-Hong Yun contributed to the protein purification. Yiqiu Wang and Fei Xia performed the MD simulation study. Lei Zhang, Zhiyu Hu, Qingyan Xu and Xiaobing Wu contributed to the analysis and interpretation of biological data. Dawang Zhou, Lanfen Chen, and Jianming Zhang gave important advice. Li Li, Yuezhou Wang, and Xianming Deng wrote the manuscript with comments from all authors. All authors read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
We thank Prof. Qiao Wu at Xiamen University for providing STAT3 plasmids. We also acknowledge the support of the NYU-ECNU Center for Computational Chemistry at NYU Shanghai as well as the ECNU Public Platform for Innovation (001) for providing computer time. This work was supported by grants from the National Key R&D Program and the National Natural Science Foundation of China (2022YFC2804100, 22025702, 82021003, 91853203, 82151211 to Xianming Deng, 82073874 to Li Li and 81903491 to Yuezhou Wang, China), the Fundamental Research Funds for the Central Universities of China (No. 20720230070 to Li Li), Xiamen Southern Oceanographic Center (No. 23YYZP020QCB26 to Xianming Deng, China), the Project “111” sponsored by the State Bureau of Foreign Experts and Ministry of Education of China (#BP2018017), and the New Cornerstone Science Foundation through the XPLORER PRIZE.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2024.10.001.
Contributor Information
Li Li, Email: lli@xmu.edu.cn.
Fei Xia, Email: fxia@chem.ecnu.edu.cn.
Xianming Deng, Email: xmdeng@xmu.edu.cn.
Appendix A. Supporting information
The following is the Supporting information to this article:
References
- 1.Mei G., Di Venere A., Rosato N., Finazzi-Agro A. The importance of being dimeric. FEBS J. 2005;272:16–27. doi: 10.1111/j.1432-1033.2004.04407.x. [DOI] [PubMed] [Google Scholar]
- 2.Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koskela H.L., Eldfors S., Ellonen P., van Adrichem A.J., Kuusanmaki H., Andersson E.I., et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366:1905–1913. doi: 10.1056/NEJMoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasan A., Kern W., Grossmann V., Haferlach C., Haferlach T., Schnittger S. STAT3 mutations are highly specific for large granular lymphocytic leukemia. Leukemia. 2013;27:1598–1600. doi: 10.1038/leu.2012.350. [DOI] [PubMed] [Google Scholar]
- 5.Ohgami R.S., Ma L., Monabati A., Zehnder J.L., Arber D.A. STAT3 mutations are present in aggressive B-cell lymphomas including a subset of diffuse large B-cell lymphomas with CD30 expression. Haematologica. 2014;99:e105–e107. doi: 10.3324/haematol.2013.101543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi M., He R., Feldman A.L., Viswanatha D.S., Jevremovic D., Chen D., et al. STAT3 mutation and its clinical and histopathologic correlation in T-cell large granular lymphocytic leukemia. Hum Pathol. 2018;73:74–81. doi: 10.1016/j.humpath.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Schust J., Sperl B., Hollis A., Mayer T.U., Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Siddiquee K., Zhang S., Guida W.C., Blaskovich M.A., Greedy B., Lawrence H.R., et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Q., Zhong Y., Dong H., Zheng Q., Shi S., Zhu K., et al. Revisiting signal transducer and activator of transcription 3 (STAT3) as an anticancer target and its inhibitor discovery: where are we and where should we go? Eur J Med Chem. 2020;187 doi: 10.1016/j.ejmech.2019.111922. [DOI] [PubMed] [Google Scholar]
- 10.Liu J., Liu A., Hu Y. Enzymatic dimerization in the biosynthetic pathway of microbial natural products. Nat Prod Rep. 2021;38:1469–1505. doi: 10.1039/d0np00063a. [DOI] [PubMed] [Google Scholar]
- 11.Stanton B.Z., Chory E.J., Crabtree G.R. Chemically induced proximity in biology and medicine. Science. 2018;359 doi: 10.1126/science.aao5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadden M.K., Blagg B.S. Dimeric approaches to anti-cancer chemotherapeutics. Anticancer Agents Med Chem. 2008;8:807–816. doi: 10.2174/187152008785914743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mak S., Li W., Fu H., Luo J., Cui W., Hu S., et al. Promising tacrine/huperzine A-based dimeric acetylcholinesterase inhibitors for neurodegenerative disorders: from relieving symptoms to modifying diseases through multitarget. J Neurochem. 2021;158:1381–1393. doi: 10.1111/jnc.15379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Listunov D., Linhares B.M., Kim E., Winkler A., Simes M.L., Weaver S., et al. Development of potent dimeric inhibitors of GAS41 YEATS domain. Cell Chem Biol. 2021;28:1716–1727. doi: 10.1016/j.chembiol.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber S.L. The rise of molecular glues. Cell. 2021;184:3–9. doi: 10.1016/j.cell.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Spencer D.M., Wandless T.J., Schreiber S.L., Crabtree G.R. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 17.Di Stasi A., Tey S.K., Dotti G., Fujita Y., Kennedy-Nasser A., Martinez C., et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIntosh J.A., Liu Z., Andresen B.M., Marzijarani N.S., Moore J.C., Marshall N.M., et al. A kinase-cGAS cascade to synthesize a therapeutic STING activator. Nature. 2022;603:439–444. doi: 10.1038/s41586-022-04422-9. [DOI] [PubMed] [Google Scholar]
- 19.Benkovics T., Peng F., Phillips E.M., An C., Bade R.S., Chung C.K., et al. Diverse catalytic reactions for the stereoselective synthesis of cyclic dinucleotide MK-1454. J Am Chem Soc. 2022;144:5855–5863. doi: 10.1021/jacs.1c12106. [DOI] [PubMed] [Google Scholar]
- 20.D'Angelo K.A., Schissel C.K., Pentelute B.L., Movassaghi M. Total synthesis of himastatin. Science. 2022;375:894–899. doi: 10.1126/science.abm6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niggemeyer G., Knyazeva A., Gasper R., Corkery D., Bodenbinder P., Holstein J.J., et al. Synthesis of 20-membered macrocyclic pseudo-natural products yields inducers of LC3 lipidation. Angew Chem Int Ed Engl. 2022;61 doi: 10.1002/anie.202114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L., Wang Y., Chen N., Li X., Li H., Jin L., et al. Exploring diversity through dimerization in natural products by a rational tandem mass-based molecular network strategy. Org Lett. 2023;25:4016–4021. doi: 10.1021/acs.orglett.3c01038. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z.L., Meng J.M., Cao Y., Yin J.L., Fang R.Q., Fan S.B., et al. A high-speed search engine pLink 2 with systematic evaluation for proteome-scale identification of cross-linked peptides. Nat Commun. 2019;10:3404. doi: 10.1038/s41467-019-11337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joung J., Konermann S., Gootenberg J.S., Abudayyeh O.O., Platt R.J., Brigham M.D., et al. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat Protoc. 2017;12:828–863. doi: 10.1038/nprot.2017.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrödinger L. 2021. The PyMOL molecular graphics system, Version 2.5.0. [Google Scholar]
- 26.Sastry G.M., Adzhigirey M., Day T., Annabhimoju R., Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aid Mol Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- 27.Maestro. [software] Schrödinger, LLC, New York, NY2018. Available from: http://www.schrodinger.com. The data of access is from Dec.3 of 2019 to Dec 1 of 2024.
- 28.Bayly C.I., Cieplak P., Cornell W., Kollman P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J Phys Chem. 1993;97:10269–10280. [Google Scholar]
- 29.Becke A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 30.Lee C., Yang W., Parr R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 31.Gaussian 09. Version revision D.01. Gaussian, Inc., Wallingford, CT2013.
- 32.Wang J., Wolf R.M., Caldwell J.W., Kollman P.A., Case D.A. Development and testing of a general amber force field. J Comput Chem. 2004;25:1157–1174. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 33.Tian C., Kasavajhala K., Belfon K.A.A., Raguette L., Huang H., Migues A.N., et al. ff19SB: amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J Chem Theor Comput. 2020;16:528–552. doi: 10.1021/acs.jctc.9b00591. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen W.L., Chandrasekhar J., Madura J.D., Impey R.W., Klein M.L. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 35.Price D.J., Brooks C.L., 3rd A modified TIP3P water potential for simulation with Ewald summation. J Chem Phys. 2004;121:10096–10103. doi: 10.1063/1.1808117. [DOI] [PubMed] [Google Scholar]
- 36.Case D.A., Berryman J.T., Betz R.M., Cerutti D.S., Cheatham I.I.I.T.E., Darden T.A., et al. University of California; San Francisco: 2020. Amber.https://ambermd.org/ [software], Available from: [Google Scholar]
- 37.Miyamoto S., Kollman P.A. Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J Comput Chem. 1992;13:952–962. [Google Scholar]
- 38.Darden T., York D., Pedersen L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 39.Pastor R.W., Brooks B.R., Szabo A. An analysis of the accuracy of Langevin and molecular dynamics algorithms. Mol Phys. 1988;65:1409–1419. [Google Scholar]
- 40.Berendsen H.J.C., Postma J.P.M., van Gunsteren W.F., DiNola A., Haak J.R. Molecular dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–3690. [Google Scholar]
- 41.Srinivasan J., Cheatham T.E., Cieplak P., Kollman P.A., Case D.A. Continuum solvent studies of the stability of DNA, RNA, and phosphoramidate−DNA helices. J Am Chem Soc. 1998;120:9401–9409. [Google Scholar]
- 42.Miller B.R., III, McGee T.D., Jr., Swails J.M., Homeyer N., Gohlke H., Roitberg A.E. MMPBSA.py: an efficient program for end-state free energy calculations. J Chem Theor Comput. 2012;8:3314–3321. doi: 10.1021/ct300418h. [DOI] [PubMed] [Google Scholar]
- 43.Karaman M.W., Herrgard S., Treiber D.K., Gallant P., Atteridge C.E., Campbell B.T., et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 44.Stark G.R., Darnell J.E., Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zamo A., Chiarle R., Piva R., Howes J., Fan Y., Chilosi M., et al. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene. 2002;21:1038–1047. doi: 10.1038/sj.onc.1205152. [DOI] [PubMed] [Google Scholar]
- 46.DeCaprio J., Kohl T.O. Cross-linking antibodies to beads with disuccinimidyl suberate (DSS) Cold Spring Harb Protoc. 2019;2019 doi: 10.1101/pdb.prot098632. Available from: [DOI] [PubMed] [Google Scholar]
- 47.Beebe J.D., Liu J.Y., Zhang J.T. Two decades of research in discovery of anticancer drugs targeting STAT3, how close are we? Pharmacol Therapeut. 2018;191:74–91. doi: 10.1016/j.pharmthera.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Furtek S.L., Backos D.S., Matheson C.J., Reigan P. Strategies and approaches of targeting STAT3 for cancer treatment. Acs Chem Biol. 2016;11:308–318. doi: 10.1021/acschembio.5b00945. [DOI] [PubMed] [Google Scholar]
- 49.Crescenzo R., Abate F., Lasorsa E., Tabbo F., Gaudiano M., Chiesa N., et al. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell. 2015;27:516–532. doi: 10.1016/j.ccell.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahmarvand N., Nagy A., Shahryari J., Ohgami R.S. Mutations in the signal transducer and activator of transcription family of genes in cancer. Cancer Sci. 2018;109:926–933. doi: 10.1111/cas.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wojtaszek J.L., Chatterjee N., Najeeb J., Ramos A., Lee M., Bian K., et al. A small molecule targeting mutagenic translesion synthesis improves chemotherapy. Cell. 2019;178:152–159. doi: 10.1016/j.cell.2019.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J., Canham S.M., Wu H., Henault M., Chen L., Liu G., et al. Activation of human STING by a molecular glue-like compound. Nat Chem Biol. 2024;20:365–372. doi: 10.1038/s41589-023-01434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song H., Wang R., Wang S., Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai L., Zhou H., Xu R., Zhao Y., Chinnaswamy K., McEachern D., et al. A potent and selective small-molecule degrader of STAT3 achieves complete tumor regression in vivo. Cancer Cell. 2019;36:498–511. doi: 10.1016/j.ccell.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.