Abstract
The innate immune sensor NLRP3 inflammasome overactivation is involved in the pathogenesis of ulcerative colitis. PGAM5 is a mitochondrial phosphatase involved in NLRP3 inflammasome activation in macrophages. However, the role of PGAM5 in ulcerative colitis and the mechanisms underlying PGAM5 regulating NLRP3 activity remain unknown. Here, we show that PGAM5 deficiency ameliorates dextran sodium sulfate (DSS)-induced colitis in mice via suppressing NLRP3 inflammasome activation. By combining APEX2-based proximity labeling focused on PGAM5 with quantitative proteomics, we identify NEK7 as the new binding partner of PGAM5 to promote NLRP3 inflammasome assembly and activation in a PGAM5 phosphatase activity-independent manner upon inflammasome induction. Interfering with PGAM5–NEK7 interaction by punicalagin inhibits the activation of the NLRP3 inflammasome in macrophages and ameliorates DSS-induced colitis in mice. Altogether, our data demonstrate the PGAM5–NEK7 interaction in macrophages for NLRP3 inflammasome activation and further provide a promising therapeutic strategy for ulcerative colitis by blocking the PGAM5–NEK7 interaction.
Key words: PGAM5, APEX2 proximity labeling, Protein–protein interaction, NEK7, NLRP3 inflammasome, Punicalagin, Macrophage, Colitis
Graphical abstract
Punicalagin directly binds to PGAM5 and blocks the interaction between PGAM5 and NEK7 to inhibit NLRP3 inflammasome activation in a PGAM5 phosphatase activity-independent manner, resulting in amelioration of ulcerative colitis.

1. Introduction
The pattern recognition receptors (PRRs) are activated by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) in innate immunity. Innate immunity serves as an incredibly effective barrier to a wide range of insults by rapidly recognizing pathogens and tissue damage. As a typical PRR, nucleotide-binding domain, leucine-rich repeat-containing receptor, pyrin domain-containing-3 (NLRP3) inflammasome is triggered and initiated by infection or cellular stress recognized by the sensors, including PAMPs and DAMPs1, 2, 3, 4. Not only exogenous microbial ligands but also cellular metabolic products and cellular damages can initiate and activate NLRP3 inflammasome3,5. NLRP3 inflammasome is a multi-protein complex, and canonical activation of NLRP3 inflammasome requires two steps. The priming step which is mediated by Toll-like receptor (TLR) agonists, such as LPS, induces activation of NF-κB signaling pathway and then induces the expression of NLRP3, pro-interleukin-1β (pro-IL-1β) and pro-IL-186. The assembly step of NLRP3 inflammasome is triggered by multiple activating signaling, including adenosine triphosphate (ATP), monosodium crystals (MSU), particulates, nigericin, silica and amyloid3. Beyond that, some bacterial pathogens have been reported to activate NLRP3 inflammasome, such as Salmonella typhimurium and Listeria monocytogenes7. Upon NLRP3 inflammasome activation, NIMA related kinase 7 (NEK7) C-lobe interacts directly with the leucine-rich repeat (LRR) domain of NLRP3 and promotes NLRP3 oligomerization8, then recruits its adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) via their pyrin domain (PYD)–PYD interaction, which leads to the formation of the speck of ASC. Subsequently, ASC interacts with pro-caspase-1 via their (caspase activation and recruitment domain) CARD–CARD domains and induces autocleavage and maturation of pro-caspase-1, which further enzymatically cleaves pro-IL-1β and pro-IL-18 into their mature forms, IL-1β and IL-18, respectively, thereby initiating pro-inflammatory cell damage and death9, 10, 11, 12. Appropriate activation of NLRP3 inflammasome can assist host cells, tissues and organs to eliminate a limited amount of PAMPs and DAMPs, thereby maintaining cellular and organismal homeostasis13,14. While excessive activation of NLRP3 inflammasome may result in severe immune inflammatory response, and even lead to overt cell death, tissue injury and inflammatory diseases, such as Alzheimer's disease (AD), diabetes, acute lung injury, acute respiratory distress syndrome (ARDS), ischemia-reperfusion injury and ulcerative colitis, etc15. Therefore, the activity of NLRP3 inflammasome must be strictly monitored by the hose cells, and a better understanding of the mechanisms that control NLRP3 inflammasome activation would be beneficial in the development of novel therapeutic strategies for the treatment of inflammatory diseases.
Ulcerative colitis is a chronic and idiopathic inflammatory disease affecting the colon, characterized by relapsing, remitting and diffusing mucosal inflammation, starting in the rectum and extending to proximal segments of the colon16. The clinical symptoms of ulcerative colitis are mainly manifested in abdominal pain, bloody diarrhea, mucopurulent, etc. Ulcerative colitis most afflicts adults aged at 30–40 years resulting in disability, and its incidence is rising worldwide16. The ultimate aim of the treatment is to induce and maintain remission, defined as resolution of symptoms and endoscopic healing17. The drugs currently available for the treatment of ulcerative colitis mainly include mesalazine, immune-suppressants, biological agents, glucocorticoids and probiotics. However, those drugs are limited due to different side effects and high relapse rates18. The main clinical therapeutic target in ulcerative colitis is the IL-12/IL-23 receptor19. Therefore, it is very urgent to discover new targets and the corresponding lead compounds for patients with ulcerative colitis.
The regulation of NLRP3 inflammasome assembly and activation remains incompletely understood. Phosphoglycerate mutase family member 5 (PGAM5) is a mitochondrial Ser/Thr phosphatase normally located in mitochondria and regulates mitochondria dynamics, mitophagy, programmed cell death, inflammation and immune response via protein–protein interactions (PPIs) and/or its specific Ser/Thr protein phosphatase activity20. Recent study had shown that PGAM5 is a novel regulator of inflammasome and caspase-1 activity21, suggesting that targeting PGAM5 may be a novel and viable therapeutic strategy in NLRP3 inflammasome-driven inflammatory diseases, such as ulcerative colitis. NLRP3 inflammasome activation is fine-tuned by post-translational modifications, including PPI, phosphorylation, alkylation, S-nitrosylation, adenosine diphosphate ribosylation, nitrosylation, ubiquitination, and SUMOylation3,6,22, 23, 24, 25. In this study, in search of PGAM5-binding proteins using APEX2 proximity labeling, we identify a new PGAM5-binding adaptor protein NEK7. We revealed that PGAM5 promotes NLRP3 inflammasome activation in a phosphatase activity-independent manner via interacting with NEK7 to further promote the NEK7 binding with NLRP3, resulting in promoting the assembly and activation of NLRP3 inflammasome. Interestingly, we found that interfering with the interaction between PGAM5 and NEK7 by a natural product punicalagin could effectively block the interaction between NEK7 and NLRP3, and eventually inhibit the NLRP3 inflammasome hyperactivation. In the dextran sodium sulfate (DSS)-induced ulcerative colitis mice model, PGAM5 deficiency or pharmacological intervention of PGAM5 and NEK7 interaction resulted in lower gut inflammation with decreased NLRP3 inflammasome activation, indicating the significant role of PGAM5 in NLRP3 inflammasome activation and ulcerative colitis.
2. Materials and methods
2.1. Reagent and resource
Reagent and resource used in this study are available in the Supporting Information.
2.2. Experimental model and subject details
Mouse works described in this manuscript have been approved and conducted under the oversight of the Administration Committee of Experimental Animals in Jiangsu Province and the Ethics Committee of China Pharmaceutical University (2020-03-010). Recombinant DNA and mammalian cell line studies comply with all relevant ethical regulations supervised by the Institutional Biosafety Committee at China Pharmaceutical University.
2.2.1. Animals and treatments
C57BL/6J mice were purchased from Hangzhou Ziyuan Laboratory Animal Technology Co., Ltd. Pgam5 knockout mice were constructed as previously reported21 and purchased from Shanghai Model Organisms Center, Inc. (Shanghai, China). Nlrp3 knockout mice were purchased from Shanghai Model Organisms Center, Inc. (Shanghai, China). After a week of acclimation, the mice were raised in a standard SPF housing environment under 12 h light/dark cycles with ad libitum access to rodent chow and water or DSS. For the DSS-induced ulcerative colitis model, the six to eight-week-old mice were fed with 3% (w/v) DSS in drinking water for one week.
To investigate the effect of PGAM5 on ulcerative colitis, the wide-type (WT) and Pgam5 knockout mice were subjected to 3% DSS for 7 days to induce ulcerative colitis in vivo. Then the body weight, diarrhea and rectal bleeding were weighed or measured every day, and the disease activity index (DAI) score was calculated using the well-established system26. After these mice were sacrificed, the colons were isolated for length measurement, hematoxylin and eosin (H&E) staining and immunohistochemistry.
To investigate whether the effect of PGAM5 on ulcerative colitis depends on gut microbiota, the fecal microbiota transplantation experiments were performed according to previous report27. Briefly, WT mice were transplanted fecal microbiota from WT or Pgam5 deficiency mice for 14 days consecutively, respectively, and were subjected to 3% DSS for 7 days to induce ulcerative colitis. Then the body weight, diarrhea and rectal bleeding were weighed or measured every day, and the DAI score was calculated using the well-established system26. After these mice were sacrificed, the colons were isolated for length measurement and H&E staining.
To investigate the effect of punicalagin on ulcerative colitis, the WT C57BL/6J mice were randomly divided into the following six groups: normal group, DSS group, punicalagin (10, 20, and 50 mg/kg) groups and mesalazine (200 mg/kg) group. Then the mice were subjected to 3% DSS for 7 days, and the punicalagin or mesalazine were orally administrated daily for 7 days. The body weight, diarrhea and rectal bleeding were weighed or measured every day, and the DAI score was calculated using the well-established system26. After these mice were sacrificed, the serums were collected, and colons were isolated for length measurement, H&E staining, immunohistochemistry, and subjected to immunoblotting analysis.
To investigate whether the effect of punicalagin on ulcerative colitis depends on PGAM5 and NLRP3, the WT and Pgam5 or Nlrp3 knockout mice were randomly divided into four groups. Then the mice were subjected to 3% DSS for 7 days, and the punicalagin (50 mg/kg) was orally administrated daily for 7 days. The body weight, diarrhea and rectal bleeding were weighed or measured every day, and the DAI score was calculated using the well-established system26. After these mice were sacrificed, the colons were isolated for length measurement and H&E staining.
To investigate the effect of NEK7 on ulcerative colitis and whether the therapeutic effect of punicalagin on ulcerative colitis depends on NEK7, the WT mice deprived of their macrophages and reconstituted of normal control or Nek7 knockdown macrophages were subjected to 3% DSS for 7 days in the absence or presence of punicalagin treatment (50 mg/kg, intragastric administration, once per day), respectively. The body weight, diarrhea and rectal bleeding were weighed or measured every day, and the DAI score was calculated using the well-established system26. After these mice were sacrificed, the colons were isolated for length measurement and H&E staining.
2.2.2. Disease activity index assessment
To assess the severity of mouse ulcerative colitis, the body weight, diarrhea and rectal bleeding were measured every day, and the DAI score was calculated according to the previous report26,28. The details of the DAI score are briefly described as follows, 1) body weight loss: 0 points = none, 1 point = 1%–5% loss, 2 points = 5%–10% loss, 3 points = 10%–20% loss, 4 points = more than 20% loss. 2) diarrhea: 0 points = normal, 2 points = loose stools, 4 points = watery diarrhea. 3) rectal bleeding: 0 points = no bleeding, 2 points = slight bleeding, 4 points = gross bleeding.
2.2.3. H&E staining
On Day 7, the mice were sacrificed, and the colons were collected. The distal colons were then fixed with 4% paraformaldehyde, embedded into paraffin, and sectioned into 3 μm for H&E staining. We scanned the slices with light microscopy (Olympus) and the histopathological scores of the image obtained were determined as previously reported26,29.
2.2.4. Immunohistochemistry
On Day 7, the mice were sacrificed, and the colons were collected. The distal colons were then fixed with 4% paraformaldehyde, embedded into paraffin, and sectioned into 3-μm-thick sections. Immunostaining was performed using the streptavidin-peroxidase immunohistochemical method as previously reported30. Briefly, the sections were incubated overnight at 4 °C with a rabbit monoclonal F4/80-specific antibody (1:100). As a blank control, phosphate-buffered saline was used in the place of the antibody. The sections were then incubated with biotin-labeled secondary antibodies at 37 °C for 30 min and were then developed with diaminobenzidine. The images of the slices were obtained by light microscopy.
2.3. Cell culture and generation of murine BMDMs
Immortalized bone marrow-derived macrophages (iBMDMs) purchased from YaJi Biological (Shanghai Yaji Biotechnology Co., Ltd.) were cultured in Dulbecco's modified Eagle's medium (DMEM, high glucose) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1% penicillin (100 U/mL) and streptomycin (100 μg/mL) (Beyotime). HEK293T cells (ATCC) were cultured in DMEM/F-12 supplemented with 10% FBS and 1% penicillin–streptomycin. Primary bone marrow-derived macrophages (BMDMs) were generated from 6–8-week-old C57BL/6J mice as described previously31,32, with minor optimizations. The femurs and tibia of mice were collected and cleaned bones were flushed with DMEM three times. The collected bone marrow suspension was then washed with DMEM and plated in DMEM supplemented with 10% FBS, 1% penicillin–streptomycin and 20 ng/mL macrophage colony-stimulating factor (M-CSF) (R&D Systems) for 6 days, and the culture medium was exchanged every 3 days, after which cells were counted and plated at 0.5 × 106 cells/mL unless otherwise stated.
2.4. Inflammasome activation
2.4.1. NLRP3 inflammasome activation
NLRP3 inflammasome was activated in primary BMDMs primed with LPS (100 ng/mL) for 3 h, and the inflammasome was further assembled using ATP (5 mmol/L) for 45 min, nigericin (10 μmol/L) for 1 h or MSU (600 μg/mL) for 6 h, according to previous reports33,34. For RNA-sequencing in iBMDMs, the cells were primed with LPS (100 ng/mL) for 3 h, and then further assembled using ATP (5 mmol/L) for 45 min. To investigate the effect of compounds in NLRP3 inflammasome activation, the LPS-primed cells were incubated with compounds for 3 h and followed by stimulation with ATP for another 45 min, nigericin for another 1 h, or MSU for another 6 h to activate NLRP3 inflammasome.
2.4.2. AIM2 inflammasome activation
AIM2 inflammasome was activated in primary BMDMs primed with LPS (100 ng/mL) for 3 h, and the inflammasome was further assembled using Poly (dA:dT) (2 μg/mL) for 6 h according to previous report35. To investigate the effect of compounds in AIM2 inflammasome activation, the LPS-primed cells were incubated with compounds for 3 h and followed by stimulation with Poly(dA:dT) for another 6 h to activate AIM2 inflammasome.
2.4.3. NLRP1 inflammasome activation
NLRP1 inflammasome was activated in primary BMDMs primed with LPS (100 ng/mL) for 3 h, and the inflammasome was further assembled using muramyl dipeptide (MDP) (500 ng/mL) for 6 h according to previous report36. To investigate the effect of compounds in NLRP1 inflammasome activation, the LPS-primed cells were incubated with compounds for 3 h and followed by stimulation with MDP for another 6 h to activate NLRP1 inflammasome.
2.4.4. Non-canonical inflammasome activation
For non-canonical inflammasome stimulation, BMDMs were primed overnight with 1 mg/mL Pam3CSK4, followed by transfection of 2 μg/mL LPS using HighGene DNA transfection reagent (Abclonal) for 16 h with or without punicalagin treatment.
2.5. APEX2 proximity labeling
APEX2 proximity labeling was performed as previously described37. In brief, HEK293T cells expressing PGAM5-FLAG-APEX2 fusion proteins cultured in DMEM/F12 complete medium were incubated with 500 μmol/L biotin-phenol (BP) in pre-warmed complete medium for 30 min at 37 °C in CO2 incubator, then treated with H2O2 (1 mmol/L) at room temperature for 1 min. Subsequently, cells were washed thrice with fresh quencher solution (10 mmol/L sodium azide, 10 mmol/L sodium ascorbate and 5 mmol/L Trolox solution in Dulbecco's phosphate-buffered saline (DPBS)). After washes, cells were lysed in fresh RIPA lysis buffer (50 mmol/L Tris, 150 mmol/L NaCl, 0.1% (w/v) SDS, 0.5% (w/v) sodium deoxycholate and 1% (v/v) Triton X-100, pH 7.5) supplemented with 1 × protease inhibitor cocktail (Thermo Scientific), 1 mmol/L PMSF (Merck) and quenchers (10 mmol/L sodium azide, 10 mmol/L sodium ascorbate and 5 mmol/L Trolox) through gentle pipetting for 10 min on ice. Whole-cell lysates were then centrifuged at 14,000 × g for 10 min at 4 °C to cleat cellular debris. The Pierce 660 nm protein assay kit (Thermo Scientific) was used to quantify the amount of protein in each sample.
To pull down biotinylated proteins by PGAM5-FLAG-APEX2 proximity labeling, streptavidin magnetic beads (Cytiva) were pre-washed twice with 1 mL of RIPA lysis buffer for each. Cleared whole-cell lysates were incubated with streptavidin magnetic beads at room temperature for 1 h. Then, the beads were washed twice with RIPA lysis buffer, once with 1 mol/L KCl, once with 0.1 mol/L Na2CO3, once with 2 mol/L urea in 10 mmol/L Tris–HCl, pH 8.0, and twice with RIPA lysis buffer. Biotinylated proteins were eluted by boiling the beads in 30 μL of 3 × protein loading buffer (6 ×: 10.5 mL dH2O, 10.5 mL 1 mol/L Tris–HCl (pH 6.8), 10.8 mL glycerol, 3.0 g sodium dodecyl sulfate, 2.79 g DTT and 3.6 mg bromophenol blue) supplemented with 5 mmol/L biotin and 20 mmol/L DTT for 10 min. The eluates were collected by pelleting the beads using a magnetic rack (MCE). For Western blotting analysis, the eluates were loaded, run and separated into 12% Tris-SDS-PAGE gels, the gels were stained with Coomassie blue, silver stain to visualize the total proteins in each lane.
2.6. Dimethyl labeling
PGAM5-FLAG-APEX2 proximity labeling was performed as mentioned above. Then, the biotinylated proteins were enriched and digested on magnetic beads with sequencing-grade modified trypsin (Promega). Digested peptides derived from labeled samples were treated with heavy isotope-labeled D13CDO and NaBH3CN to methylate –NH2 groups, consequently leading to a mass shift of +34.0631 Da per addition. In parallel, digested peptides from the control sample were treated with light isotope-labeled HCHO and NaBH3CN, resulting in N-dimethylation with a mass shift of +28.0313 Da for each substituted group. After the addition of formaldehyde, 4 μL of freshly prepared 0.6 mol/L NaBH3CN was added. After incubation at room temperature for 1 h, the dimethyl labeling reaction was terminated by adding ammonium hydroxide (16 μL, 1% in water). Subsequently, 8 μL of 5% (v/v) formic acid was added to acidify the reaction. The heavy and light samples were mixed in a 1:1 ratio and desalted before injection on the LC–MS/MS.
2.7. Recombinant protein purification and in vitro PGAM5 phosphatase assay
Recombinant His-tagged PGAM5 proteins were purified as described previously38. Briefly, His-tagged proteins (ΔN21 PGAM5, ΔN90 PGAM5, ΔN21 PGAM5 (C168A), ΔN21 PGAM5 (C229A) and ΔN21 PGAM5 (C239A)) were purified from Escherichia coli BL21 (DE3) competent cells with Ni-NTA resin (GE Healthcare), eluted by imidazole and further purified by size-exclusion chromatography (SEC). Purified recombinant proteins were dialyzed against protein buffer containing 20 mmol/L HEPES and 150 mmol/L NaCl with pH 7.2–7.4 using a 10 kDa cutoff concentrator (Millipore) to meet the needs of in vitro PGAM5 phosphatase assay and surface plasmon resonance (SPR) assay.
For in vitro PGAM5 phosphatase assay, compounds or DMSO diluted in reaction buffer (250 mmol/L imidazole, pH 7.2, 1 mmol/L EGTA, 0.1% β-mercaptoethanol, 0.5 mg/mL BSA) were mixed and incubated with equal volume of ΔN21 PGAM5 (The finial concentration was 0.5 μmol/L) for 20 min at 25 °C, then 100 μmol/L of Ser/Thr phosphopeptide (NFEDH(pSER)APPSP) was added and mixer to each reaction followed by incubation for another 20 min at 25 °C. BIOMOL Green (Enzo Life Sciences) was added and mixed to each reaction. Upon brief incubation for 20 min at 25 °C, the PGAM5 phosphatase activity was related quantified spectrophotometrically at 620 nm.
2.8. SPR assay
The binding kinetics or affinity of compounds with PGAM5 was assessed via SPR using a Biacore T200 instrument and manufacturer-provided software (GE Healthcare), and all measurements were performed at 25 °C. The CM5 chips were activated with 75 mg/mL N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride and 10 mg/mL N-hydroxysuccinimide. ΔN21 PGAM5 and ΔN90 PGAM5 were diluted to 10 μg/mL with 10 mmol/L sodium acetate (pH 5.0), and then covalently immobilized on CM5 chips by amino coupling. The protein coupling solutions were injected over the activated CM5 chip surface to achieve an immobilization level of about 10,000 resonance units (RU), and a blank surface which was similarly treated but without immobilization with PGAM5 protein solutions was used as a reference surface flow cell. The coupling procedure was run at the flow rate of 10 μL/min. Binding kinetics or affinity measurements were performed at flow rate of 30 μL/min using PBS-P+ buffer, and the running parameters were 90 s of binding with compound or DMSO solution, 90 s of dissociation with PBS-P+ buffer, and 180 s of regeneration with Glycine-HCl (pH 1.5) buffer. The values of RU responses were recorded, and sensor grams were processed and analyzed using Biacore T200 control and evaluation software and the binding curves were fitted to determine the equilibrium dissociation constant (KD).
2.9. Target engagement analysis
For drug affinity responsive target stability (DARTS) assay experiments, BMDMs or HEK293T lysates were used. BMDMs were lysed with NP-40 lysis buffer and the supernatant was collected by centrifugation at 14,000 × g for 10 min at 4 °C. HEK293T cells were transfected with pcDNA3.1(+)-PGAM5-Myc or pcDNA3.1(+)-PGAM5 (C239A)-Myc for 24 h, and then the cells were washed with PBS and lysed with NP-40 lysis buffer, and the supernatant was collected by centrifugation at 14,000 × g for 10 min at 4 °C. Punicalagin dissolved in 1 × TNC buffer (50 mmol/L Tris, 50 mmol/L NaCl, 10 mmol/L CaCl2, pH 7.4) was added into the cell lysate to reach the indicated concentrations and gently mixed, and further incubated for 1 h on a rotator at 20 rpm at 25 °C to allow ligand–protein target interactions sufficiently. Then, the lysates were digested by pronase (Roche, dissolved in 1 × TNC buffer) at a ratio of 1: 200 (w/w) for 30 min at 25 °C. Reactions were stopped by adding protease inhibitor cocktail, and the samples were analyzed by Western blotting.
The cellular thermal shift assay (CETSA) measures the binding efficiency between a compound and its target proteins in the cell and operates on the principle that target proteins usually stabilize when combined with a ligand39. The CETSA protocol was adapted from that of the previous literature40. Briefly, BMDMs were incubated with punicalagin (20 μmol/L) or DMSO (Sigma–Aldrich) for 3 h in CO2 humidified incubator at 37 °C. BMDMs were then harvested and washed thrice with PBS and resuspended in 1 mL of PBS containing protease inhibitor cocktail. The cells were then divided into 12 aliquots for heat treatment at the designated temperature (37–81 °C) for 3 min, followed by 3 min at 25 °C. Heated cells were freeze–thawed three times by using liquid nitrogen and a heating block set at 25 °C to lyse the cells. The lysates were centrifuged at 14,000 × g for 30 min at 4 °C to remove cellular debris, and protein aggregates and the supernatants were transferred into another microtube for Western blotting analysis.
For solvent-induced protein precipitation assay (SIP) experiments with BMDMs treated in culture, the target proteins will become more resistant to the denaturation and precipitation induced by the treatment of organic solvent (acetone/ethanol/acetic acid = 50:50:0.1, v/v/v, abbreviated as A.E.A.) after binding with punicalagin41. Briefly, BMDMs were incubated with punicalagin (20 μmol/L) or DMSO, then harvested and washed thrice with PBS as above. The cells were lysed in NP-40 lysis buffer (Thermo Scientific) and the supernatant was collected and divided into 12 aliquots followed by addition of A.E.A with ratio of 50:5:0.1 (v/v/v) to reach the final percentage of A.E.A ranging from 12% to 32%. Subsequently, the mixtures were equilibrated at 500 × g for 30 min at 37 °C, followed by centrifugation at 14,000 × g for 30 min at 4 °C to separate the soluble fraction from precipitates, and the supernatants were transferred to another microtube for Western blotting analysis.
2.10. RNA-sequencing and bioinformatics analysis
The iBMDMs were pre-incubated with DMSO or punicalagin (20 μmol/L) for 3 h, then primed with LPS (100 ng/mL, Sigma–Aldrich) for another 3 h and assembled with ATP (5 mmol/L, Sigma–Aldrich) for 45 min. The samples were used for RNA-seq library preparation. After cluster generation, transcriptome sequencing was carried out on an Illumina HiSeq™ 2500/Illumina HiSeq X Ten platform that generated raw reads. After removing adaptor sequences, ambiguous ‘N’ nucleotides, and low-quality sequences, the remaining clean reads were assembled using Trinity software as described for de novo transcriptome assembly with a reference genome. The mapped clean read number was normalized to RPKM. The edgeR package was used to determine the StringTie genes. The threshold of significant difference was |log2foldchange| ≥ 1, P < 0.05. The Gene Ontology (GO) enrichment analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and heatmap analysis were conducted at https://cloud.oebiotech.cn/task/.
2.11. Cell viability assay
LDH cytotoxicity colorimetric assay kit (Beyotime) and Cell counting kit-8 (CCK-8) (Beyotime) were used to measure cell viability after activation of NLRP3 inflammasome per manufacturer's instructions.
2.12. Small interfering RNA (siRNA) transfection
For siRNA transfection, siRNAs (GenePharma) were mixed with jetPRIME transfection reagent (Polyplus) as per the manufacturer's instructions and added to the cells at a final concentration of 80 nmol/L. After incubation with the siRNA complex for 24 h, the medium was changed, and the cells were used for further experiments. The following siRNAs were used: scramble siRNA, ACGUGACACGUUCGGAGAA; si-PGAM5 (Human), CCAUAGAGACCACCGAUAUTT; si-NEK7 (Human), CCAGAAUGAUCAAGCAUUUTT.
2.13. Plasmids
PGAM5 (NM_001170543.2)-FLAG-APEX2 cDNAs, PGAM5-Myc, PGAM5 (C239A)-Myc and PGAM5 (H105A)-Myc cDNAs were all synthesized from Sangon Biotech and cloned into CMV-driven pcDNA3.1(+) (Sangon Biotech) or pLVX-CMV-MCS-RES-ZGreen. ΔN21 PGAM5 in pET-28a(+) and ΔN90 PGAM5 in pSJ2 were cloned according to the previous report38. ΔN21 PGAM5 (C168A), ΔN21 PGAM5 (C229A) and ΔN21 PGAM5 (C239A) in pET-28a(+) point mutations were generated by site-directed mutagenesis. Lentiviral plasmids encoding shRNA targeting mouse Nek7 (shNek7-1: GCATCATTCATTGAGGATAAT, shNek7-2: GAGCTACGACAGCTAGTTAAT and shNek7-3: CACTGTAATGCAAGCATATAT) and nontargeting control shRNA (GGTTCTCCGAACGTGTCACGT) were inserted into the pLenti-U6-MCS-CMV-GFP-2A-Puro vector. All plasmids were verified by sequencing.
2.14. Lentiviral infection
Lentivirus particles were generated from co-transfection with lentiviral vectors and packaging plasmids in HEK293T cells, filtered through 0.45-μm filters, and immediately used for infections. BMDMs were incubated with lentivirus for 36 h. Then, BMDMs were subjected to further experiments.
2.15. Reconstruction of NLRP3 inflammasome system in HEK293T cells
HEK293T cells were plated in microplates at a density of 2 × 105 cells per milliliter and were incubated overnight. The cells were transfected with plasmids expressing FLAG-IL-1β (200 ng/mL) and NLRP3 inflammasome components FLAG-NLRP3 (200 ng/mL), FLAG-ASC (20 ng/mL) and FLAG-Pro caspase-1 (100 ng/mL) using jetOPTIMUS DNA transfection reagent (Polyplus). These modified cells were then co-transfected with plasmids encoding HA-NEK7 and/or PGAM5-Myc or PGAM5 (C239A)-Myc or PGAM5 (H105A)-Myc or specific siRNA to knock down endogenous NEK7 or PGAM5 as indicated. The total amount of DNA was adjusted to a concentration of 1 μg per milliliter with empty vector and these cells were washed with culture medium 24 h after transfection and were further incubated with ATP for 45 min. For compound treatment, the cells were incubated with LFHP-1c (20 μmol/L) or Punicalagin (20 μmol/L) or DMSO 6 h after transfection as indicated. Activation of NLRP3 inflammasomes in HEK293T was assessed based on immunoblotting analysis of cleaved caspase-1 in whole cell lysates by standard techniques.
2.16. Quantitative real-time reverse transcription PCR (qRT-PCR)
The total mRNA was extracted using Trizol reagent (Vazyme) according to the manufacturer's instructions. Isolated mRNA was reverse transcribed into cDNA using a cDNA synthesis kit (Vazyme). The qRT-PCR was performed on the Stepone Software v2.3 Detection System using synthetic primers and SYBR Green Master Mix (Vazyme).
2.17. ELISA
Mouse serum, colon tissue and cell culture supernatants were assayed by ELISA kit (R&D Systems) for IL-1β according to the manufacturer's instructions using a Thermo Scientific plate reader.
2.18. Immunoprecipitation
For regular immunoprecipitations, cell and colonic tissue samples were harvested and lysed in IP lysis buffer (Thermo Scientific) in the presence of protease and phosphatase inhibitor cocktail (Thermo Scientific). The lysates were centrifuged (14,000 × g, 10 min at 4 °C), and supernatants were collected. The equal amounts of protein lysates were incubated with 4 μg IgG or the indicated primary antibodies on a rotator at 20 rpm at 4 °C overnight, followed by the addition of 50 μL protein A/G magnetic beads (Bimake) at 4 °C for another 4–6 h. Subsequently, the beads were washed with IP lysis buffer 6 times, and pull-down beads were eventually boiled and analyzed by Western blotting.
For protein tag immunoprecipitations in HEK293T, cells were lysed in the same manner as above. The cell lysates were pre-cleaned with control IgG magnetic beads, and then the lysates were incubated with Myc or HA-tag magnetic beads (Beyotime) as indicated on a rotator overnight at 4 °C. Then, the magnetic beads were washed with IP lysis buffer 7 times followed by the addition of 60 μL SDS-PAGE sample loading buffer and boiled and analyzed by immunoblotting.
For streptavidin beads-based pull-down, PGAM5-FLAG-APEX2 proximity labeling was performed as mentioned above, then the biotinylated proteins were pulled down and the eluates were analyzed by immunoblotting using indicated antibodies.
2.19. In vitro pull-down assay
His-tagged PGAM5 (5 μg/mL) was mixed with 5 μg/mL full-length GST-NEK7 in buffer containing 30 mmol/L HEPES at pH 7.5, 150 mmol/L NaCl, and incubated for 30 min at room temperature. The mixture was further incubated for 1 h with 50 μL Anti-GST Magnetic Beads (Beyotime) and washed six times with 1 mL of the same buffer, followed by the addition of 60 μL SDS-PAGE sample loading buffer, and the pull-down beads were eventually boiled and analyzed by Western blotting.
2.20. Western blotting
Cell and colonic tissue proteins were lysed in RIPA lysis buffer (Thermo Scientific) containing protease and phosphatase inhibitor cocktail. Cell supernatants were precipitated with 4 volumes of pre-chilled acetone (−20 °C), then the precipitated proteins were subsequently collected by centrifugation (14,000 × g, 10 min, 4 °C), washed thrice with 1 mL of prechilled methanol (−20 °C), and the precipitated proteins were lysed by 1% SDS in PBS buffer supplemented with protease and phosphatase inhibitor cocktail. The extracted total protein concentration was quantified with BCA protein assay kit (Thermo Scientific) for the normalization assayed samples, and then equal amounts of proteins were separated by 4%–12% SDS-PAGE gels, followed by transfer onto polyvinylidene difluoride (PVDF) membranes (Millipore). The membranes were blocked with 5% non-fat milk for 1–2 h at room temperature and further incubated with HRP-labeled streptavidin (1:5000) or the indicated primary antibodies solution overnight at 4 °C followed by incubation with horseradish peroxidase-conjugated secondary antibodies or anti-mouse IgG for IP (HRP) (Abcam). Then, the membranes were visualized with ChemiDoc System (Bio-Rad).
2.21. Fluorescence microscopy
The HEK293T cells and primary BMDMs were seeded in cell dish and fixed with 4% paraformaldehyde solution for 15 min at room temperature, followed by permeabilization with 0.15% Triton X-100 for 20 min at room temperature. Cells were then incubated in 10% goat serum for 1 h and followed by incubation with the primary antibodies overnight at 4 °C. After incubation with secondary and/or Cy5-labeled streptavidin (Solarbio) or phalloidin (Thermo Scientific) or mitoSOX (Yeasen) for 60 min and counterstained with Hoechst 33342 (Beyotime) to label all cell nuclei for 15 min at room temperature, the cells were photographed with FV 3000 fluorescence microscope (Olympus).
2.22. Docking
For PGAM5-NEK7 docking, ClusPro 2.0 was used to perform a global search on PGAM5 (PDB: 3MXO) and NEK7 (PDB: 2WQM), and then use RosettaDock 4.0 for local ensemble docking of the predicted conformation, with the default parameters. The best prediction results were obtained according to the recommended criteria42. In addition, superposition of PGAM5–NEK7 docking results with crystal (6NPY) followed by energy optimization using Rosetta Relax43 to obtain the ternary complex model.
For punicalagin–PGAM5 docking, molecular dynamics simulations (200 ns) and trajectory clustering as previously described44 were used to explore more conformations of PGAM5 monomer. The binding pocket was predicted using the D3pocket algorithm45. Covalent molecular docking was performed using Autodock4 with default parameters except for the reaction rules which refer to the latest research46.
2.23. Protein thermal shift assay
Protein thermal shift assay was performed according to the manufacturer's instructions (Thermo Scientific). Briefly, His-tagged ΔN21 PGAM5 proteins were incubated with punicalagin for 5 min at room temperature, subsequently, the mixtures were subjected to protein thermal shift assay on the Stepone Software v2.3 Detection System according to the manufacturer's instructions.
2.24. Depletion and reconstitution of macrophages in mice
The depletion and reconstitution of macrophages in mice were performed according to previous protocol47, with minor optimizations. Briefly, mice were injected intraperitoneally with clodronate-containing liposomes or control liposomes 3 days prior to the onset of experiments to ensure depletion of resident macrophages and every 3 days during the protocol to deplete new infiltrating macrophages (200 μL per mouse). The normal control or Nek7 knockdown macrophages were refused back into mice onset of experiments by intravenous injection every 3 days during the experiments to reconstitute macrophages in vivo (106 macrophages in a total volume of 100 μL per mouse).
2.25. Macrophages sorting in the colon
The single-cell suspensions of colon were prepared via MACS tissue dissociation kit (130-097-410, Miltenyi Biotec) according to the manufacturer's instructions. Briefly, the colons were isolated from mice and then washed thrice with HBSS to remove residual fat tissue. Then, the colons were cut into pieces of approximately 0.5-cm length and subjected to digestion after adding predigestion solution on MACSmix Tube Rotator. Then, the tissue was further digested via gentle MACS Octo Dissociator after adding enzyme mix. The macrophages were sorted from the digested single cell suspension using Mouse F4/80 Positive Selection Kit (720205, Pbmedicals).
2.26. Fecal microbiota transplantation (FMT)
FMT was performed based on an established protocol27, with minor optimizations. Briefly, WT and Pgam5 deficiency C57BL/6J donor mice were raised in a standard SPF housing environment under 12 h light/dark cycles with ad libitum access to rodent chow and water. Stool was collected daily from WT and Pgam5 deficiency C57BL/6J donor mice and pooled respectively. The recipient mice were transplanted with the fecal microbiota from WT and Pgam5 deficiency C57BL/6J donor mice one week prior to DSS treatment and maintained for 2 weeks. Donor stool (100 mg) was diluted with normal saline and homogenized for 1 min using a vortex to achieve a liquid slurry, and then centrifuged at 500 × g for 5 min to remove particulate matter to facilitate administration. Fresh transplant material was prepared within 10 min before oral gavage to prevent changes in bacterial composition. Oral gavage with FMT material was conducted daily through the 2-week experiment.
2.27. Statistical analysis
All results are shown as mean ± standard error of the mean (SEM). Data analyses were performed via t-test for two groups, or via one-way followed by Tukey's post hoc test or Two-way ANOVA followed by Bonferroni post hoc test for multiple groups using Prism software (GraphPad, San Diego, CA, USA). Differences were considered statistically significant at P < 0.05.
3. Results
3.1. Pgam5 deficiency attenuates canonical NLRP3 inflammasome assembly and activation in macrophages
PGAM5 is highly expressed in immune tissues and cells, which is closely associated with inflammation and immune response. Although PGAM5 has been reported to regulate NLRP3 inflammasome activation in macrophages21, its role and molecular mechanism in regulating NLRP3 inflammasome have been less understood. To address this, BMDMs from Pgam5 knockout (KO) mice and WT littermates were generated and then primed with LPS followed by ATP, nigericin or MSU stimulation to induce NLRP3 inflammasome assembly and activation. In line with the previous reports21, the positive effect of PGAM5 in regulating canonical NLRP3 inflammasome was further confirmed by immunoblotting assay, indicating decreased production and secretion of mature IL-1β and cleaved caspase-1 (a cleaved form of caspase-1) in the Pgam5-deficient BMDMs upon stimulation by LPS and ATP, nigericin or MSU (Supporting Information Fig. S1A–S1C). Besides, there is no difference in LDH release between Pgam5 KO and WT BMDMs stimulated by LPS and ATP, demonstrating that the role of Pgam5 deficiency in inhibiting the secretion of IL-1β in BMDMs was not the results of differences in cell death (Fig. S1D). Moreover, we found that Pgam5 deficiency also inhibited N-terminal cleavage of gasdermin D (Fig. S1A and S1C). In addition, Pgam5 deficiency did not inhibit the mRNA expression of Nlrp3 and Il-1β in BMDMs primed with LPS (Fig. S1E and S1F), suggesting that PGAM5 regulating NLRP3 inflammasome activation may be caused by its PPI or post-translational modifications rather than transcriptional modulation of NLRP3 inflammasome components. Moreover, knockout of Pgam5 could also inhibit AIM2 inflammasome activation, but did not affect the activation of NLRP1 inflammasome (Supporting Information Fig. S2). All of these results suggest that PGAM5 could not be a pan-regulator of ASC or caspase-1.
3.2. Pgam5 deficiency attenuates the activation of NLRP3 inflammasome in vivo and protects against DSS-induced colitis
PGAM5 plays an important role in regulating NLRP3 inflammasome activation, and the excessive activation of NLRP3 inflammasome activation has been reported to be involved in the pathogenesis and progression of ulcerative colitis26. However, whether knockdown of Pgam5 can improve the disease progression of ulcerative colitis by inhibiting the activation of NLRP3 inflammasome has not been reported. In this study, in order to systematically investigate pathological functions of PGAM5-regulated NLRP3 inflammasome in the colon, we employed a DSS-induced mouse model of colitis (Fig. 1A). DSS-induced ulcerative colitis is a widely acceptable model to resemble the symptoms of human clinical ulcerative colitis, including body weight loss, rectal bleeding and diarrhea. Analyses of the GEO data48 indicate that DSS treatment upregulated Pgam5 and Nlrp3 mRNA expression in the colon of mice (Supporting Information Fig. S3A and S3B). Meanwhile, our immunoblotting experiments also showed that DSS treatment upregulated PGAM5 expression and activated NLRP3 inflammasome in WT mice (Fig. S3C). Besides, the F4/80 immunohistochemistry assay suggested that a massive macrophage infiltration was observed in the colon tissue of mice with ulcerative colitis (Fig. S3D). We generated Pgam5 deficiency mice to study the effect of PGAM5 on NLRP3 inflammasome activation and resulting ulcerative colitis. The knockout efficiency of PGAM5 in the colon of mice was validated via immunoblotting assay (Fig. S3E). As expected, Pgam5 KO mice demonstrated less DSS-induced ulcerative colitis as evidenced by decreased loss of body weights and disease activity index (DAI), and lengthened colon length compared to their littermates (Fig. 1B–E). Seven days after DSS-induced colitis model in mice, the colons collected from DSS-induced mice were subjected to H&E staining and histological analysis. The results suggested that Pgam5 KO mice exhibited less colon histological damage with the decreased loss of goblet cells and crypts and attenuated inflammatory cell infiltration in the colon compared with their WT littermates (Fig. 1F and G). In addition, immunoblotting analysis revealed that there was less production of NLRP3 and cleaved caspase-1 proteins in colons of Pgam5 KO mice than in those of WT littermates induced by DSS (Fig. 1H). Moreover, the expression of IL-1β in serum and colon in Pgam5 KO mice were also lower than that in WT littermates treated with DSS (Fig. 1I and J). In addition, Pgam5 deficiency did not inhibit the mRNA expression of Il-1β, Il-18 and Caspase-1 in colon tissue of mice with ulcerative colitis, which further indicated that PGAM5 regulating NLRP3 inflammasome activation may be caused by its PPI or post-translational modifications rather than transcriptional modulation (Fig. S3F). Altogether, these data indicate that Pgam5 deficiency protects against DSS-induced colitis by inhibiting NLRP3 inflammasome activation, and PGAM5 may be a potential target for the therapy of ulcerative colitis.
Figure 1.
PGAM5 deficiency attenuates the activation of NLRP3 inflammasome in vivo and protects against DSS-induced colitis. (A) Schematic representation of evaluation of the biological activity of PGAM5 deficiency on DSS-induced ulcerative colitis. (B, C) The change of body weight and disease activity index (DAI) of WT and Pgam5 deficiency mice in (A) (n = 8). The DAI value was calculated as described in Methods. (D, E) Colon length in WT and Pgam5 deficiency mice was measured after being treated with DSS for 7 days (n = 8). (F, G) H&E staining and histological score of colons from DSS-induced WT and Pgam5 deficiency mice at day 7 (n = 8). (H) Immunoblot analysis and statistical analysis of PGAM5, NEK7, NLRP3 and cleaved caspase-1 protein expression of mouse colon from WT and Pgam5 deficiency mice treated with DSS for 7 days (n = 8). (I, J) ELISA analysis of IL-1β protein in serum and colons from WT and Pgam5 deficiency mice treated with DSS for 7 days (n = 8). All data are represented as mean ± SEM in (B, C, E, G–J). Statistical analysis was carried out using unpaired t-test for (B, C, E, G–J). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
3.3. Proteomic proximity analysis of PGAM5 reveals PGAM5-interacting proteins
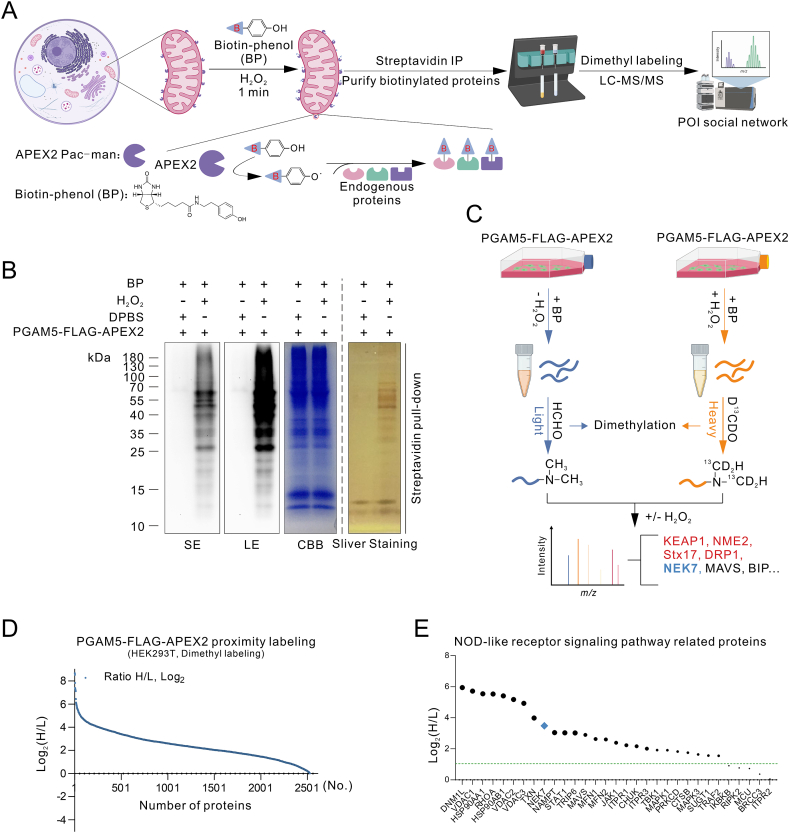
We next investigated the binding partners of PGAM5 for mechanisms regarding how PGAM5 regulates NLRP3 inflammasome activation. An APEX2 proximity labeling strategy to identify proteins that interact with PGAM5 in cells was employed (Fig. 2A). We genetically fused APEX2 with mitochondrial-localized PGAM5 and FLAG tag (Supporting Information Fig. S4A–S4C). Then, to examine the biotinylation activity and location of APEX2-based proximity labeling of the PGAM5-FLAG-APEX2 fusion construct, PGAM5-FLAG-APEX2 proximity labeling was conducted. The streptavidin-horseradish peroxidase (HRP) blotting analysis of the whole cell lysate subjected to PGAM5-FALG-APEX2 proximity labeling suggested that endogenous neighboring proteins of PGAM5 were biotinylated by APEX2, and this biotinylation signal exerts high signal to noise (S/N) ratio compared to the biotinylation signal caused by APEX2 self-biotinylation, endogenously present H2O2 and endogenous peroxidase activity (negative controls that omit APEX2, BP or H2O2) (Fig. S4D). Subsequently, we performed fluorescence imaging to detect the cellular sites where the PGAM5-FLAG-APEX2 proximity labeling occurs. This recombinant fusion protein of PGAM5-FLAG-APEX2 was localized in the mitochondria (Fig. S4E), which is consistent with the localization of PGAM5. Also, the PGAM5-FLAG-APEX2 proximity labeling mainly occurs in the mitochondria (Fig. S4E), which is consistent with the localization of PGAM5-FLAG-APEX2, suggesting that the labeling was not diffusing. Thus, the PGAM5-FLAG-APEX2 fusion proteins target the mitochondria and can biotinylate neighboring proteins of PGAM5.
Figure 2.
Proteomics analysis of PGAM5-interacting proteins using APEX2-based proximity labeling. (A) Schematic overview of PGAM5-FLAG-APEX2 catalyzed biotinylation in the mitochondria. (B) Streptavidin blot and streptavidin pull-down analysis of biotinylated proteins from PGAM5-FLAG-APEX2 proximity labeling, and the right shows CBB staining of the same samples. HEK293T cells were transfected with PGAM5-FLAG-APEX2 fusion construct for 24 h, and then biotin phenol (0.5 mmol/L, 30 min, 37 °C) was added, followed by with or without H2O2 (1 mmol/L, 1 min, RT). Then, quickly aspirate the labeling solution and wash cells three times with quencher buffer. The biotinylated proteins were enriched using streptavidin beads and then analyzed by silver staining (streptavidin pull-down) and streptavidin blotting analysis (Input). Negative control is shown with omission of H2O2 treatment. (C) Illustration depicting the strategy to identify PGAM5 interaction partners by dimethyl labeling and quantitative LC–MS/MS-based proteomics. The control group was labeled with light isotope-labeled HCHO and the PGAM5-FLAG-APEX2 group was labeled with heavy isotope-labeled D13CDO. (D) The rank plot of streptavidin magnetic beads enriched proteins, according to the ranking of H/L ratios. (E) The rank plot of enriched proteins related with Nod-like receptor signaling pathway based on KEGG analysis. Similar results were obtained from three independent experiments.
Then, we performed the PGAM5-FLAG-APEX2 proximity labeling and the biotinylation neighboring proteins of PGAM5 were enriched by streptavidin magnetic beads, which were further subjected to streptavidin-HRP blotting analysis, Coomassie brilliant blue staining (CBB) and silver staining (Fig. 2B). In the meantime, the sample that omits H2O2 treatment was used as a negative control. Abundant biotinylated neighboring proteins of PGAM5 were enriched and no or very little biotinylation proteins signal was observed in the negative control (Fig. 2B). Subsequently, to identify the neighboring proteomes of PGAM5, the samples were prepared in the same way above mentioned and the biotinylation proteins were enriched and digested on streptavidin magnetic beads with sequencing grade modified trypsin (Promega), followed by dimethyl labeling via using CH2O or 13CD2O (Fig. 2C). Lots of unique proteins were identified and would be considered to be the neighboring proteins of PGAM5 (Fig. 2D). Among them, Bcl-xL, KEAP1, RIPK1, NME2, STX17 and DRP1 have been reported as PGAM5 interaction partners, which extremely supported the success of PGAM5-FLAG-APEX2 proximity labeling strategy. Then, our research oriented toward the biological function of PGAM5 in regulating NLRP3 inflammasome activation, and 31 unique proteins involved in NOD-like receptor signaling pathway were enriched by KEGG analysis (Fig. 2E).
3.4. NEK7 directly interacts with PGAM5
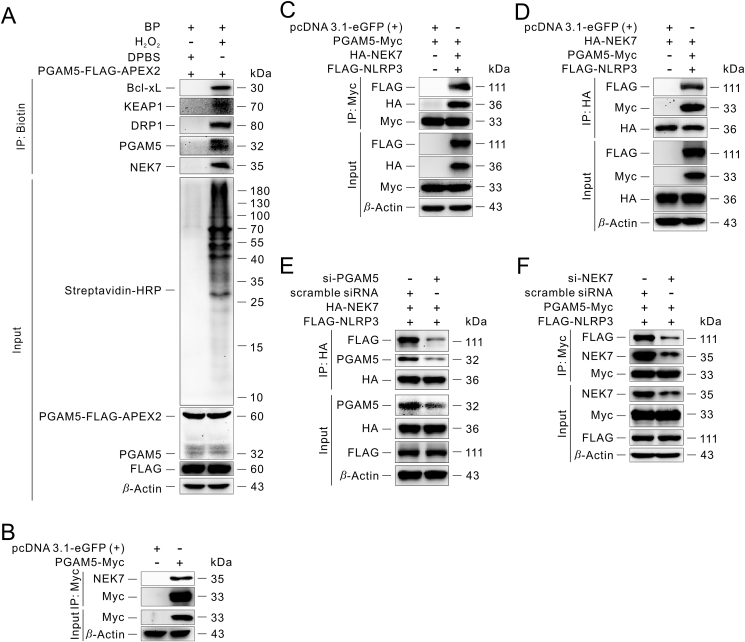
We then analyzed the proteins above involved in NOD-like receptor signaling pathway and compared them with STRING analysis of NLRP3 (Fig. 2E; and Supporting Information Fig. S5A), and found that HSP90AA1, NEK7, MAVS, SUGT1 and BRCC3 were closely associated with NLRP3 inflammasome activation. It has been shown that there is no direct physical interaction between PGAM5 and ASC21. Moreover, our immunoprecipitation experiments also showed no direct interaction between PGAM5 and ASC (Fig. S5B), which is consistent with previous reports21. Given the structural basis of NLRP3 inflammasome activation, NEK7 drew attention to us. Previous studies have shown that NEK7 can directly interact with NLRP3 and further promote NLRP3 oligomerization and NLRP3 inflammasome activation8,49. To study NLRP3 inflammasome activation at the level of post-translational modifications, predominantly via PPI, we reconstructed NLRP3 inflammasome in HEK293T cells to eliminate the impact of transcriptional regulation. We found that overexpression of NEK7 significantly increases the cleaved form of caspase-1, also known as cleaved caspase-1, which is used to characterize the activation of NLRP3 inflammasome (Fig. S5C). On the contrary, knockdown of NEK7 extremely decreases NLRP3 inflammasome activation (Fig. S5D and S5E). These results above reveal that NEK7 is an important component of NLRP3 inflammasome activation, which is consistent with previous research49.
Immunoblotting analysis of the elution from streptavidin magnetic beads after PGAM5-FLAG-APEX2 proximity labeling suggested that NEK7 is one of the neighboring proteins of PGAM5 (Fig. 3A). To confirm the interaction between PGAM5 and NEK7, we transfected a PGAM5-Myc fusion construct into HEK293T for 24 h, followed by co-immunoprecipitation with anti-Myc magnetic beads, and NEK7 was detected in the elution, supporting that PGAM5 interacts with NEK7 (Fig. 3B and Fig. S5F). Meanwhile, an in vitro pull-down assay also suggested that PGAM5 could interact with NEK7 (Fig. S5G). Previous reports have suggested that the C-lobe of NEK7 interacts with the LRR domain of NLRP3 directly and further promotes NLRP3 oligomerization and NLRP3 inflammasome activation8,9. Therefore, we co-transfected the PGAM5-Myc, HA-NEK7 and FLAG-NLRP3 fusion constructs into HEK293T cells for 24 h and then co-immunoprecipitated the lysates using anti-Myc magnetic beads or anti-HA magnetic beads, respectively (Fig. 3C and D). We found that PGAM5 could interact with NEK7 and NLRP3, suggesting that they may exist as a protein complex in the cells. At the same time, fluorescence imaging showed that the PGAM5 signal overlapped with the NLRP3 signal or NEK7 signal, respectively (Fig. S5H and S5I). When PGAM5 was knocked down in cells, the interaction between NEK7 and PGAM5 or the interaction between NEK7 and NLRP3 was impaired (Fig. 3E and Fig. S5J). Also, knockdown of NEK7 attenuates the interaction between PGAM5 and NEK7, similarly between PGAM5 and NLRP3 (Fig. 3F and Fig. S5D). Given that HEK293T cells do not express endogenous NLRP3, the above results suggest that NEK7 may act as a bridge for the interaction of PGAM5 and NLRP3.
Figure 3.
NEK7 is a new binding partner of PGAM5. (A) Verification of the PGAM5-NEK7 interaction by streptavidin pull-down assay in HEK293T. The Bcl-xL, KEAP1 and DRP1 proteins serve as positive controls for PGAM5-FLAG-APEX2 proximity labeling. (B–D) Immunoprecipitation of the interaction between PGAM5 and NEK7 in the absence or presence of NLRP3 in HEK293T. (E) Immunoprecipitation analysis of the effect of knockdown of PGAM5 on the interaction between PGAM5 and NEK7, similarly between NEK7 and NLRP3 in HEK293T. (F) Immunoprecipitation analysis of the effect of knockdown of NEK7 on the interaction between PGAM5 and NEK7, similarly between PGAM5 and NLRP3 in HEK293T. Similar results were obtained from three independent experiments (A–F).
3.5. PGAM5–NEK7 interaction promotes NLRP3 inflammasome activation
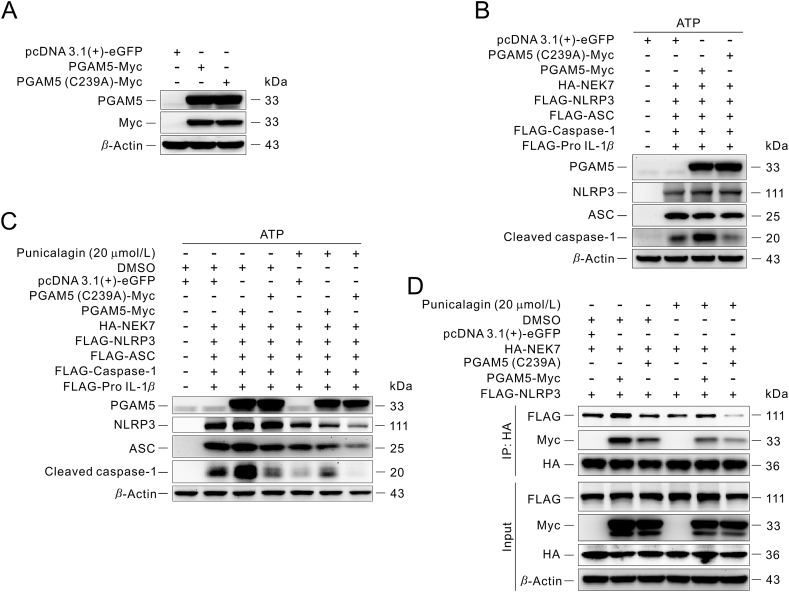
Previous study had reported that PGAM5 could promote mitochondrial ROS (mtROS) production, and further promote inflammasome activation in a complex way of signal transduction50. Our confocal images suggested that Pgam5 deficiency attenuated the production of mtROS (Supporting Information Fig. S6A). Moreover, the interaction between PGAM5 and NEK7 was not affected after pre-treated with antioxidant NAC (Fig. S6B), suggesting that mtROS may not directly participate in the interaction between PGAM5 and NEK7. Meanwhile, efflux of potassium and leakage of cathepsins plays a crucial role as an upstream event in the activation of NLRP3 inflammasome, and previous study had reported that NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux49, suggesting that efflux of potassium or leakage of cathepsins may affect the interaction between PGAM5 and NEK7. However, our confocal images indicate that the interaction between PGAM5 and NEK7 was not affected after pre-treated with potassium inhibitor PAP-1 or lysosomal inhibitor HCQ (Fig. S6C and S6D), suggesting that efflux of potassium and leakage of cathepsins may not directly participate in the interaction between PGAM5 and NEK7. More importantly, in this study, to determine the effect of PGAM5 on NLRP3 inflammasome activation at the post-translation level, NLRP3 inflammasome system in HEK293T cells was reconstructed to eliminate the regulation of transcriptional modifications according to the previous researches51,52. In line with previous reports, NEK7 is necessary for NLRP3 inflammasome activation at the post-translation level (Fig. S5C–S5E). Intriguingly, knockdown of PGAM5 could decrease the expression of cleaved caspase-1, which suggested that the activation of NLRP3 inflammasome was inhibited (Fig. 4A), while PGAM5 overexpression could promote NLRP3 inflammasome activation (Fig. 4B). These results indicate that PGAM5–NEK7 interaction promotes NLRP3 inflammasome activation. PGAM5 is a mitochondrial Ser/Thr phosphatase and regulates multiple biological functions, such as mitochondria dynamics, mitophagy, cell death, organelle homeostasis and immune response, in a phosphatase activity-dependent or independent manner20. We therefore examined whether the phosphatase activity of PGAM5 was involved in regulating NLRP3 inflammasome activation. We genetically constructed a PGAM5-Myc with mutation of histidine to alanine at position 105 of PGAM5 fusion construct, abbreviated as PGAM5 (H105A)-Myc (Supporting Information Fig. S7). H105 is required for phosphatase activity of PGAM5 and substitution of an alanine residue for H105 completely abolishes its catalytic activity53,54. However, this mutation of PGAM5 did not affect the protein conformation55. Compared to overexpression of PGAM5, overexpression of PGAM5 (H105A) or treatment with PGAM5 phosphatase inhibitor LFHP-1c38 could not inhibit NLRP3 inflammasome activation (Fig. 4C). Further studies showed that overexpression of PGAM5 (H105A) or LFHP-1c treatment did not affect the interaction between PGAM5 and NEK7, and similarly did not affect the interaction between NEK7 and NLRP3 (Fig. 4D), suggesting that PGAM5 phosphatase activity-independent interaction of PGAM5 and NEK7 is responsible for NLRP3 inflammasome activation.
Figure 4.
PGAM5 phosphatase activity-independent interaction of PGAM5 and NEK7 is responsible for NLRP3 inflammasome activation. (A) Immunoblot analysis of the effect of knockdown of PGAM5 on the activation of NLRP3 inflammasome in HEK293T. (B) Immunoblot analysis of the effect of PGAM5 overexpression on the activation of NLRP3 inflammasome in HEK293T. (C) Immunoblot analysis of the effect of the phosphatase catalysis activity of PGAM5 on the activation of NLRP3 inflammasome in HEK293T. (D) Immunoprecipitation analysis of the effect of the phosphatase catalysis activity of PGAM5 on the interaction between PGAM5 and NEK7, similarly between NEK7 and NLRP3 in HEK293T. Similar results were obtained from three independent experiments (A–D).
3.6. Natural product punicalagin directly binds to PGAM5 and blocks PGAM5–NEK7 interaction
Due to the significant role of PGAM5 in promoting NLRP3 inflammasome activation independent of its phosphatase activity, we were intrigued by whether there are compounds that could pharmacologically block this process and thus inhibit NLRP3 inflammasome activation. In comparison with synthetic chemical small molecule drugs, natural products have been naturally selected and optimized during the long-term evolution process, and exert obvious advantages in the novelty of structure, biocompatibility and functional diversity. Besides, statistics show that more than 50% of the drugs approved by FDA between 1936 and 2019 contain molecular fragments of natural products, and some of them are even derived directly from natural products56. All of this suggests that natural products play an important role in disease treatment. Therefore, we first screened compounds from the natural compound library (TargetMol, Shanghai) with clear binding to PGAM5 by SPR and then detected whether these compounds could inhibit the activation of NLRP3 inflammasome. We finally screened and obtained the compound punicalagin, which not only binds to PGAM5, but also inhibits the activation of NLRP3 inflammasome (Supporting Information Fig. S8A and S8B). Western blotting data show that treatment of LPS-primed BMDMs with punicalagin prior to activation of NLRP3 with ATP, nigericin or MSU resulted in concentration-dependent reduction in cleaved caspase-1 in whole cell lysates, as well as IL-1β and caspase-1 p20 in cell supernatant (Fig. 5A and B, and Supporting Information Fig. S9A–S9C), which is used as a measure marker for NLRP3 inflammasome activation. CCK-8 assay showed that punicalagin protected BMDMs against cell damage caused by LPS plus ATP (Fig. 5C). In addition, we found that punicalagin also inhibited the activation of AIM2 inflammasome and non-canonical inflammasome, but had no effect on NLRP1 inflammasome activation (Fig. S9D–S9F). Also, to exclude the regulation effect of punicalagin on the assembly and activation of NLRP3 inflammasome at the post-translation level, we evaluated the effect of punicalagin on NLRP3 inflammasome activation in the NLRP3 inflammasome system-reconstructed HEK293T cells. As expected, treatment with punicalagin after reconstruction of NLRP3 inflammasome system in HEK293T cells could effectively decrease the expression of cleaved caspase-1, suggesting that punicalagin blocks the assembly and activation of NLRP3 inflammasome in cells (Fig. 5D). Also, immunofluorescence staining experiments of phalloidin showed that punicalagin treatment ameliorated the morphological damage of LPS-primed BMDMs which further stimulated with ATP (Fig. S9G). The immunoprecipitation assay further revealed that punicalagin could block the interaction between PGAM5 and NEK7, and thus decrease the interaction between NEK7 and NLRP3 in BMDMs (Fig. 5E), ultimately inhibiting the activation of NLRP3 inflammasome, which is similar to Pgam5 deficiency in BMDMs (Fig. 5F; and Fig. S1A–S1C). More importantly, the further SPR assay results confirmed that there was a strong direct covalent binding between punicalagin and ΔN21 PGAM5 or ΔN90 PGAM5 with the KD value of about 0.3 nmol/L or 9.5 nmol/L (Fig. 5G and H), respectively. To further validate the interaction of punicalagin with PGAM5, we performed DARTS, CETSA and SIP experiments in BMDMs. The results suggest that punicalagin significantly restrained the proteolysis of PGAM5 by pronase, further supporting that punicalagin could bind to PGAM5 in BMDMs (Fig. 5I). Meanwhile, the protein stability of PGAM5 was also increased after treatment with punicalagin in CETSA and SIP assay, supporting the binding of punicalagin with PGAM5 (Fig. 5J and K). Subsequently, we performed RNA-sequencing experiment and differential expression analysis. Compared to the cells treated with negative control DMSO, a number of genes related to TNF signaling pathway, which is the main signaling pathway regulated by PGAM5, were regulated by punicalagin (Fig. S9H–S9J). Besides, KEGG pathway analysis showed that NOD-like receptor signaling pathway was also significantly enriched among the downregulated genes by punicalagin treatment in cells (Fig. S9I), and the downregulated genes are shown in Fig. S9K. In addition to inflammation-related signaling pathways, a great number of virus- and infection-associated signaling pathways were all significantly enriched after punicalagin treatment, which suggested that regulation of NLRP3 inflammasome activation by PGAM5 or by blocking the interaction between PGAM5 and NEK7 may play a significant role in viral infections. Collectively, all these results reveal that natural product punicalagin directly binds to PGAM5 and blocks PGAM5–NEK7 interaction. Moreover, punicalin and urolithin A are the metabolites of punicalagin57. Interestingly, we found that there was a similar direct interaction between punicalin and ΔN21 PGAM5 or ΔN90 PGAM5 compared with punicalagin (Fig. S9L and S9M). However, urolithin A neither binds with PGAM5 nor inhibits the activation of NLRP3 inflammasome (Fig. S9N–S9P). Besides, punicalin also could decrease IL-1β secretion in BMDMs primed with LPS and subsequently stimulated with ATP (Fig. S9P).
Figure 5.
Natural product punicalagin blocks PGAM5–NEK7 interaction and inhibits NLRP3 inflammasome activation. (A) Immunoblot analysis of the effect of punicalagin treatment on the activation of NLRP3 inflammasome in BMDMs. (B) ELISA analysis of IL-1β secretion in supernatants of LPS-primed and ATP-activated BMDMs with punicalagin treatment. (C) Cell viability analysis of BMDMs subjected to LPS and ATP stimulation followed by punicalagin treatment. (D) Immunoblot analysis of the effect of punicalagin treatment at the level of post-translational modifications on the activation of NLRP3 inflammasome in HEK293T cells. (E, F) Immunoprecipitation analysis of the effect of punicalagin treatment or PGAM5 deficiency on the interaction between PGAM5 and NEK7, similarly between NEK7 and NLRP3 in BMDMs subjected to LPS and ATP stimulation. (G, H) SPR analysis of the binding between punicalagin and PGAM5. (I–K) Immunoblot analysis of the binding between punicalagin and PGAM5 in BMDMs via DARTS (I), CETSA (J) and SIP (K). Similar results were obtained from three independent experiments (A, D–K). All data are represented as mean ± SEM in (B, C). Statistical analysis was carried out using One-way ANOVA followed by Tukey's post hoc test for (B, C). ∗∗∗P < 0.001; ##P < 0.01, and ###P < 0.001.
3.7. Cysteine 239 residue of PGAM5 is responsible for punicalagin activity and is necessary for the interaction between PGAM5 and NEK7
Given that punicalagin is a polyphenolic compound possibly covalent binding to PGAM5, we speculated that the cysteine residues in PGAM5 might be the binding sites between punicalagin and PGAM5. Thus, we checked the ΔN21 PGAM5 and ΔN90 PGAM5 protein amino acid sequences and identified three cysteine residues (Supporting Information Fig. S10A and S10B). Besides, we performed a conserved PGAM5 sequence analysis in human beings and a multitude of model animals, such as mice, fruit flies, rats, caenorhabditis elegans and zebrafish, which suggested that cysteine 239 residue of human PGAM5 protein is highly conserved in human beings and a wide range of animals (Fig. S10C). To confirm which of the cysteine residues may be modified by punicalagin, we incubated recombinant PGAM5 protein with or without punicalagin followed by Nano LC–Q-TOF-MS analysis. The MS analysis showed that PGAM5 had a 1083 Da mass shift in the presence of punicalagin; however, the shift mass was almost eliminated after treatment with DTT (Fig. S10D and S10E). Due to the molecular weight of punicalagin reaching 1084, and it is easy to break during the electron process of secondary MS, therefore, it is very difficult to identify the specific amino acid binding site or fragment that binds with punicalagin by LC–MS/MS. However, these above results suggest that cysteine residues of PGAM5 may play important roles in the interaction between punicalagin and PGAM5. To further illustrate which cysteine residue is the binding site between punicalagin and PGAM5, we established a method to detect the phosphatase activity of PGAM5 and mutate each cysteine residue of PGAM5 into alanine, respectively. Interestingly, punicalagin still inhibited the phosphatase activity of PGAM5 C168A or C229A protein mutant, but did not affect the mutation of Cys239 residue into alanine of PGAM5, suggesting that punicalagin may bind to Cys239 residue of PGAM5 (Fig. S10F–S10H). Previous report had shown that the dimer interface created by β6, α4, and the neighboring β3–α3 loop are necessary for the formation of PGAM5 conformations58. Therefore, we genetically constructed a PGAM5–Myc with mutation of cysteine to alanine at the position 239 of PGAM5 fusion construct, abbreviated as PGAM5 (C239A)–Myc, and confirmed the overexpression of PGAM5 compared with PGAM5–Myc fusion construct. The overexpression efficiency of PGAM5 is the same as that of PGAM5–Myc construct (Fig. 6A). We found that PGAM5 (C239A)–Myc could not promote the expression of cleaved caspase-1 compared with PGAM5–Myc (Fig. 6B), suggesting that PGAM5 (C239A)–Myc may not promote NLRP3 inflammasome activation, similar to punicalagin treatment (Fig. 5D and 6C). Interestingly, punicalagin treatment still inhibited the expression of cleaved caspase-1 compared with cells transfected with PGAM5 (C239A) construct (Fig. 6C), which may be due to the fact that punicalagin could also bind with endogenous PGAM5 in cells. Besides, the immunoprecipitation assay revels that PGAM5 (C239A)–Myc and/or punicalagin treatment could block the interaction between PGAM5 and NEK7, and then further block the interaction between NEK7 and NLRP3 (Fig. 6D), resulting in inhibition of the activation of NLRP3 inflammasome. Meanwhile, the DARTS assay also showed that punicalagin binds to Cys239 residue of PGAM5 (Fig. S10I). Most importantly, overexpression of PGAM5 (H105A) in Pgam5-deficient BMDMs could promote NLRP3 inflammasome activation at similar level compared to overexpression of PGAM5 wide-type, but overexpression of PGAM5 (C239A) in Pgam5-deficient BMDMs had less effects on NLRP3 inflammasome activation compared to PGAM5 wide-type (Supporting Information Fig. S11A and S11B). All these results suggest that cysteine 239 residue of PGAM5 is necessary for NLRP3 inflammasome activation, but PGAM5 phosphatase enzyme activity was not involved in NLRP3 inflammasome activation.
Figure 6.
Cysteine 239 residue of PGAM5 is responsible for punicalagin activity and is necessary for the interaction between PGAM5 and NEK7. (A) Immunoblot analysis of overexpression of PGAM5 (C239A)–Myc compared with PGAM5–Myc in HEK293T. (B and C) Immunoblot analysis of the effect of PGAM5 (C239A)-Myc or punicalagin treatment compared with or without PGAM5–Myc on the activation of NLRP3 inflammasome at the level of post-translational modifications in HEK293T. (D) Immunoprecipitation analysis of the effect of PGAM5 (C239A)–Myc or punicalagin treatment compared with or without PGAM5–Myc on the interaction between PGAM5 and NEK7, similarly between NEK7 and NLRP3 in HEK293T. Similar results were obtained from three independent experiments (A–D).
In this study, we conducted global protein–protein docking using Cluspro combined with local ensemble docking using RosettaDock4.0 to obtain a visible binding model of PGAM5 with NEK742,59. The algorithm predicts that PGAM5 binds to the N-terminal lobe of NEK7 in a dimer form, and six hydrogen bonds (Tyr224, Gly139, Arg273, His109, Asp111 and Arg288 of PGAM5 with Arg76, Ala73, Asp69, Ala192, Lys39 and Asp69, respectively) and two electrostatic interactions (Arg273 and Arg288 of PGAM5 with Asp69 of NEK7) occur at this interface (Supporting Information Fig. S12A). Furthermore, when we superimposed and relaxed this model with the known complex crystals of NEK7 and NLRP3, no steric conflicts were found, indicating that the ternary complex is also plausible. Besides, by performing molecular dynamics simulations on PGAM5, it was found that the cysteine 239 of PGAM5 protein located in the α4 helix of the structure of PGAM5, and the destabilization of the α4–β5-Loop would expose the cysteine 239 of PGAM5 as a possible covalent binding site. Further molecular docking results showed that punicalagin may occupy this pocket, thereby hindering the dimerization of PGAM5, which is necessary for its interaction with NEK7 (Fig. S12B). In addition, the dimer interface was created by β6, α4, and the neighboring β3–α3 loop, which is necessary for the formation of PGAM5 structures58. Meanwhile, the protein thermal shift assay suggested that punicalagin could bind with the hydrophobic residues and then destabilize PGAM5 (Fig. S12C), suggesting that punicalagin binding to PGAM5 would affect the natural physiological structure of PGAM5.
3.8. Punicalagin inhibits the activation of the NLRP3 inflammasome in vivo and protects against DSS-induced colitis depending on PGAM5–NEK7–NLRP3 complex
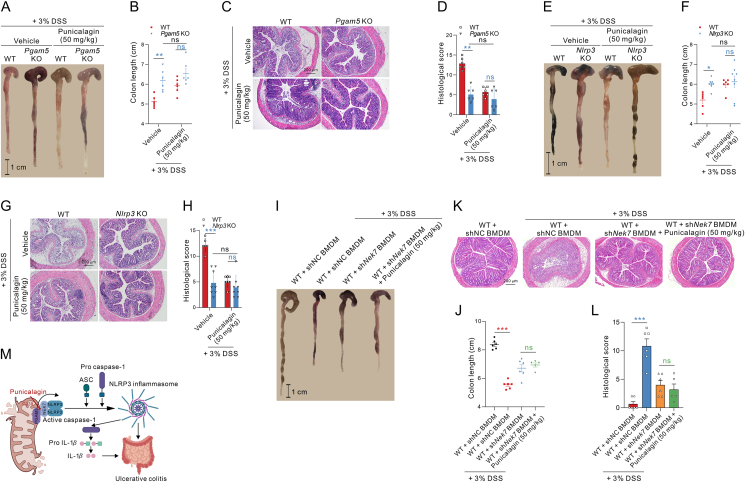
Due to punicalagin binding with PGAM5 blocks the interaction between PGAM5 and NEK7 and further inhibits the activation of NLRP3 inflammasome. Therefore, we evaluated the effect of the pharmacological intervention of PGAM5 and NEK7 interaction by punicalagin on DSS-induced ulcerative colitis. To evaluate the therapeutic effect of punicalagin on ulcerative colitis, the C57BL/6J mice were subjected to 3% DSS for 7 days, punicalagin (10, 20, and 50 mg/kg) and mesalazine (200 mg/kg) were orally administrated daily for 7 days, and the body weight and DAI were monitored every day (Supporting Information Fig. S13A). Punicalagin administration markedly ameliorated the body weight loss and decreased the DAI scores in a dose-dependent manner during DSS-induced ulcerative colitis (Fig. 7A and B). Besides, colon shortening, which is the macroscopic pathological feature of colitis, was also ameliorated by punicalagin administration (Fig. 7C and D). Moreover, the colon histological analysis showed that punicalagin administration decreased the colon damage, the infiltration of immune cells and destruction of the intestinal epithelium (Fig. 7E and F). Moreover, the F4/80 immunohistochemistry assay suggested that punicalagin treatment could improve macrophage infiltration in mouse colon tissue with ulcerative colitis (Fig. S13B). The immunoblotting assay data indicated that punicalagin administration could decrease the expression of NLRP3 and cleaved caspase-1 proteins in a dose-dependent manner in colons of mice induced by DSS (Fig. 7G and H). Besides, the levels of IL-1β protein in colon and serum were also decreased after punicalagin administration (Fig. 7I and J). Moreover, the immunoprecipitation experiments data reveal that punicalagin could block the interaction between PGAM5 and NEK7, then block the interaction between NEK7 and NLRP3 (Fig. 7K), and finally inhibit the activation of NLRP3 inflammasome in colon.
Figure 7.
Punicalagin inhibits the activation of NLRP3 inflammasome in vivo and protects against DSS-induced colitis. (A, B) The change of body weight and DAI of mice administrated with or without punicalagin in DSS-induced ulcerative colitis. The DAI value was calculated as described in the Methods (n = 6). (C, D) Colon length of mice administrated with or without punicalagin was measured after induced by DSS for 7 days (n = 6). (E, F) H&E staining and histological score of colons from DSS-induced mice administrated with or without punicalagin on Day 7 (n = 6 for E and n = 3 for F). (G, H) Immunoblot analysis and statistical analysis of PGAM5, NEK7, NLRP3 and Cleaved caspase-1 protein expression of mouse colon from mice administrated with or without punicalagin and subjected to DSS for 7 days (n = 6). (I, J) ELISA analysis of IL-1β protein in serum and colon from DSS-induced mice administrated with or without punicalagin for 7 days (n = 6). (K) Immunoprecipitation analysis of the effect of punicalagin on the interaction between PGAM5 and NEK7, similarly between NEK7 and NLRP3 in colon of mice subjected to DSS. All data are represented as mean ± SEM in (D, F and H–J). Statistical analysis was carried out using One-way ANOVA followed by Tukey's post hoc test for (D, F and H–J). ∗∗P < 0.01, and ∗∗∗P < 0.001; #P < 0.05, ##P < 0.01, and ###P < 0.001; &&P < 0.01, and &&&P < 0.001; $$$P < 0.001.
Moreover, to determine whether punicalagin improving DSS-induced colitis depends on PGAM5–NEK7–NLRP3 complex, we firstly evaluated the therapeutic effect of punicalagin on Pgam5 knockout mice or Nlrp3 knockout mice. The data showed that knockout of Pgam5 improved ulcerative colitis; however, punicalagin treatment did not further ameliorate the body weight loss and decrease the DAI scores in Pgam5 knockout mice (Supporting Information Fig. S14A and S14B), and punicalagin also did not further increase colon length and ameliorate colonic tissue damage in Pgam5 knockout mice (Fig. 8A–D). The roles of NLRP3 inflammasome in the pathogenesis of ulcerative colitis have been extensively studied, with some results showing that loss of epithelial integrity and massive leukocyte infiltration appear to associate with Nlrp3 knockout mice for developing more severe colitis13, but other studies showed that knockout of Nlrp3 could ameliorate ulcerative colitis60. The researchers attribute this difference to the experimental environment and the genetic background of the mice. In our study, we found that knockout of Nlrp3 could ameliorate the body weight loss and decrease the DAI scores (Fig. S14C and S14D), as well as increase colon length and ameliorate colonic tissue damage (Fig. 8E–H). In addition, punicalagin treatment could not further improve ulcerative colitis in Nlrp3 knockout mice (Fig. 8E–H; Fig. S14C and S14D). These results reveal that punicalagin treatment improves ulcerative colitis mainly through PGAM5 and NLRP3. In addition, the depletion and reconstitution of Nek7 knockdown macrophages experiments in mice were performed to reveal the role of NEK7 in ulcerative colitis. Colon macrophages were depleted about 70% after injection with clodronate-containing liposomes compared to injection with control liposomes (Fig. S14E and S14F), and the NEK7 expression in macrophages isolated from the colon of mice deprived of their macrophages and reconstituted of Nek7 knockdown macrophages was decreased than that of isolated from colon of mice deprived of their macrophages and reconstituted of normal control macrophages (Fig. S14G), which suggested that NEK7 was knocked down in the macrophages of colon in mice. The data show that the WT mice deprived of their macrophages and reconstituted of Nek7 knockdown macrophages could protect against ulcerative colitis induced by DSS, which indicated that Neh7 deficiency could improve the symptoms of ulcerative colitis induced by DSS, such as weight loss, DAI scores (Fig. S14H and S14I), colon length and colon histological damage with the decreased loss of goblet cells and crypts and inflammatory cells infiltration (Fig. 8I–L) in the colon compared with WT mice reconstituted of normal control macrophages. Moreover, we found that mice deprived of their macrophages and reconstituted of Nek7 knockdown macrophages, followed by treatment with punicalagin, simultaneously did not exert significant improvements effect compared with the mice that were only deprived of their macrophages and reconstitution of Nek7 knockdown macrophages, but with an improving tendency to ameliorate colitis between these two groups (Fig. S14H and S14I, Fig. 8I–L). Altogether, punicalagin could inhibit the NLRP3 inflammasome activation through blocking the PGAM5 interaction with NEK7, and effectively improve DSS-induced colitis in mice, indicating that punicalagin exerts a potential clinical application as a lead compound for the treatment of ulcerative colitis.
Figure 8.
Punicalagin improves DSS-induced ulcerative colitis depending on PGAM5–NEK7–NLRP3 complex. (A, B) Colon length of Pgam5 knockout or WT mice administrated with or without punicalagin was measured after induced by DSS for 7 days (n = 6). (C, D) H&E staining and histological score of colons from DSS-induced Pgam5 knockout or WT mice administrated with or without punicalagin on Day 7 (n = 6). (E, F) Colon length of Nlrp3 knockout or WT mice administrated with or without punicalagin was measured after induced by DSS for 7 days (n = 6–8). (G, H) H&E staining and histological score of colons from DSS-induced Nlrp3 knockout or WT mice administrated with or without punicalagin on Day 7 (n = 6–8). (I, J) Colon length of mice reconstituted of Nek7 knockdown macrophages in the presence or absence of punicalagin treatment was measured after DSS administration for 7 days (n = 5–6). (K, L) H&E staining and histological score of colons from mice reconstituted of Nek7 knockdown macrophages in the presence or absence of punicalagin treatment (n = 5–6). (M) Schematic diagram for the PGAM5-NEK7 interaction playing a critical role for the NLRP3 inflammasome activation in colitis. All data are represented as mean ± SEM in (B, D, F, H, J and L). Statistical analysis was carried out using Two-way ANOVA followed by Bonferroni's post hoc test for (B, D, F and H). Statistical analysis was carried out using unpaired t-test between mice reconstituted of normal control macrophages group and mice reconstituted of Nek7 knockdown macrophages group, or mice reconstituted of Nek7 knockdown macrophages group and mice reconstituted of Nek7 knockdown macrophages followed by treatment with punicalagin (50 mg/kg) group, respectively for (J, L). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001; ns, no significance.
Together, in this work, we characterized a critical role for PGAM5 and its novel binding partner, NEK7, in NLRP3 inflammasome activation and ulcerative colitis. Our study for the first time showed that PGAM5 may act as a bracket to recruit NEK7 and NLRP3, then enhance the interaction between NEK7 and NLRP3, and further facilitate the assembly and overactivation of NLRP3 inflammasome. In addition, we for the first time discovered a natural product punicalagin as the blocker of the PGAM5 interaction with NEK7. We found that the activation of NLRP3 inflammasome could be mitigated by PGAM5 depletion or pharmacological intervention of the interaction between PGAM5 and NEK7 by punicalagin, and PGAM5 deficiency or punicalagin treatment could ameliorate NLRP3 inflammasome activation-driven ulcerative colitis (Fig. 8M). Our study first pointed out a new potential therapeutic target of PGAM5 interaction with NEK7 and a lead compound targeting PGAM5 for the treatment of ulcerative colitis. Moreover, our research reveals that the conformational structure of PGAM5, rather than its phosphatase activity, is necessary for the interaction between PGAM5 and NEK7 and further formation of PGAM5/NEK7/NLRP3 complex, resulting in NLRP3 inflammasome hyperactivation.
3.9. Gut microbiota is not involved in the regulation of PGAM5 on DSS-induced ulcerative colitis
A recent study revealed that Pgam5 deficiency alters the gut microbiota in mice61, and gut microbiota plays an extremely intricate role in colitis62,63. Therefore, we collected the fecal microbiota from WT and Pgam5 deficiency mice, respectively, and then transplanted them to WT mice subjected to 3% DSS treatment. The data of FMT experiments indicated that the mice transplanted the fecal microbiota from Pgam5 deficiency mice, did not improve the symptoms of ulcerative colitis induced by DSS, such as weight loss, DAI scores (Supporting Information Fig. S15A and S15B), colon length (Fig. S15C and S15D), colon histological damage with the decreased loss of goblet cells and crypts and inflammatory cells infiltration in colon (Fig. S15E and S15F) compared with that transplanted the fecal microbiota from WT mice. Besides, we also collected the fecal microbiota from WT and Pgam5 deficiency mice, respectively, and then transplanted them to Pgam5 deficiency mice subjected to 3% DSS treatment. The results show that there was no difference between the Pgam5 deficiency mice transplanted with fecal microbiota from WT mice or Pgam5 deficiency mice, mainly in terms of weight loss, DAI scores (Fig. S15G and S15H), colon length (Fig. S15I and S15J) and colon histological damage (Fig. S15K and S15L). These results suggest that gut microbiota is not involved in the regulation of PGAM5 on DSS-induced ulcerative colitis.
4. Discussion
NLRP3 is an intracellular pattern recognition receptor, that recognizes a broad range of PAMPs and DAMPs64. The sensor NLRP3, the adapter ASC, and the effector caspase-1 are all the components of the NLRP3 inflammasome, and the formation of the NLRP3 inflammasome results in the cleavage and subsequent release of pro-inflammatory cytokines IL-1β and IL-18. As a key component of innate immunity, the NLRP3 inflammasome plays an important role in inflammatory diseases, such as ulcerative colitis. Although the NLRP3 inflammasome is essential for host defense against pathogenic infection, increasing evidence has suggested that its hyperactivation contributes to a variety of inflammatory and autoimmune diseases, such as AD, PD, ulcerative colitis, etc15,65. The NLRP3 inflammasome has been shown to affect the pathogenesis of DSS-induced ulcerative colitis13,66. Several studies have indicated that the NLRP3 inflammasome, as well as downstream IL-1β and IL-18 cytokines, may have various, even double-side effects on experimental colitis in mice13,14,67. The role of NLRP3 inflammasome involved in the pathophysiology of DSS-induced colitis has also been widely examined, with the findings suggesting that loss of epithelial integrity and massive leukocyte infiltration appear to be associated with NLRP3 or caspase-1 deficiency in mice for developing more severe colitis13,68. Possibly it was because the NLRP3 inflammasome and downstream IL-1β and IL-18 cytokines are also required for the proliferation of epithelial cells, the functions of T-cell development, the survival of macrophage and dendritic cells, and the development of gut barrier13,14. However, upon NLRP3 inflammasome hyperactivation, it would promote the massive maturation and secretion of IL-1β and IL-1869, and then further exacerbate the colon inflammatory response and damage. Recently, the roles of NLRP3 inflammasome in the pathogenesis of ulcerative colitis have been extensively studied, with some results showing that loss of epithelial integrity and massive leukocyte infiltration appear to associate with Nlrp3 knockout mice for developing more severe colitis13, but other studies showed that knockout of Nlrp3 could ameliorate ulcerative colitis. In addition to differences in experimental design, the inherent characteristics of the knockout mouse model are possible reasons for the differences in results between laboratories. The knockout mice are susceptible to the genetic background of the mice and the gut microbiota70. In addition to the influence of external factors, the complex physiological effects of the NLRP3 inflammasome itself are also responsible for the contradictory experimental results. It is now believed that activation of NLRP3 inflammasomes at different states may produce opposite effects. In a healthy state, intestinal epithelial cells sense intestinal commensal bacteria through NLRP3 inflammasomes, produce appropriate amounts of IL-1β and IL-18, promote epithelial cell proliferation, and maintain the integrity of the intestinal barrier. When the epithelial barrier is disrupted, microorganisms enter the lamina propria and activate the NLRP3 inflammasome of macrophages, dendritic cells, and neutrophils, which produce a large number of cytokines and chemokines and exacerbate the inflammatory response70,71. The current opinion is that normal NLRP3 inflammasome activation appears to assist homeostatic healing and restoration after traumatic tissue injuries, nevertheless, its hyperactivation is often associated with the progression of inflammatory diseases, especially ulcerative colitis72. Recently, a study showed that depletion of PGAM5 inhibited NLRP3 inflammasome activation in BMDMs21. However, until now, the exact molecular mechanism of the regulation role of PGAM5 in NLRP3 inflammation activation has not been elaborated. Interestingly, previous report had shown that PGAM5 promotes lipid metabolism and colorectal tumorigenesis in mice73. However, whether PGAM5 regulates the disease progression of ulcerative colitis has not been reported so far.
In this study, we first reported that PGAM5 is upregulated in the colon of mice with colitis induced by DSS administration. We further constructed Pgam5 knockout mice and for the first time found that PGAM5 deficiency could alleviate NLRP3 inflammasome activation, and the disease progression and symptoms of ulcerative colitis. We also found that PGAM5 deficiency did not affect the mRNA expression of Nlrp3 and Il-1β in BMDMs under LPS stimulation. Therefore, APEX2 proximity labeling method was used to investigate the regulation mechanism of PGAM5 on NLRP3 inflammasome activation in PPI aspect. A novel binding partner of PGAM5, NEK7, was identified and drew attention to us. Previous reports had reported that NEK7 is a serine/threonine kinase that mainly regulates mitotic spindle formation and centrosome separation during mitosis and centrosome duplication during interphase74,75. Besides, it was shown that NEK7 regulates telomere DNA replication via promoting a multi-protein complex with telomeric shelterin proteins76. Recent research has revealed that the binding between NEK7 and NLRP3 is necessary for the assembly and activation of NLRP3 inflammasome49. Protein co–crystal structure indicates that the C-lobe of NEK7 directly interacts with the LRR domain of NLRP3, further promoting the assembly and activation of NLRP3 inflammasome8. In our study, we found that PGAM5 may act as a bracket to recruit NEK7 and NLRP3, then enhance the interaction between NEK7 and NLRP3, and further facilitate the assembly and over-activation of NLRP3 inflammasome. Furthermore, we found that this process was independent of the phosphatase activity of PGAM5. In agreement with our study, Andreeva et al.77 showed that NLRP3 is basically localized on membrane structure organelles and this localization of NLRP3 could promote the formation of oligomeric double-ring cages, and facilitate the rapid activation of NLRP3 inflammasome under stimulation, suggesting the possibility of existence of a PGAM5/NEK7/NLRP3 protein complex. Our results indicate that the protein structure of PGAM5 rather than the phosphatase activity of PGAM5 is necessary for the interaction between PGAM5 and NEK7. However, due to the complexity of the protein structure of PGAM5, we are currently unable to obtain a co–crystal structure of PGAM5 and NEK7 proteins, or even a longer protein crystal structure than ΔN90 PGAM5. Besides, both the long and short form of PGAM5 could interact with NEK7 (data not shown). Therefore, the detailed mode of action between PGAM5 and NEK7 still requires new techniques and strategies for further investigation. Besides, our data revealed that the PPI between PGAM5 and NEK7 is crucial for NLRP3 inflammasome overactivation, but whether PGAM5 regulates the activation of NLRP3 inflammasome in other post-translational manner still needs further investigation. How NEK7 is switched from cell cycle regulator to NLRP3 inflammasome regulator during NLRP3 inflammasome activation remains elusive. Yang et al.6 thought that LPS priming alters the structure of the centrosome and the function of NEK7 residing inside. In our study, we found that NLRP3 inflammasome activation promoted the interaction between PGAM5 and NEK7, but how PGAM5 recruits NEK7 into mitochondria still needs further study. The results of our study combined with previous report77 indicate the presence of PGAM5/NEK7/NLRP3 protein complexes. Besides, through virtual docking, we found that the structure of PGAM5 is essential for the formation of the PGAM5/NEK7/NLRP3 protein complexes (Fig. S12A and S12B).
However, whether other proteins are interacting with PGAM5 to regulate the activation of NLRP3 inflammasome directly or indirectly needs further study. In our quantitative proteomics data, several proteins associated with NLRP3 inflammasome activation are identified, such as VDAC1/2/3, TXN, MAVS, etc. A recent study has shown that mitochondrial DNA (mtDNA) can activate NLRP3 inflammasome and interferon signaling via mPTP- and VDAC-dependent channels34. Interestingly, PGAM5 can promote the release of mtDNA by dephosphorylating the pro-apoptotic protein Bax78. This suggests that PGAM5 may regulate the activation of NLRP3 inflammasome by regulating mtDNA release. Apart from that, PGAM5–MAVS interaction can regulate antiviral responses79, while MAVS interacts with NLRP3 to enhance host antiviral response. Whether PGAM5 can regulate NLRP3 inflammasome activation via MAVS is also unclear and needs further investigation.
Based on SPR screening, we screened and identified the natural product punicalagin that directly binds to PGAM5 and blocks the interaction between PGAM5 and NEK7, then further inhibits NLRP3 inflammasome activation and alleviates ulcerative colitis for the first time. Punicalagin is an ellagitannin found in the peel of pomegranate (Punica granatum). It is a polyphenol compound with antioxidant, hepatoprotective, anti-atherosclerotic and chemopreventive activities, as well as antiproliferative activity against tumor cells80 and is mainly extracted from pomegranate fruit peel81. A recent study has shown that punicalagin is a reversible and non-competitive 3CLpro inhibitor and inhibits SARS-CoV-2 replication in vitro82. Besides, it has been reported that P. granatum juice and punicalagin could inhibit the activation of NF-κB signaling pathway in inflammatory bowel disease83, but its protein target and related mechanisms against ulcerative colitis are still unknown. In our work, we found that punicalagin could directly bind with PGAM5 to block the interaction between PGAM5 and NEK7, and then inhibit the interaction of NEK7 and NLRP3. In turn, punicalagin inhibited the activation of NLRP3 inflammasome in the colon of mice with ulcerative colitis. However, due to the complexity of the structure of punicalagin, it is difficult to optimize and modify its structure and also very hard to obtain the co–crystal forms of punicalagin and PGAM5. But, in this study, we found that punicalagin binding to the cysteine 239 of PGAM5 protein could affect the formation of the dimer interface created by β6, α4, and the neighboring β3–α3 loop and further disrupt the original structure of PGAM5, which in turn inhibited the interaction between PGAM5 and NEK7. When the cysteine 239 residue of human PGAM5 was bound or modified by compounds, it would affect the hydrophobic binding sites of PGAM5, and further affect the formation of PGAM5/NEK7/NLRP3 complex, in turn affecting the function of PGAM5. All these results would provide insights into the development of PGAM5 function-regulating compounds. Therefore, further screening of lead compounds that directly bind to PGAM5 or targeted design of small molecular compounds via interfering with the protein interaction of PGAM5 and NEK7 are needed, which may discover a new promising therapeutic drug for regulating NLRP3 inflammasome activation and provide a therapy for ulcerative colitis.
In summary, our findings identified a new binding partner of PGAM5, NEK7 for its regulating NLRP3 inflammasome activation. The interaction between PGAM5 and NEK7 could promote NLRP3 inflammasome activation, and the interference in the interaction between PGAM5 and NEK7 by punicalagin or depletion of PGAM5 could in turn attenuate the association of NEK7 with NLRP3 and further lead to the inhibition of abnormal hyperactivation of NLRP3 inflammasome. Our study would shed new insight into the understanding of the mitochondria as a platform for the assembly and activation of NLRP3 inflammasome, and also provide a novel potential therapeutic target and a lead compound for the treatment of NLRP3 inflammasome-driven inflammatory diseases, such as ulcerative colitis.
5. Conclusions
Ulcerative colitis is one of the inflammatory bowel diseases associated with NLRP3 inflammasome overactivation, which has few effective therapeutics. The mitochondrial phosphatase PGAM5 promotes NLRP3 inflammasome activation in macrophages, indicating the potential target for colitis. This work investigates whether PGAM5 regulating macrophage inflammation plays an important role in the pathogenesis of ulcerative colitis and the molecular mechanism for PGAM5 promoting NLRP3 inflammasome activation. The finding that mitochondrial PGAM5 is an effective target in ulcerative colitis may help address an unmet clinical need. Most importantly, the APEX2 proximity labeling combined with quantitative proteomics reveals the PGAM5–NEK7 interaction promoting NLRP3 assembly and activation, which provides insight into the specific mechanism of NLRP3 activation in macrophages and an attractive target for NLRP3 inflammasome-driven inflammatory diseases including ulcerative colitis. Finally, the natural product punicalagin represents as a promising blocker of the PGAM5–NEK7 interaction, which may have broader application for the treatment of inflammatory diseases associated with NLRP3 overactivation.
Author contributions
Cheng-Long Gao: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Jinqian Song: Methodology, Investigation, Formal analysis, Data curation. Haojie Wang: Visualization, Software, Methodology, Investigation, Data curation. Qinghong Shang: Resources, Methodology, Investigation. Xin Guan: Resources, Methodology, Investigation. Gang Xu: Resources, Methodology, Investigation. Jiayang Wu: Software, Resources, Methodology, Investigation. Dalei Wu: Validation, Formal analysis, Data curation. Yueqin Zheng: Writing – review & editing, Resources, Investigation. Xudong Wu: Writing – review & editing, Investigation, Formal analysis, Data curation. Feng Zhao: Software, Methodology, Investigation. Xindong Liu: Writing – review & editing, Validation, Supervision, Resources, Project administration. Lei Shi: Writing – review & editing, Supervision, Resources, Investigation. Tao Pang: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82174010 and 81973512), the Open Fund of the State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University (Grant No. KF-202206, China), and the Double First-Class Project of China Pharmaceutical University (CPUQNJC22_02, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2024.11.019.
Contributor Information
Lei Shi, Email: leishi@dmu.edu.cn.
Tao Pang, Email: tpang@cpu.edu.cn.
Appendix A. Supporting information
The following is the Supporting Information to this article.
References
- 1.Broz P., Dixit V.M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 2.Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 3.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 5.Zindel J., Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol. 2020;15:493–518. doi: 10.1146/annurev-pathmechdis-012419-032847. [DOI] [PubMed] [Google Scholar]
- 6.Yang X.D., Li W., Zhang S., Wu D., Jiang X., Tan R., et al. PLK4 deubiquitination by Spata2-CYLD suppresses NEK7-mediated NLRP3 inflammasome activation at the centrosome. EMBO J. 2020;39 doi: 10.15252/embj.2019102201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J.J., Jo E.K. NLRP3 inflammasome and host protection against bacterial infection. J Korean Med Sci. 2013;28:1415–1423. doi: 10.3346/jkms.2013.28.10.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharif H., Wang L., Wang W.L., Magupalli V.G., Andreeva L., Qiao Q., et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–343. doi: 10.1038/s41586-019-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Sharif H., Vora S.M., Zheng Y., Wu H. Structures and functions of the inflammasome engine. J Allergy Clin Immunol. 2021;147:2021–2029. doi: 10.1016/j.jaci.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Davis B.K., Wen H., Ting J.P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latz E., Xiao T.S., Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaki M.H., Boyd K.L., Vogel P., Kastan M.B., Lamkanfi M., Kanneganti T.D. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowarski R., Jackson R., Gagliani N., de Zoete M.R., Palm N.W., Bailis W., et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell. 2015;163:1444–1456. doi: 10.1016/j.cell.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakhshi S., Shamsi S. MCC950 in the treatment of NLRP3-mediated inflammatory diseases: latest evidence and therapeutic outcomes. Int Immunopharmacol. 2022;106 doi: 10.1016/j.intimp.2022.108595. [DOI] [PubMed] [Google Scholar]
- 16.Ungaro R., Mehandru S., Allen P.B., Peyrin-Biroulet L., Colombel J.F. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi T., Siegmund B., Le Berre C., Wei S.C., Ferrante M., Shen B., et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. doi: 10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 18.Feuerstein J.D., Moss A.C., Farraye F.A. Ulcerative colitis. Mayo Clin Proc. 2019;94:1357–1373. doi: 10.1016/j.mayocp.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Gottlieb Z.S., Sands B.E. Personalised medicine with IL-23 blockers: myth or reality?. J Crohns Colitis. 2022;16:ii73–94. doi: 10.1093/ecco-jcc/jjab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng M., Lin N., Dong D., Ma J., Su J., Sun L. PGAM5: a crucial role in mitochondrial dynamics and programmed cell death. Eur J Cell Biol. 2021;100 doi: 10.1016/j.ejcb.2020.151144. [DOI] [PubMed] [Google Scholar]
- 21.Moriwaki K., Farias Luz N., Balaji S., De Rosa M.J., O'Donnell C.L., Gough P.J., et al. The mitochondrial phosphatase PGAM5 is dispensable for necroptosis but promotes inflammasome activation in macrophages. J Immunol. 2016;196:407–415. doi: 10.4049/jimmunol.1501662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin Y., Li Q., Liang W., Yan R., Tong L., Jia M., et al. TRIM28 SUMOylates and stabilizes NLRP3 to facilitate inflammasome activation. Nat Commun. 2021;12:4794. doi: 10.1038/s41467-021-25033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seok J.K., Kang H.C., Cho Y.Y., Lee H.S., Lee J.Y. Regulation of the NLRP3 inflammasome by post-translational modifications and small molecules. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.618231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik S., Kim J.K., Silwal P., Sasakawa C., Jo E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. 2021;18:1141–1160. doi: 10.1038/s41423-021-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bose S., Segovia J.A., Somarajan S.R., Chang T.H., Kannan T.R., Baseman J.B. ADP-ribosylation of NLRP3 by Mycoplasma pneumoniae CARDS toxin regulates inflammasome activity. mBio. 2014;5 doi: 10.1128/mBio.02186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv Q., Xing Y., Liu J., Dong D., Liu Y., Qiao H., et al. Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation. Acta Pharm Sin B. 2021;11:2880–2899. doi: 10.1016/j.apsb.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng S.L., Li S.Z., Xiao P.T., Cai Y.Y., Chu C., Chen B.Z., et al. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci Adv. 2020;6 doi: 10.1126/sciadv.aax6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cen L., Yi T., Hao Y., Shi C., Shi X., Lu Y., et al. Houttuynia cordata polysaccharides alleviate ulcerative colitis by restoring intestinal homeostasis. Chin J Nat Med. 2022;20:914–924. doi: 10.1016/S1875-5364(22)60220-6. [DOI] [PubMed] [Google Scholar]
- 29.Tamaki H., Nakamura H., Nishio A., Nakase H., Ueno S., Uza N., et al. Human thioredoxin-1 ameliorates experimental murine colitis in association with suppressed macrophage inhibitory factor production. Gastroenterology. 2006;131:1110–1121. doi: 10.1053/j.gastro.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Li X., Cao Y., Li M., Jin F. Upregulation of HES1 promotes cell proliferation and invasion in breast cancer as a prognosis marker and therapy target via the AKT pathway and EMT process. J Cancer. 2018;9:757–766. doi: 10.7150/jca.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trouplin V., Boucherit N., Gorvel L., Conti F., Mottola G., Ghigo E. Bone marrow-derived macrophage production. J Vis Exp. 2013 doi: 10.3791/50966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ying W., Cheruku P.S., Bazer F.W., Safe S.H., Zhou B. Investigation of macrophage polarization using bone marrow derived macrophages. J Vis Exp. 2013;23 doi: 10.3791/50323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooftman A., Angiari S., Hester S., Corcoran S.E., Runtsch M.C., Ling C., et al. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 2020;32 doi: 10.1016/j.cmet.2020.07.016. 468-78.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xian H., Watari K., Sanchez-Lopez E., Offenberger J., Onyuru J., Sampath H., et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022;55:1370–1385. doi: 10.1016/j.immuni.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidler T.P., Xue C., Yalcinkaya M., Hardaway B., Abramowicz S., Xiao T., et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature. 2021;592:296–301. doi: 10.1038/s41586-021-03341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C., Zhou Y., Ning X., Li S., Xue D., Wei C., et al. Directly targeting ASC by lonidamine alleviates inflammasome-driven diseases. J Neuroinflammation. 2022;19:315. doi: 10.1186/s12974-022-02682-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung V., Udeshi N.D., Lam S.S., Loh K.H., Cox K.J., Pedram K., et al. Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nat Protoc. 2016;11:456–475. doi: 10.1038/nprot.2016.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao C., Xu Y., Liang Z., Wang Y., Shang Q., Zhang S., et al. A novel PGAM5 inhibitor LFHP-1c protects blood–brain barrier integrity in ischemic stroke. Acta Pharm Sin B. 2021;11:1867–1884. doi: 10.1016/j.apsb.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jafari R., Almqvist H., Axelsson H., Ignatushchenko M., Lundback T., Nordlund P., et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc. 2014;9:2100–2122. doi: 10.1038/nprot.2014.138. [DOI] [PubMed] [Google Scholar]
- 40.Gao C.L., Hou G.G., Liu J., Ru T., Xu Y.Z., Zhao S.Y., et al. Synthesis and target identification of benzoxepane derivatives as potential anti-neuroinflammatory agents for ischemic stroke. Angew Chem Int Ed Engl. 2020;59:2429–2439. doi: 10.1002/anie.201912489. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X., Wang Q., Li Y., Ruan C., Wang S., Hu L., et al. Solvent-induced protein precipitation for drug target discovery on the proteomic scale. Anal Chem. 2020;92:1363–1371. doi: 10.1021/acs.analchem.9b04531. [DOI] [PubMed] [Google Scholar]
- 42.Marze N.A., Roy Burman S.S., Sheffler W., Gray J.J. Efficient flexible backbone protein-protein docking for challenging targets. Bioinformatics. 2018;34:3461–3469. doi: 10.1093/bioinformatics/bty355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyskov S., Chou F.C., Só Conchúir, Der B.S., Drew K., Kuroda D., et al. Serverification of molecular modeling applications: the Rosetta online server that includes Everyone (ROSIE) PLoS One. 2013;8 doi: 10.1371/journal.pone.0063906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y., Fan W., Gong Q., Tian J., Zhou M., Li Q., et al. Structure-based optimization of 3-phenyl-N-(2-(3-phenylureido)ethyl)thiophene-2-sulfonamide derivatives as selective Mcl-1 inhibitors. J Med Chem. 2021;64:10260–10285. doi: 10.1021/acs.jmedchem.1c00690. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z., Zhang X., Peng C., Wang J., Xu Z., Chen K., et al. D3Pockets: a method and web server for systematic analysis of protein pocket dynamics. J Chem Inf Model. 2019;59:3353–3358. doi: 10.1021/acs.jcim.9b00332. [DOI] [PubMed] [Google Scholar]
- 46.Su H., Yao S., Zhao W., Zhang Y., Liu J., Shao Q., et al. Identification of pyrogallol as a warhead in design of covalent inhibitors for the SARS-CoV-2 3CL protease. Nat Commun. 2021;12:3623. doi: 10.1038/s41467-021-23751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weisser S.B., van Rooijen N., Sly L.M. Depletion and reconstitution of macrophages in mice. J Vis Exp. 2012;(66):4105. doi: 10.3791/4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang K., Bruce M., Pattillo C.B., Zhang S., Stone R., Clifford J., et al. Temporal genomewide expression profiling of DSS colitis reveals novel inflammatory and angiogenesis genes similar to ulcerative colitis. Physiol Genomics. 2011;43:43–56. doi: 10.1152/physiolgenomics.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Y., Zeng M.Y., Yang D., Motro B., Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moriwaki K., Farias Luz N., Balaji S., De Rosa M.J., O'Donnell C.L., Gough P.J., et al. The mitochondrial phosphatase PGAM5 is dispensable for necroptosis but promotes inflammasome activation in macrophages. J Immunol. 2016;196:407–415. doi: 10.4049/jimmunol.1501662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi H., Wang Y., Li X., Zhan X., Tang M., Fina M., et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17:250–258. doi: 10.1038/ni.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng Z., Yang Z., Cui C., Wei H., Wang L., Tian D., et al. NR4A1 suppresses pyroptosis by transcriptionally inhibiting NLRP3 and IL-1β and co-localizing with NLRP3 in trans-Golgi to alleviate pathogenic bacteria-induced colitis. Clin Transl Med. 2021;11:e639. doi: 10.1002/ctm2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tipton P., Su T., Hannink M. Assembly of PGAM5 into multimeric complexes provides a mechanism for allosteric regulation of phosphatase activity. Methods Enzymol. 2018;607:353–372. doi: 10.1016/bs.mie.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 54.Wilkins J.M., McConnell C., Tipton P.A., Hannink M. A conserved motif mediates both multimer formation and allosteric activation of phosphoglycerate mutase 5. J Biol Chem. 2014;289:25137–25148. doi: 10.1074/jbc.M114.565549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz K., Thaker T.M., Agnew C., Miller-Vedam L., Trenker R., Herrera C., et al. Functional role of PGAM5 multimeric assemblies and their polymerization into filaments. Nat Commun. 2019;10:531. doi: 10.1038/s41467-019-08393-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodrigues T., Reker D., Schneider P., Schneider G. Counting on natural products for drug design. Nat Chem. 2016;8:531–541. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- 57.Cerdá B., Espín J.C., Parra S., Martínez P., Tomás-Barberán F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004;43:205–220. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- 58.Chaikuad A., Filippakopoulos P., Marcsisin S.R., Picaud S., Schroder M., Sekine S., et al. Structures of PGAM5 provide insight into active site plasticity and multimeric assembly. Structure. 2017;25:1089–1099. doi: 10.1016/j.str.2017.05.020. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desta I.T., Porter K.A., Xia B., Kozakov D., Vajda S. Performance and its limits in rigid body protein–protein docking. Structure. 2020;28:1071–1081. doi: 10.1016/j.str.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng B., Huang Y., Chen S., Xu R., Xu L., Qiu J., et al. Dextran sodium sulfate potentiates NLRP3 inflammasome activation by modulating the KCa3.1 potassium channel in a mouse model of colitis. Cell Mol Immunol. 2022;19:925–943. doi: 10.1038/s41423-022-00891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y., Chen J., Wei H., Gong K., Meng J., Long T., et al. Akkermansia muciniphila-Nlrp3 is involved in the neuroprotection of phosphoglycerate mutase 5 deficiency in traumatic brain injury mice. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1172710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glassner K.L., Abraham B.P., Quigley E.M.M. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145:16–27. doi: 10.1016/j.jaci.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Ni J., Wu G.D., Albenberg L., Tomov V.T. Gut microbiota and IBD: causation or correlation?. Nat Rev Gastroenterol Hepatol. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conforti-Andreoni C., Ricciardi-Castagnoli P., Mortellaro A. The inflammasomes in health and disease: from genetics to molecular mechanisms of autoinflammation and beyond. Cell Mol Immunol. 2011;8:135–145. doi: 10.1038/cmi.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L., Hauenstein A.V. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol Aspects Med. 2020;76 doi: 10.1016/j.mam.2020.100889. [DOI] [PubMed] [Google Scholar]
- 66.Huang X., Feng Z., Jiang Y., Li J., Xiang Q., Guo S., et al. VSIG4 mediates transcriptional inhibition of and β in macrophages. Sci Adv. 2019;5 doi: 10.1126/sciadv.aau7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin Y., Li Z., Wang Y., Tian T., Jia P., Ye Y., et al. CCDC50 suppresses NLRP3 inflammasome activity by mediating autophagic degradation of NLRP3. EMBO Rep. 2022;23 doi: 10.15252/embr.202154453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui S., Wang C., Bai W., Li J., Pan Y., Huang X., et al. CD1d1 intrinsic signaling in macrophages controls NLRP3 inflammasome expression during inflammation. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jo E.K., Kim J.K., Shin D.M., Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13:148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaki M.H., Lamkanfi M., Kanneganti T.D. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32:171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lissner D., Siegmund B. The multifaceted role of the inflammasome in inflammatory bowel diseases. SWorldJournal. 2011;11:1536–1547. doi: 10.1100/tsw.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strowig T., Henao-Mejia J., Elinav E., Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 73.Zhu Y., Gu L., Lin X., Liu C., Lu B., Cui K., et al. Dynamic regulation of ME1 phosphorylation and acetylation affects lipid metabolism and colorectal tumorigenesis. Mol Cell. 2020;77:138–149. doi: 10.1016/j.molcel.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 74.O'Regan L., Blot J., Fry A.M. Mitotic regulation by NIMA-related kinases. Cell Div. 2007;2:25. doi: 10.1186/1747-1028-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim S., Kim S., Kim S., Rhee K. NEK7 is essential for centriole duplication and centrosomal accumulation of pericentriolar material proteins in interphase cells. J Cell Sci. 2011;124:3760–3770. doi: 10.1242/jcs.078089. [DOI] [PubMed] [Google Scholar]
- 76.Tan R., Nakajima S., Wang Q., Sun H., Xue J., Wu J., et al. Nek7 protects telomeres from oxidative DNA damage by phosphorylation and stabilization of TRF1. Mol Cell. 2017;65:818–831. doi: 10.1016/j.molcel.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andreeva L., David L., Rawson S., Shen C., Pasricha T., Pelegrin P., et al. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell. 2021;184:6299–6312. doi: 10.1016/j.cell.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J., Sun Xa, Yang N., Ni J., Xie H., Guo H., et al. Phosphoglycerate mutase 5 initiates inflammation in acute kidney injury by triggering mitochondrial DNA release by dephosphorylating the pro-apoptotic protein Bax. Kidney Int. 2023;103:115–133. doi: 10.1016/j.kint.2022.08.022. [DOI] [PubMed] [Google Scholar]
- 79.Yu Y.-Q., Zielinska M., Li W., Bernkopf D.B., Heilingloh C.S., Neurath M.F., et al. PGAM5–MAVS interaction regulates TBK1/IRF3 dependent antiviral responses. Sci Rep. 2020;10:8323. doi: 10.1038/s41598-020-65155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Venusova E., Kolesarova A., Horky P., Slama P. Physiological and immune functions of punicalagin. Nutrients. 2021;13:2150. doi: 10.3390/nu13072150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith A.D., George N.S., Cheung L., Bhagavathy G.V., Luthria D.L., John K.M., et al. Pomegranate peel extract reduced colonic damage and bacterial translocation in a mouse model of infectious colitis induced by Citrobacter rodentium. Nutr Res. 2020;73:27–37. doi: 10.1016/j.nutres.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Du R., Cooper L., Chen Z., Lee H., Rong L., Cui Q. Discovery of chebulagic acid and punicalagin as novel allosteric inhibitors of SARS-CoV-2 3CL. Antivir Res. 2021;190 doi: 10.1016/j.antiviral.2021.105075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shah T.A., Parikh M., Patel K.V., Patel K.G., Joshi C.G., Gandhi T.R. Evaluation of the effect of Punica granatum juice and punicalagin on NFκB modulation in inflammatory bowel disease. Mol Cell Biochem. 2016;419:65–74. doi: 10.1007/s11010-016-2750-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.