Abstract
Kupffer cells (KCs), as residents and sentinels of the liver, are involved in the formation of hepatic fibrosis (HF). However, the biological functions of circular RNAs (circRNAs) in KCs to HF have not been determined. In this study, the expression levels of circRNAs, microRNAs, and messenger RNAs (mRNAs) in KCs from a mouse model of HF mice were investigated using microarray and circRNA-Seq analyses. circDcbld2 was identified as a candidate circRNA in HF, as evidenced by its up-regulation in KCs. Silver staining and mass spectrometry showed that Wtap and Igf2bp2 bind to cirDcbld2. The suppression of circDcbld2 expression decreased the KC inflammatory response and oxidative stress and inhibited hepatic stellate cell (HSCs) activation, attenuating mouse liver fibrogenesis. Mechanistically, Wtap mediated the N6-methyladenosine (m6A) methylation of circDcbld2, and Igf2bp2 recognized m6A-modified circDcbld2 and increased its stability. circDcbld2 contributes to the occurrence of HF by binding miR-144-3p/Et-1 to regulate the inflammatory response and oxidative stress. These findings indicate that circDcbld2 functions via the m6A/circDcbld2/miR-144-3p/Et-1 axis and may act as a potential biomarker for HF treatment.
Key words: Kupffer cells, circDcbld2, miR-144-3p, Et-1, N6-methyladenosine, Wtap, Igf2bp2, Hepatic fibrosis
Graphical abstract
The authors discovered that Wtap mediates the N6-methyladenosine (m6A) methylation of circDcbld2, and then Igf2bp2 recognized m6A-modified circDcbld2 and increased its stability. circDcbld2 is involved in the occurrence of liver fibrosis by binding miR-144-3p/Et-1 to regulate inflammatory response and oxidative stress.
1. Introduction
Hepatic fibrosis (HF), a chronic liver disease, is characterized by excessive extracellular matrix (ECM) deposition1,2. HF can progress to cirrhosis or even cancer resulting in persistent liver injury3, 4, 5, and is caused by various conditions, such as alcohol abuse and non-alcoholic steatohepatitis (NASH)6. Macrophage infiltration in the liver accompanies persistent liver injury7, 8, 9. Interestingly, the uncontrolled secretion of inflammatory factors and chemokines by macrophages are important driving force for HF formation10. In addition, inflammation-induced changes in the liver microenvironment lead to dysregulation of liver function, reducing the capacity for self-repair and accelerating HF progression11.
Kupffer cells (KCs) and recruited macrophages are crucial regulators of inflammation in the organ and are considered potential new targets for the treatment and prevention of HF12. Activated macrophages synthesize and release various inflammatory cytokines and chemokines that accelerate inflammatory infiltration and collectively drive activated hepatic stellate cells (HSCs) to transform into myofibroblasts12,13, while simultaneously producing enormous amounts of actin alpha 2, smooth muscle, aorta (α-SMA)14. Therefore, it is essential to explore the mechanisms by which macrophage activation affects HSC activation during HF formation. Recent work has shown that liver macrophages promote HF by enhancing HSC activation in a nuclear factor kappa B (NF-κB)-dependent manner15. However, the precise molecular mechanisms by which macrophages are involved in HF remain largely unclear.
N6-methyladenosine (m6A), a common modification of messenger RNA (mRNA)16,17, has recently gained attention owing to its potential involvement in Circular RNAs (circRNA) function. circRNAs, are small non-coding RNAs (ncRNAs) with a covalently closed single-stranded loop configuration produced by exon skipping or direct back-splicing of precursor mRNA (pre-mRNA)18, exhibit different manifestation m6A modification patterns from those of traditional mRNA19,20.
Recent advances in RNA sequencing technology have revealed that most circRNAs exhibit tissue-specific expression profiles21. circRNAs act as competing endogenous RNAs (ceRNAs) and specifically adsorb microRNAs (miRNAs) to affect downstream target gene expression or regulate gene expression at the levels of transcription and splicing22, 23, 24. Differential circRNA expression is associated with various pathological processes, including HF25. circRNAs are also dysregulated in macrophages during disease development26,27. Our group has studied the roles of non-coding RNAs in the development of HF extensively28, 29, 30, 31, 32. We have shown that circFbxw4 (F-box and WD repeat domain containing 4) affects HF progression via the miR-18b-3p/Fbxw7 (F-box and WD repeat domain containing 7) axis in HSCs28. Furthermore, circUbe2k33 (ubiquitin conjugating enzyme E2 K), circPsd329 (Pleckstrin and Sec7 domain containing 3), and circMcph134 (Microcephalin 1) play significant roles in HF. The functions and mechanisms of circRNAs that are differentially expressed in macrophages during HF formation have been a focus of our research35.
In this study, the expression patterns of circRNAs, miRNAs, and mRNAs in KCs extracted from a mouse model of HF were analyzed to explore the potential biomarkers. We identified a novel abnormally expressed circRNA, circDcbld2, derived from the Discoidin, CUB and LCCL domain containing 2 (Dcbld2) gene locus. circDcbld2 expression levels were significantly increased in HF formation and were elevated in patients with HF. The inhibition of circDcbld2 reduced the expression of inflammatory factors in macrophages, attenuated oxidative stress, and affected HSC activation to mitigate liver fibrogenesis in mice. Mechanistically, we discovered that circDcbld2 binds to miR-144-3p to regulate Endothelin 1 (Et-1) expression. These findings indicate that the circDcbld2/miR-144-3p/Et-1 axis may have important functions in macrophages and contribute to the pathogenesis of HF. Furthermore, our investigation revealed an interaction between circDcbld2 and WT1 associated protein (Wtap), a pivotal m6A writer protein, and the stability of circDcbld2 was increased via insulin-like growth factor 2 mRNA binding protein 2 (Igf2bp2), a pivotal m6A reader protein. Therefore, our results support the value of circDcbld2 as a novel potential therapeutic biomarker for HF.
2. Materials and methods
2.1. Animals and model establishment
C57BL/6J mice (6–8 weeks of age) were obtained from the Animal Experiment Center of Anhui Medical University (Anhui, China). Before the experiment, mice were housed in an environment with adequate food and water for one week. Euthanasia was performed 3 days after the last injection for modeling. A set of mice was used for the perfusion extraction of primary cells and detection of related indicators, and the remaining mice were used for histopathological examination of tissue following paraformaldehyde embedding. The animal studies were approved and reviewed by the Animal Experimentation Ethics Committee of Anhui Medical University.
Various methods were used for HF model were establishment. Firstly, for carbon tetrachloride (CCl4)-induced HF model, as described previously33. Carbon tetrachloride (CCl4) and olive oil (1:4, v/v) were administered to mice by intraperitoneal injection (1 mL/kg), biweekly for 6 weeks to trigger HF. Vehicle mice were injected with olive oil (same volume).
Secondly, the model of cholestatic HF was established through bile duct ligation (BDL) as described previously36. The cholestatic liver fibrosis model was housed in a specific pathogen-free (SPF)-class experimental animal room at the Experimental Animal Center of Anhui Medical University. Mice in the BDL group were established by common bile duct ligation, operated by double ligation using non-resorbable surgical sutures. The sham group underwent identical procedures without ligation. After 15 days, mice were sacrificed to observe fibrosis.
Thirdly, another model of HF was established through the injection of Thioacetamide (TAA) (200 mg/kg, diluted in saline) 3 times weekly for 8 weeks37. Mice were sacrificed 24 h after the last administration, and fractional liver tissues were collected for histopathological examination.
2.2. Generation of macrophage-specific Wtap knockout mice
Wtap-cKO (Wtap-conditional knockout) (C57BL/6J background) mice were generated in which Wtap was specifically depleted from macrophages (global Wtap knockout is embryonically lethal). WtapF/F (WtapFlox/Flox) mice were purchased from GemPharmatech Co., Ltd. (Nanjing, China). WtapF/F;Lyz-Cre+ (Wtap-cKO) mice were generated by mating WtapF/F mice with macrophage-specific promoter (Lysozyme 2)-driven Cre (Lyz-Cre) mice.
2.3. circRNA expression analysis
2.3.1. RNA extraction and quality control
Primary macrophages were extracted from the vehicle and CCl4-treated mice. Each group of samples contained primary macrophages from six mice for sequencing. Total RNAs of KCs were extracted using the MIRNeasy Mini Kit (Qiagen, Hilden, Germany). Then, the RNase-Free DNase Set and RNA Clean XP Kit (Beckman Coulter, Brea, CA, USA) were used to purify the extracted RNA. Quantitative detection was performed using the NanoDrop 2000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA) and Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA).
2.3.2. Library preparation and high-throughput sequencing
Following the manufacturer's instructions, RNA-seq libraries were constructed using the TruSeq® Stranded Total RNA Sample Preparation Kit (Illumina, USA). Subsequently, the Qubit® 2.0 Fluorometer (Life Technologies, USA) and Agilent 2100 Bioanalyzer (Agilent Technologies, USA) were used for quantification and validation. The library was diluted to 10 pmol/L and sequenced using the Illumina HiSeq 2500 system (Illumina, USA). Sequencing and library validation and construction were performed by Origin Biotech (Shanghai, China).
2.3.3. Data analysis
Quality control for RNA-Seq reads was performed using FastQC1 (v.0.11.3). Ribosomal RNA reads, poor reads and Illumina TruSeq adapter sequences were trimmed using seqtk. Then, BWA-MEM (v.2.0.4) was used to map mouse reference genes and trimmed reads. circRNA sequencing data were analyzed using CIRI, and matched against the circBase database. The counts were normalized to SRPBM. The thresholds for differential expression were adjusted P-value <0.05 and |log2 (fold change)| ≥ 1.0. All of the dysregulated genes were evaluated using a heat map and KEGG pathway enrichment analyses.
2.4. RNA sequencing and functional enrichment analysis
RNA samples were isolated and purified using TRIzol (Thermo Fisher Scientific, USA) according to the manufacturer's protocol. PolyA mRNA was specifically captured through two rounds of purification using oligo (dT) magnetic beads (Thermo Fisher, USA). The fragmented RNA was subjected to reverse transcription to synthesize cDNA (Invitrogen SuperScript™ II Reverse Transcriptase, Cat. 1896649, CA, USA). These compound DNA and RNA duplexes were then converted to DNA duplexes by double-strand synthesis using E. coli DNA polymerase I (NEB, USA) and RNase H (NEB, USA). The two strands were digested with UDG enzymes (NEB, cat.m0280, MA, US) to form a library with a fragment size of 300 bp ± 50 bp (strand-specific library). Then, the Illumina NovaSeq™ 6000 platform (LC Bio Technology Co., Ltd., Hangzhou, China) was used for paired-end sequencing following the standard operation in PE150 mode. Differentially expressed mRNAs were identified using adjusted P-value <0.05 and |log2 (fold change)| ≥ 1.0 as thresholds. Gene functions were evaluated using Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) functional enrichment analyses.
2.5. Microarray analysis
According to the instructions provided, the Affymetrix GeneChip® miRNA 4.0 Array (Affymetrix, USA) and FlashTag™ Biotin HSR Labeling Kit (Affymetrix, USA) were used. The GeneChip Hybridization Wash and Stain Kit (Thermo Fisher Scientific, USA) was used to dye the miRNA array for imaging. Differentially expressed miRNAs were identified using |log2 (fold change)| ≥ 1.0), P-value <0.05, and volcano plot filtering. The targets of miRNAs were obtained using two databases TargetScan and miRanda.
2.6. ceRNA network (ceRNET) snalysis
A ceRNET (circRNAs–miRNAs–mRNAs) was generated based on dysregulated circRNAs, miRNAs, and mRNAs. The interaction ceRNET was established using Cytoscape.
2.7. Human samples
Human liver samples were obtained from the First Affiliated Hospital of Anhui Medical University (Anhui, China). This study was approved by the Biomedical Ethics Committee of Anhui Medical University. The volunteers were undertaken with the understanding and written consent. All protocols adhered to the principles outlined in the Declaration of Helsinki. Characteristics of participants are shown in Supporting Information Table S1.
2.8. circDcbld2 suppression in mice
pHBAAV-circDcbld2 (circDcbld2-KD) and control were designed and synthesized by Hanbio Biotechnology (Hanbio Biotechnology, China). Mice were injected with circDcbld2-KD and the vector at 1 × 1012 vg/mL into the tail vein. After a week of observation, the model was established by circDcbld2-KD administration. The transfection efficiency for circDcbld2 was measured by qRT-PCR in primary macrophages.
2.9. Flow cytometry analysis
Cells were incubated with anti-Cd11b-FITC (BD Biosciences, USA) and anti-F4/80-PE (BD Biosciences, USA) antibodies. Next, the CytoFLEX flow cytometer (Beckman Coulter, USA) was used to detect compounds. Cd11b+ and F4/80+ cells were considered macrophages for subsequent experiments, and CytExpert software (Beckman Coulter, USA) was used to analyze data.
2.10. Histology and immunohistochemistry
Paraffin-embedded and 4% paraformaldehyde-fixed liver tissues (4 μm) were used for immunofluorescence (IF) and immunohistochemical (IHC) staining of Et-1 (Abcam, USA), F4/80 (Bioss, bsm-34028M), and α-SMA (Abcam, USA). Liver pathology was evaluated by Sirius red and H&E staining. Sections were scanned using a digital slide scanner (Pannoramic MIDI, 3DHISTECH, Hungary).
2.11. Examination of oxidative stress markers
Commercial assay kits for the detection of Tatalase (CAT; #A007-1-1), glutathione (GSH; #A006-2-1), malondialdehyde (MDA; #A003-1-3), and superoxide dismutase (SOD; #A001-3-2) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Oxidative stress damage of ROS in liver tissue for the HF model was examined by ROS assay kit (Bestbio, #BB-470534) following the manufacturer's protocols.
2.12. DNA sequencing
RNA was reverse-transcribed into cDNA by using PrimeScriptTMRT Master Mix (Takara, Japan). Polymerase chain reaction (PCR) was performed using 2 × Taq Master Mix (Takara, Japan) following the manufacturer's instructions. The PCR products were identified by using DNA sequencing (ABI3730XL, USA).
2.13. Pull-down assay
A biotinylated circDcbld2 probe was designed for binding to the junction site of circDcbld2. Bone marrow-derived macrophages (BMDMs) were washed with PBS, and the cell lysate was incubated with 3 μg of biotinylated probe for 4 h. Lysates were incubated with RNase-free BSA (10 mg/mL) and Pierce™ Streptavidin Magnetic Beads (Thermo Fisher Scientific, USA) at 4 °C (3 h) to decreasing nonspecific binding. RNA complexes were rotated with probe-bead complexes at 4 °C overnight. After washing the beads three times, relative protein levels were analyzed through Western blotting.
2.14. Fluorescence in situ hybridization (FISH)
In situ hybridization probes, i.e., FAM-labelled circDcbld2 and FAM-labelled miR-144-3p probes, were established by GenePharma (Shanghai, China). Briefly, cells were washed three times with sterile PBS and fixed with 4% paraformaldehyde at room temperature. A FISH Kit (GenePharma, China) was employed for protein detection according to the manufacturer's instructions, with hybridization at 37 °C overnight in a dark moist chamber. DAPI was used to stain nuclei. Images were collected by an inverted fluorescence microscope (Leica, Japan).
2.15. Isolation of bone marrow-derived macrophages, Kupffer cells, hepatocytes, and hepatic stellate cells
Previously described methods were used to isolate KCs38, hepatocytes, HSCs28, and BMDM39. In situ perfusion was performed using collagenase followed by differential centrifugation according to a density gradient. A catheter was inserted in the liver portal vein of the mouse and dissect the inferior vena cava and perfusion. The digested liver was passed through a sieve (200-mesh). Then, 25% and 50% Percoll were used to separate macrophages. The isolated cells were resuspended in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS). Non-adherent cells and culture medium were discarded after the liver macrophages adhered to the flask surface for 40 min. Macrophages were identified by using flow cytometry40. Hepatocytes were isolated from the mouse liver by in situ collagenase perfusion. The liver was perfused with HBSS (2%FBS) via the portal vein, followed by 0.27% collagenase IV. Perfused livers were dissected and teased through 70 μm nylon mesh cell strainers. Hepatocytes were collected following centrifugation at 50 × g (2 min three times). Primary HSCs were isolated through two-step collagenase (Sigma)–pronase (Sigma) perfusion of mouse livers. Then, OptiPrep (Axis Shield, Norway) was used to for density gradient centrifugation (11.5% and 20%)41. Furthermore, tibias and femurs were isolated from mice via cutting at the knee joint. Bone marrows were collected and cultured for 7 days and terminally differentiated into macrophages.
2.16. siRNA-circDcbld2 and over-expression circDcbld2 plasmid transfection
Small interfering RNAs (siRNAs) and an over-expression plasmid for circDcbld2 were obtained from Hanheng Biotechnology (Shanghai, China). BMDMs were transfected with siRNA-circDcbld2 with Lipofectamine 2000 (Invitrogen, USA). The culture medium was replaced, after 6 h, followed by incubation for an additional 24 h. The transfection efficiency of circDcbld2 was detected using qRT-PCR. Sequences are shown in Supporting Information Table S2.
2.17. miR-144-3p mimics and inhibitor transfection
miR-144-3p mimics and the inhibitor were structured in Hanheng Biotechnology (Shanghai, China). The transfection methods were consistent with those for circDcbld2. A qRT-PCR analysis was used to measure the transfection efficiency of the miR-144-3p mimics and inhibitor. Related sequences are shown in Supporting Information Table S3.
2.18. RNA immunoprecipitation (RIP)
The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore Sigma, 17-701) was used for an RIP analysis following the manufacturer's protocols. Magnetic beads precoated with Igf2bp2 or IgG (Millipore) were incubated with the cell suspension at 4 °C overnight. Proteinase K was used to treat RNA–protein complexes to remove protein impurities. Finally, RNA was purified using TRIzol and detected using qRT-PCR.
2.19. m6A MeRIP-qRT-PCR
RNA samples were collected in Wtap-cKO or WtapF/F BMDMs under lipopolysaccharide (LPS) stimulation. The RNA was sonicated to obtain 100–150 nt fragments, which were incubated with m6A antibody to analyze m6A enrichment using qRT-PCR. Briefly, fragmented RNA was combined with the m6A antibody in RIP immunoprecipitation buffer and incubated at 4 °C overnight, followed by incubation in proteinase K buffer at 55 °C for 30 min. TRIzol was used to extract RNA, and m6A immunopurification of mRNA was detected using qRT-PCR.
2.20. Dot blot
Extracted RNA was boiled in a metal bath at 90 °C for 5 min and cooled immediately. The RNA sample point on the nylon membrane (UV lamp for 2 h) was blocked with 5% skim milk (2 h) and incubated with the m6A antibody overnight. The nylon membrane was washed with TBST (Tris-buffered saline containing 0.1% Tween 20) three times on the next day. The secondary antibody was applied, followed by detection using the Amersham Imager 600 System. Methylene blue was used to stain the membranes.
2.21. RNA extraction and qRT-PCR analysis
Total RNA was extracted with TRIzol from cells or tissues. The NanoDrop 2000 Spectrophotometer was employed to detect the purity and concentration of extracted RNA. PrimeScrip™ RT Master Mix was used to synthesize cDNA. Gapdh and β-actin expression were used as internal controls. Primer sequences are listed in Supporting Information Tables S4–S6.
2.22. Nuclear and cytoplasmic fractionation analysis
The Cytoplasmic & Nuclear RNA Purification Kit (#BB-36021, BestBio) was used for a nuclear and cytoplasmic fractionation analysis. The nuclear and cytoplasmic components were extracted through centrifugation (1200 × g for 5 min). Then, each fraction was collected separately.
2.23. Actinomycin D (Act-D) and RNase R treatment
Linear RNA was removed with RNase R (Epicentre Technologies, USA). The samples (RNase R+ and RNase R–) were supplemented with reaction buffer (10 ×, 0.6 μL) and DEPC-water (0.2 μL) for 20 min at 37 °C. circRNA and mRNA exposed to RNase R were detected with CFX96 qRT-PCR (Bio-Rad, CA). BMDM transcription was blocked with Actinomycin D (4, 8, 16, and 24 h) (2 μg/mL, Sigma, USA). qRT-PCR was used to evaluate the stability of circDcbld2 and the mRNA level of linear Dcbld2.
2.24. Luciferase reporter assay
circDcbld2 sequences with miR-144-3p target sites were synthesized and cloned into the pSI-Check2 reporter vector downstream of firefly luciferase (circDcbld2-wild type and circDcbld2-mutant) by Hanheng Biotechnology (Shanghai, China), respectively. The reporter vector, miR-144-3p mimics, or negative control were transfected in HEK-293T cells using Lipofectamine 3000 (Invitrogen, CA). Activity levels of firefly and Renilla luciferase were measured using a Dual-Luciferase system (Promega, USA) according to the manufacturer's protocol and detected by GloMax Multi Jr (Promega, USA).
2.25. Western blotting
Whole-protein samples were transferred to a PVDF membrane (Millipore, USA). The antibodies used in the analysis were as follows: β-actin (1:500, Sino Biological lnc., 100166-MM10), Wtap (1:500; Cell Signaling Technology, #56501), Igf2bp2 (1:500; Abcam, ab124930). α-SMA (1:500; Abcam, ab7817), Col1α1 (1:500; Bioss, bs-0578R). Then, membranes were incubated with secondary antibodies (HRP-coupled) (1:1000, ZSGB-Bio, China) for 60 min at room temperature. Finally, protein signals were analyzed using an enhanced chemiluminescence (ECL) system (Bio-Rad, USA).
2.26. Statistical analysis
Data are shown as the mean ± standard error of mean (SEM) and were analyzed by one-way analysis of variance (ANOVA), followed by Newman–Keuls post hoc tests implemented in Prism 8.0 (GraphPad Software, USA). Correlations in expression levels were evaluated using Pearson's correlation coefficients. P value < 0.05 were considered statistically significant.
3. Results
3.1. circDcbld2 up-regulated in mouse KCs by high-throughput sequencing
The secretion of inflammatory factors and chemokines from KCs is critical during HF42. To identify candidate circRNAs involved in HF, primary KCs (isolated from vehicle or HF mice) were examined by circRNA high-throughput sequencing. The progression of HF formation is shown in Supporting Information Fig. S1A. We first successfully established BDL, CCl4, and TAA-induced HF mouse models and confirmed injury and pathological characteristics (Fig. 1A and Fig. S1B–S1D). Inflammatory factors were increased in KCs (Fig. S1E) and tissues (Fig. S1F) from HF mice than from control mice. The serological indicator alanine/aspartate-transaminase (ALT/AST) in CCl4-induced HF mice is summarized in Fig. S1G. These results indicate successful model construction.
Figure 1.
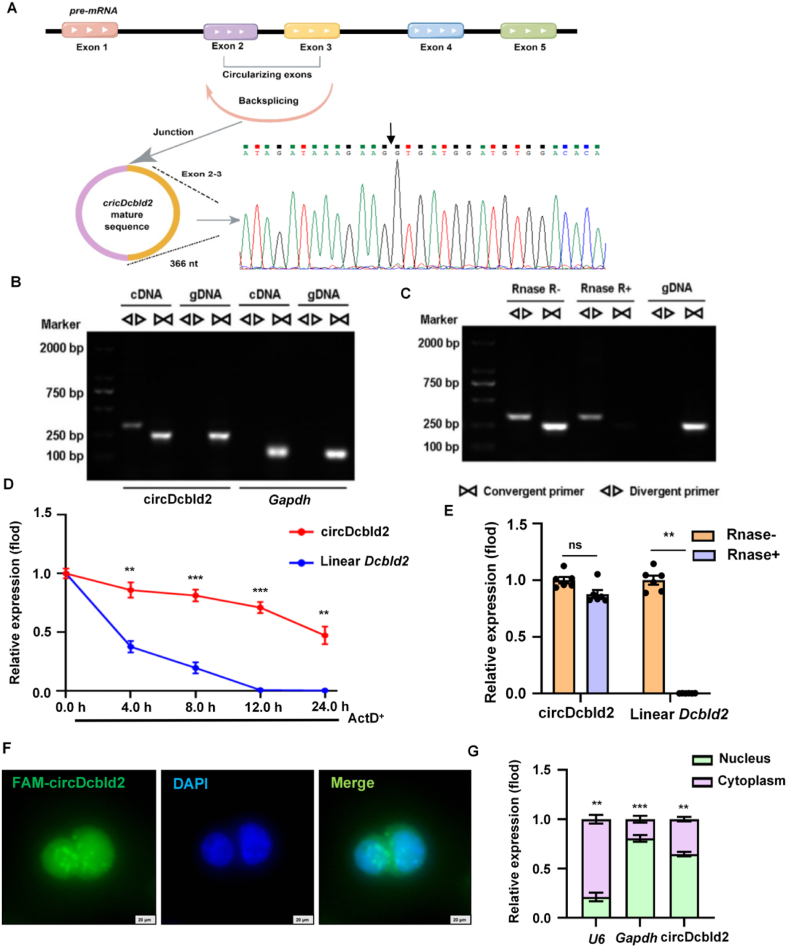
circDcbld2 up-regulated in mouse KCs by high-throughput sequencing. (A) Pathology observation stained with Sirius red staining were performed in CCl4-induced, BDL-induced, TAA-induced and vehicle mouse liver tissues sections. Scale bar = 100 μm. (B) The up-regulated of circDcbld2 in HF mice induced by CCl4, TAA and BDL (n = 6). (C) The process of BMDM extraction. (D) KCs were identified by flow cytometry. (E) The expression of circDcbld2 in KCs, BMDM, HSCs and Hepatocytes (n = 6). (F) Pathology observation of human fibrotic liver tissues sections stained with H&E and IHC of α-SMA. (G) circDcbld2 expressed in human fibrotic liver tissues. The bar shows the mean ± SEM. ∗P < 0.05, ∗∗∗P < 0.001 vs vehicle group.
We detected differential expression of 3138 circRNAs between KCs extracted from HF mice and control mice, of which 457 circRNAs were recorded in circBase. The circRNAs with log2FC ≥ 1.0 were selected for subsequent analyses. Further analysis indicated 99 circRNAs were expressed differently in HF (Supporting Information Files S1 and S2), including 39 up-regulated and 60 down-regulated circRNAs (Supporting Information File S3). These dysregulated circRNAs, after validation, may be related to the development of HF. The relative expression levels of circRNAs are shown in Supporting Information Fig. S2A and the circRNA gene distribution is shown in Fig. S2B. Back-splicing from host genes is considered the source of most circRNAs18. The biological characteristics (including length, localization, host genes, and exons/introns) of the circRNAs were evaluated. We selected 13 circRNAs for model validation (Fig. S2C). Based on validation and expression intensity results, we selected circDcbld2 for further analysis. Notably, we found that circDcbld2 was up-regulated in HF mice induced by CCl4, TAA, and BDL (Fig. 1B). We also detected circDcbld2 expression in BMDMs (Fig. 1C), KCs (Fig. 1D), HSCs, and hepatocytes, extracted from CCl4-induced HF mice. Levels of circDcbld2 were significantly elevated in KCs and BMDMs in HF, and did not differ significantly in HSCs and hepatocytes (Fig. 1E). To explore the expression changes of circDcbld2 (hsa_circ_0066631) in human liver fibrosis, we extracted RNA from human liver tissues for detection, revealing that circDcbld2 as up-regulated (Fig. 1F–G and Fig. S2D). These findings indicate that the expression pattern of circDcbld2 corresponds with the pathology of HF; accordingly, circDcbld2 is a potential biomarker during HF formation.
3.2. Characterization of circDcbld2
circDcbld2 (mmu_circ_0000693) is derived from the host gene Dcbld2 located on chromosome 16 (58424673–58433576) (366 nt). Genomic structure and back-splicing point analyses revealed that circDcbld2 consists of two exons from the Dcbld2 (exons 2–3) (Fig. 2A). Furthermore, the qRT-PCR product of circDcbld2 verified that the circRNA matched the circDcbld2 sequence from circBase and showed head-to-tail splicing. Using convergent and divergent primers designed for circDcbld2, and genomic DNA (gDNA) and complementary DNA (cDNA) as templates, 1% agarose gel electrophoresis revealed a distinct, single product of circDcbld2 using divergent primers from cDNA only, while no product was obtained from gDNA (Fig. 2B). To verify the circRNA characteristics of circDcbld2, we confirmed resistance to RNase R digestion (Fig. 2C). Furthermore, an Actinomycin D (Act D) analysis showed that circDcbld2 had greater stability than that of the linear Dcbld2 transcript in KCs (Fig. 2D). We also treated circDcbld2 and linear Dcbld2 with RNase R, revealing that circDcbld2 was resistant to RNase R digestion (Fig. 2E). FISH and cytoplasmic and nuclear fractionation assays, with U6 and Gapdh as controls, revealed that circDcbld2 localized to both the nucleus and cytoplasm (Fig. 2F and G).
Figure 2.
Characterization of circDcbld2. (A) The genomic structure and backsplicing point of circDcbld2. (B) Divergent primers amplified circDcbld2 from cDNA by PCR and an agarose gel electrophoresis, rather than from gDNA, Gapdh was used as a linear control. (C) circDcbld2 from cDNA was analyzed by divergent primers even exposed to Rnase R digestion, the opposite result showed from gDNA. (D) Actinomycin D was added to detected the circDcbld2 and linear Dcbld2 expression in KCs at the indicated time points. (E) circDcbld2 resisted to Rnase R digestion (n = 6). (F, G) Fluorescence in situ hybridization and Cytoplasmic and nuclear fractionation assay revealed that circDcbld2 localized to both the nucleus and cytoplasm. Scale bar = 20 μm. The bar shows the mean ± SEM. ns, no significance; ∗∗P < 0.01, ∗∗∗P < 0.001 vs Linear Dcbld2 group (D); Rnase− group (E) and Nucleus group (F).
3.3. Wtap mediates circDcbld2 m6A modification and increases the stability viaIgf2bp2
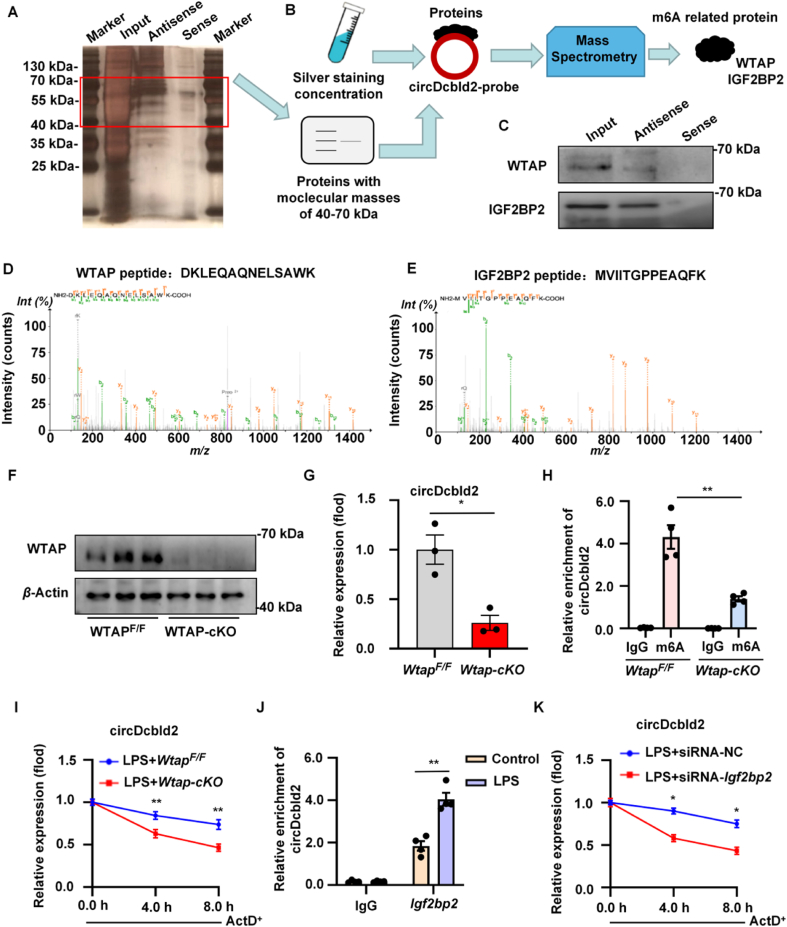
Cytoplasmic circRNAs act as miRNA sponges or as RNA-binding proteins (RBPs), while nuclear circRNAs mainly interact with RBPs43. Given the location of circDcbld2, RNA pull-down and mass spectrometry (MS) were employed to explore its functions (Fig. 3A). We detected m6A-related proteins with protein molecular weights of 40–70 kDa and found two proteins, WTAP and IGF2BP2, were consistently pulled down by the biotin-labeled circDcbld2 antisense probe (Fig. 3B and C). The pull-down concentration and silver staining of the polypeptide sites of Wtap and Igf2bp2 are shown in Fig. 3D and E, and Supporting Information Fig. S3A and S3B. We then constructed WtapF/F;Lyz-Cre+ (Wtap-cKO) mice and confirmed that the knockout efficiency through Western blotting (Fig. 3F and Fig. S3C). Colorimetric and dot blot assays indicated the m6A methylation level was lower in Wtap-cKO mice than in WtapF/F (Fig. S3D and S3E). We predicted a credible methylation site of circDcbld2 using SRAMP (http://www.cuilab.cn/sramp) (Fig. S3F). Luciferase reporter gene constructs with wild-type or mutant circDcbld2 further verified the m6A modification of circDcbld2 by Wtap (Fig. S3G). The luciferase reporter assay indicated that the level of wild-type circDcbld2 decreased significantly after Wtap-cKO administration but not in response to mutant circDcbld2 (Fig. S3H). Furthermore, circDcbld2 levels were significantly decreased in Wtap-cKO than in WtapF/F (Fig. 3G). MeRIP-qPCR results proved that circDcbld2 was enriched for m6A modifications in the LPS-stimulated WtapF/F group, while this enrichment was decreased in the LPS-stimulated Wtap-cKO group (Fig. 3H). These results demonstrated that the Wtap-related m6A modification regulated circDcbld2 expression. Recently, Igf2bp2 has been identified as an m6A-related “reader” protein involved in the stability of downstream genes by recognizing m6A modifications44. Silver staining and mass spectrometry revealed enrichment for the stability-related protein Igf2bp2; therefore, we further evaluated whether m6A modification affects circDcbld2 stability. The results showed that Wtap-cKO significantly reduced the stability of circDcbld2, as determined using Act D treatment (Fig. 3I). An RIP analysis further proved that circDcbld2 and Igf2bp2 interact in LPS-stimulated BMDMs (Fig. 3J). RNA stability analysis indicated that Igf2bp2 inhibition reduced circDcbld2 stability following Act D treatment (Fig. 3K), and shortened the RNA half-life of circDcbld2 (Fig. S3I), indicating that Igf2bp2 participated in the maintenance of circDcbld2 stability. Furthermore, RIP-qPCR analysis showed that an Igf2bp2-specific antibody resulted in significantly greater circDcbld2 enrichment in the WtapF/F group than in the IgG control, and this increase was obviously reduced in the Wtap-cKO group (Fig. S3J). Therefore, the increase in circDcbld2 expression in the model group may be due to the modified and identified of circDcbld2 m6A site by Wtap and Igf2bp2 to enhance the stability of circDcbld2, this clarifying the mechanism underlying circDcbld2 up-regulation in liver fibrosis.
Figure 3.
Wtap mediates circDcbld2 m6A modification and increases the stability via Igf2bp2. (A, B) The process of RNA pull-down and MS. (C) Western blot analysis of WTAP and IGF2BP2. (D, E) The polypeptide sites of Wtap and Igf2bp2 in pull-down concentration and silver staining. (F) The Western blot analysis of knockout efficiency of WTAP. (G) Relative expression of circDcbld2 decreased in Wtap-cKO group. (H) Wtap-mediated circDcbld2 m6A modifications was detected with MeRIP-qPCR analysis. The m6A modification of circDcbld2 was decreased following Wtap-cKO (n = 4). (I) The stability of circDcbld2 was weaken by Wtap-cKO. (J) qRT-PCR analysis of RIP in LPS-stimulated BMDMs indicated the binding of Igf2bp2 protein and circDcbld2 (n = 4). (K) The stability of circDcbld2 was decreased following Igf2bp2 administration. The data represent the mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, vs WtapF/F group (G), m6A group in WtapF/F (H), LPS + WtapF/F group (I), Control group (J) and LPS + siRNA-NC-Igf2bp2 group (K).
3.4. circDcbld2 increases inflammation and oxidative stress of BMDMs
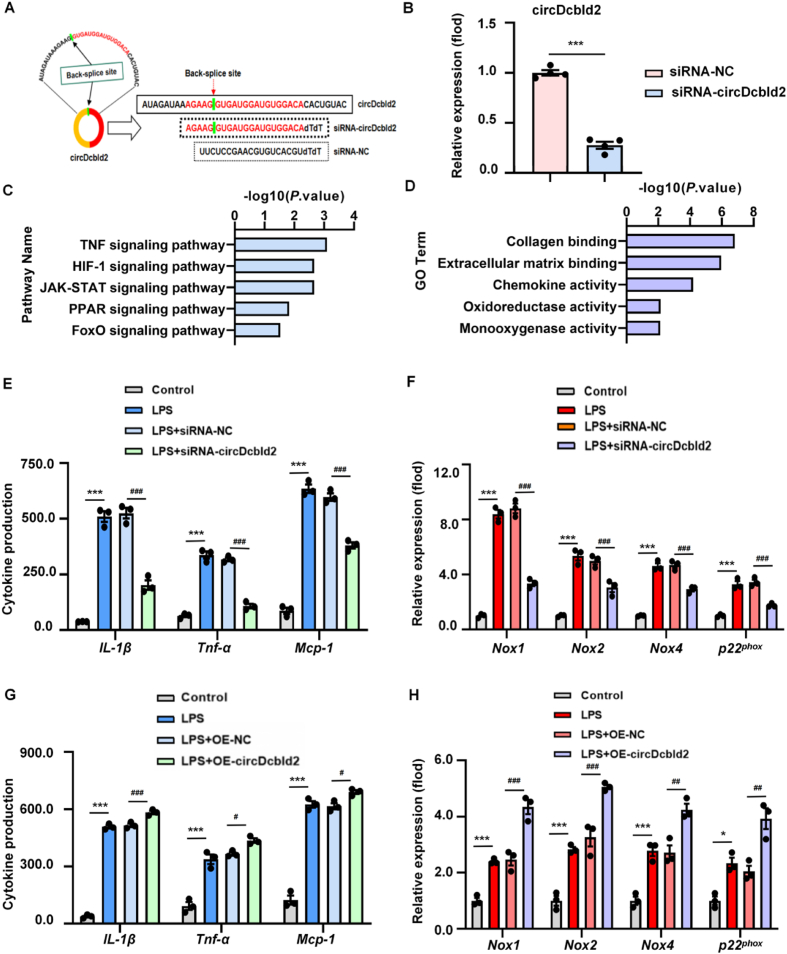
To analyze the function of circDcbld2 in BMDMs, we constructed siRNA of circDcbld2 (Fig. 4A). After analyzing the efficiency of siRNA-circDcbld2 transfection in BMDMs (Fig. 4B), we performed RNA-seq (Supporting Information Fig. S4A). Genes associated with KEGG pathways (TNF, HIF-1, and FoxO signaling pathways), and GO terms (inflammatory response, oxidoreductase activity) were affected by circDcbld2 silencing (Fig. 4C and D). In addition, circDcbld2 suppression decreased the secretion of inflammatory factors and chemokines such as IL-1β, Tnf-α, and Mcp-1, as determined by ELISA (Fig. 4E). We further found that circDcbld2 silencing reduced the mRNA levels of oxidative stress markers, such as Nox1, Nox2, Nox4, and p22phox (Fig. 4F). Moreover, while SOD and GSH were decreased in LPS-stimulated BMDMs compared with controls, these decreases were attenuated by circDcbld2 knockdown (Fig. S4B and S4C). In addition, the over-expression of circDcbld2 had the opposite effects compared with those for circDcbld2 silencing (Fig. 4G and H, Fig. S4D and S4E). Taken together, these results show that circDcbld2 silencing effectively reduces LPS-induced inflammation and oxidative stress in BMDM.
Figure 4.
circDcbld2 increases inflammation and oxidative stress of BMDMs. (A) A siRNA target site of circDcbld2 was constructed. (B) Silencing efficiency of siRNA-circDcbld2 in BMDMs following transfection. (C, D) GO and KEGG enrichment analysis in BMDMs following circDcbld2 knock-down. (E) circDcbld2 suppression decreased the release of IL-1β, Tnf-α, and Mcp-1 by ELISA (n = 3). (F) mRNA expression of Nox1, Nox2, Nox4, and p22phox were reduced by circDcbld2 administration (n = 3). (G) circDcbld2 over-expression enhanced the release of IL-1β, Tnf-α, and Mcp-1 by ELISA (n = 3). (H) mRNA expression of Nox1, Nox2, Nox4, and p22phox were increased following circDcbld2 over-expression (n = 3). The data represent the mean ± SEM. ∗P < 0.05, ∗∗∗P < 0.001 vs siRNA-NC group (B) and control group (E, F, G, H); #P < 0.05, ##P < 0.01, ###P < 0.001 vs LPS + siRNA-NC group (E, F) and LPS + OE-NC group (G, H).
3.5. Pro-fibrogenic activities and pro-oxidative activities effects of circDcbld2 in HF mice
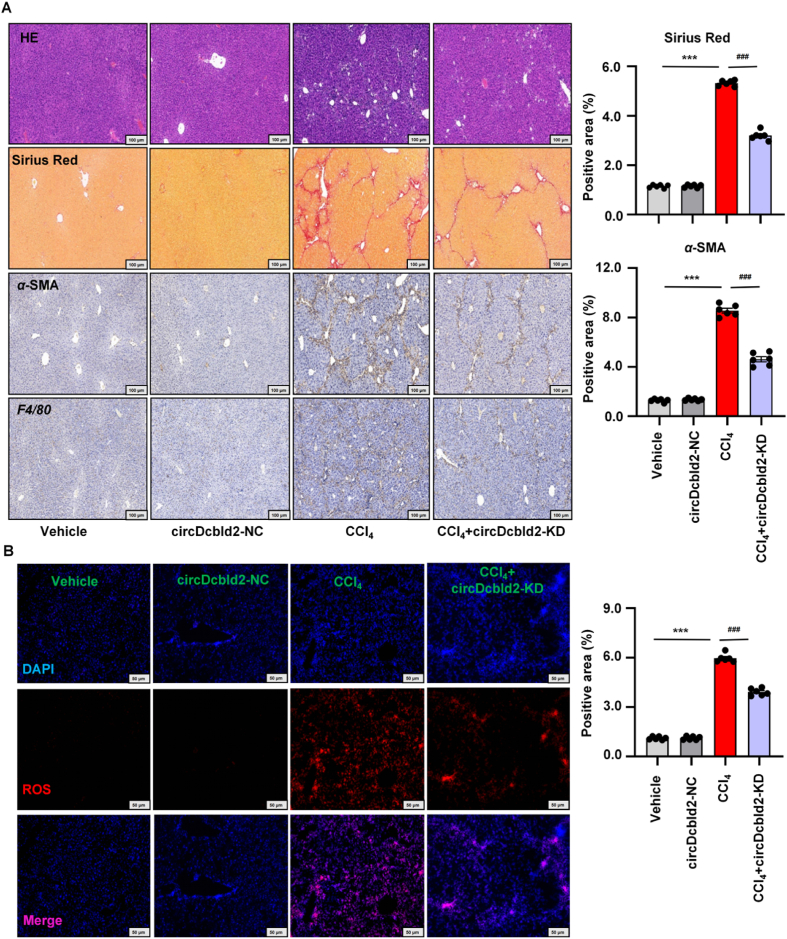
To further explore the influence of circDcbld2 on HF, circDcbld2-KD was injected into HF mice through the tail vein. We verified the efficiency of circDcbld2 inhibiting in KCs (Supporting Information Fig. S5A). Functionally, the degrees of liver collagen deposition and parenchymal distortion were reduced, vascular architecture was altered, and F4/80+ macrophagocytes and α-SMA+ myofibroblasts were reduced in HF following circDcbld2-KD treatment (Fig. 5A and Fig. S5B). We further evaluated HF-related damage indicators, Timp-1, α-SMA, Col1α1, and Tgf-β1, in liver tissues and the inflammation markers IL-1β, Tnf-α, and Mcp-1 in KCs (Fig. S5C). The ALT/AST indicator in CCl4-induced HF mice decreased following circDcbld2-KD administration (Fig. S5D). circDcbld2-KD administration decreased the levels of inflammatory factors and chemokines (IL-1β, Tnf-α, and Mcp-1), as analyzed by ELISA (Fig. S5E). Immunofluorescence staining showed that fibrogenic factors (Col1α1), and macrophage factors (iNos and F4/80) were increased in the HF model and decreased following circDcbld2-KD administration (Supporting Information Fig. S6A). NAD(P)H oxidase subunits (Nox1, Nox2, Nox4, and p22phox) increased in primary KCs from HF mice, and these increases were mitigated by circDcbld2-KD treatment (Fig. S6B). These results suggest that circDcbld2-KD could effectively reduce inflammatory injury and fibrosis in HF mice. Moreover, while CCl4 induction increased oxidative stress hallmarks significantly (MDA), CCl4-induced increases in expression were attenuated by circDcbld2-KD treatment. Levels of antioxidants, such as CAT, GSH, and SOD, were restored in CCl4-induced HF mice after circDcbld2-KD administration (Fig. S6C). Immunofluorescence staining confirmed that ROS levels were high in CCl4-induced HF mice and were reduced in circDcbld2-KD mice (Fig. 5B). These results show that circDcbld2 affects hepatic oxidative stress and inflammatory infiltration in HF model mice.
Figure 5.
Pro-fibrogenic activities and pro-oxidative activities effects of circDcbld2 in HF mice. (A) Pathology observation of H&E staining and Sirius red staining in HF with circDcbld2-KD, IHC stain of α-SMA and F4/80. Representative images were presented, scale bar, 100 μm. Quantification of Sirius red and α-SMA (n = 6). (B) IF staining confirmed that the level of oxidative stress activation (ROS) was highly in HF mice and decreased following circDcbld2-KD (n = 6). Representative images were presented, scale bar = 50 μm. The data represent the mean ± SEM. ∗∗∗P < 0.001 vs vehicle group; ###P < 0.001 vs CCl4-induced liver fibrosis group.
3.6. Silencing circDcbld2 reduces HSC activation in HF mice
HSCs, which are the primary source of mature myofibroblasts, play a crucial role in HF formation. To explore the influence of inflammatory factors and chemokines secreted by activated macrophages on HSCs, we co-cultured primary HSCs with the BMDM supernatant, transfected with circDcbld2-siRNA (Fig. 6A). We detected α-SMA, Col1α1, Timp-1, and Tgf-β1 mRNA expression (Fig. 6B) and analyzed the Col1α1 and α-SMA protein levels in HSCs (Fig. 6C). Silencing circDcbld2 in BMDMs could effectively reduce HSC activation. Furthermore, Immunofluorescence staining showed that macrophage factors (F4/80) and HSC activation factors (α-SMA) were elevated in the HF model but reduced following circDcbld2-KD administration in vivo (Fig. 6D). Finally, we extracted primary HSCs from HF mice and explored the expression levels of the HSC activation factor α-SMA and fibrogenic factor Col1α1 following circDcbld2-KD administration. Silencing circDcbld2 through circDcbld2-KD administration effectively reduced the expression of α-SMA and Col1α1 in primary HSCs (Fig. 6E). Furthermore, the α-SMA, Col1α1, Timp-1, and Tgf-β1 mRNA levels were decreased following circDcbld2-KD treatment (Fig. 6F). Taken together, silencing circDcbld2 alleviated the secretion of inflammatory factors and chemokines in macrophages, reduced HSC activation, inhibited the differentiation of activated HSCs into myofibroblasts, decreased collagen deposition, and attenuated CCl4-induced HF injury.
Figure 6.
Silencing circDcbld2 reduces HSC activation in HF mice. (A) Schematic representation of the co-culture of primary HSCs and BMDMs. (B) Timp-1, α-SMA, Col1α1, and Tgf-β1 mRNA level for primary HSCs, which affected by BMDM with siRNA-circDcbld2 (n = 6). (C) The protein expression of α-SMA and Col1α1 in HSCs, which affected by BMDMs with siRNA-circDcbld2 administration. (D) Immunofluorescent staining indicated α-SMA and F4/80 were enhanced in the CCl4-induced HF model and decreased following circDcbld2-KD administration. Representative images were presented, scale bar = 50 μm. (E) The protein expression level of α-SMA and Col1α1 in primary HSCs by circDcbld2-KD administration. (F) The mRNA expression level of α-SMA, Col1α1, Timp-1 and Tgf-β1 in primary HSCs by circDcbld2-KD administration (n = 6). The data represent the mean ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001 vs LPS + siRNA-NC-circDcbld2 group (B) and Vehicle group (F); ###P < 0.001 vs CCl4-induced liver fibrosis group (F).
3.7. Microarray analysis and identification of circDcbld2/miR-144-3p interaction
circRNAs commonly function as miRNA sponges. To identify potential miRNAs that bind circDcbld2, we performed a miRNA microarray analysis using primary KCs. The results revealed 138 dysregulated miRNAs in HF samples; the abnormal miRNAs were shown in the heatmap (Supporting Information Fig. S7A, Supporting Information Files S4 and S5). A circRNA-miRNA network was constructed based on the dysregulated miRNAs and circRNAs (Fig. S7B). These results were combined with target prediction tools (TargetScan) and a microarray analysis to evaluate potential miRNAs targets of circDcbld2 and to identify potential miRNAs related to HF (Fig. S7C). We detected eight miRNAs that may interact with circDcbld2 (Fig. S7D). Sequence pairing and expression analysis further revealed that circDcbld2 potentially interacts with miR-144-3p (Fig. 7A). Subsequently, circDcbld2 fragments with the wild-type (WT) sequence or mutations in putative binding sites as well as negative control and miRNA mimics were used to explore circDcbld2 and miR-144-3p binding (Fig. 7A). circDcbld2-WT luciferase reporter activity was suppressed in the presence of miR-144-3p (Fig. 7B). Furthermore, miR-144-3p was predominantly localized in the cytoplasm in a FISH analysis (Fig. 7C). miR-144-3p expression, as determined by qRT-PCR, was lower in LPS-stimulated BMDMs than in controls, consistent with the results of the microarray analysis (Fig. 7D). Interestingly, miR-144-3p was increased in BMDMs following siRNA-circDcbld2 administration compared with the LPS + siRNA-NC group (Fig. 7E). Furthermore, transfection with miR-144-3p mimics effectively reduced the expression levels of inflammatory factors (IL-1β, Tnf-α, and Mcp-1) and oxidative stress markers (Nox1, Nox2, Nox4, and p22phox) (Supporting Information Fig. S8A). miR-144-3p mimics could effectively increase levels of indicators of oxidative stress (SOD and GSH) (Fig. S8B). miR-144-3p inhibition had opposite effects on markers of inflammation and oxidative stress compared with those of miR-144-3p mimics (Fig. S8C and S8D). Cotransfected with siRNA-circDcbld2 and miR-144-3p mimics resulted in further reductions in the expression levels of inflammatory markers (IL-1β) (Fig. 7F) and oxidative stress-related indicators (Nox1, Nox2, and Nox4) than those for siRNA-circDcbld2 single transfection (Supporting Information Fig. S9A). In addition, SOD and GSH contents were further increased (Fig. S9B). These results indicate that circDcbld2 may bind to and regulate the expression of miR-144-3p in BMDMs.
Figure 7.
Microarray analysis and identification of circDcbld2/miR-144-3p interaction. (A) Schematic of miR-144-3p binding site in circDcbld2. (B) Renilla luciferase activity analysis of wild-type or mutant circDcbld2 and miRNAs mimics, respectively. (C) miR-144-3p (FAM) predominantly localized in the cytoplasm with FISH assay. Representative images were presented, scale bar = 20 μm. (D) miR-144-3p suppression was confirmed by qRT-PCR in LPS-stimulated BMDMs (n = 3). (E) Lower level of miR-144-3p in BMDMs was increased following siRNA-circDcbld2 administration (n = 3). (F) IL-1β mRNA expression was decreased in BMDMs following siRNA-circDcbld2 administration. The influence of siRNA-circDcbld2 was further decreased following miR-144-3p mimics (n = 3). The data represent the mean ± SEM. ns, no significance; ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs control group (D, E) and LPS group (F); ###P < 0.001 vs LPS + siRNA-NC-circDcbld2 group (E) and LPS + siRNA-circDcbld2 group (F).
3.8. circDcbld2 up-regulates Et-1 expression by sponging miR-144-3p
miRNAs regulate gene expression by binding the 5′ or 3′ untranslated regions of mRNAs to suppress translation. Whole-transcriptome-seqencing was employed to explore candidate genes regulated by circDcbld2/miR-144-3p in KCs in HF (Supporting Information File S6). Differentially expressed mRNAs in KCs extracted from vehicle and HF mice are shown in scatter plots in Supporting Information Fig. S10A. These differentially expressed genes were evaluated through GO classification (Fig. S10B) and KEGG pathway enrichment analyses (Fig. S10C). Following miRNA microarray and RNA-seq analyses (Fig. S10D), Et-1 was identified as a candidate target mRNA of miR-144-3p. The whole-transcriptome-seq analysis revealed that Et-1 expression is elevated in KCs (Supporting Information File S7). Et-1 binding sites in the miR-144-3p sequence were identified (Fig. 8A). We found that Et-1 was increased in LPS-stimulated BMDMs compared with controls (Fig. 8B). Additionally, the expression of Et-1 decreased with the inhibition of circDcbld2 and further decreased after the administration of miR-144-3p mimics (Fig. 8C). In both immunohistochemical and immunofluorescent analyses of liver tissue, Et-1 expression was decreased in the CCl4-induced HF model following circDcbld2-KD suppression (Fig. 8D). These results indicate that the circDcbld2/mir-144-3p/Et-1 axis plays a role in HF; in particular, circDcbld2 sponges miR-144-3p, thereby affecting Et-1 expression.
Figure 8.
circDcbld2 up-regulates Et-1 expression by sponging miR-144-3p. (A) Binding sites of miR-144-3p and 3′UTR of Et-1. (B) Et-1 was increased in LPS-stimulated BMDMs (n = 3). (C) Et-1 decreased with the siRNA-circDcbld2 administration and further reduced with miR-144-3p mimics (n = 3). (D) Immunohistochemistry and immunofluorescence analysis of liver tissue, Et-1 expression was reduced in HF model following circDcbld2-KD administration. Quantification of fibrosis based on immunohistochemistry analysis of Et-1. Representative images were presented, scale bar = 100 μm/50 μm. The data represent the mean ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001 vs Control group (B) and LPS group (C) and Vehicle group (D); #P < 0.05, ###P < 0.001 vs LPS + siRNA-circDcbld2 group (C) and CCl4-induced liver fibrosis group (D).
4. Discussion
4.1. Stability of circDcbld2 is regulated by m6A modification
Advances in RNA sequencing technology provide a basis for elucidating the mechanisms underlying the effects of m6A methylation45. In cancer, m6A modifications play significant roles in RNA stability, interactions, and production46. Altering m6A levels in Mettl3 and Alkbh5 affects circRNA biosynthesis47,48. However, m6A modifications on circRNAs have not been explored in KCs during HF formation. In this study, we showed that circDcbld2 is an important promoter of HF and the presence of methylation sites in the circDcbld2 sequence was predicted. In exploring the upstream regulation mechanism of circDcbld2, we found a m6A methylation site in circDcbld2 and further confirmed that circDcbld2 could bind to Wtap and Igf2bp2. The m6A sequence motif ‘RRm6ACH’ (R = G or A; H = A, C, or U) is the consensus recognition sequence for Igf2bp2. We speculated that Igf2bp2 binds circDcbld2 via the m6A motif. Furthermore, the increase in circDcbld2 expression in the model group may be due to the modified and identified circDcbld2 m6A site by Wtap and Igf2bp2, which enhances the stability of circDcbld2, in turn affecting inflammation and oxidative stress. This explained the mechanism of circDcbld2 up-regulation in HF. These findings show for the first time that circDcbld2 binds key m6A writer and reader proteins, suggesting the potential for m6A modification on circRNAs.
4.2. circDcbld2 contributes to inflammation, oxidative stress activation, and HSC activation, and differentiation in HF
Imbalances in inflammation and oxidation play pivotal roles in HF formation49. In this study, circDcbld2 levels were significantly higher in the HF model than in controls and were elevated in patients with HF. The differential expression of circDcbld2 prompted us to further investigate its function and mechanism of action. We discovered that circDcbld2 suppression significantly reduces the secretion of inflammatory cytokines in macrophages, alleviates liver fibrogenesis injury, and inhibits chemokine expression. The down-regulation of circDcbld2 protects against oxidative stress in macrophages, suggesting that it contributes to HF by regulating oxidative stress within macrophages. Additionally, HSC trans-differentiation into myofibroblasts is a central event during HF formation13. Therefore, investigating the impact of inflammatory factors and chemokines secreted by macrophages on HSC production represents an important direction for our future research. In this study, silencing circDcbld2 in macrophages effectively reduced HSC activation and trans-differentiation, alleviated collagen deposition, and reduced liver injury. Collectively, these results indicate that circDcbld2 exerts pro-fibrotic effects through increased inflammatory factor and chemokine secretion, while influencing the oxidative stress balance; furthermore, it facilitates HSC trans-differentiation into myofibroblasts, thereby accelerating HF progression.
4.3. Identification of the circDcbld2/miR-144-3p/Et-1 axis in KCs
Despite increasing research on circRNAs in HF, the functions and mechanisms of circRNAs in KCs remain elusive. HF is characterized by progressive inflammation and extracellular matrix (ECM) deposition. Liver macrophages play a central role in driving inflammation and HSC activation to trigger HF12. Our previous studies have demonstrated that the aberrant expression of circFbxw4 may inhibit HF progression by modulating HSC activation28. Although numerous circRNAs have been investigated in HSCs, their functions and expression profiles within liver macrophages remain largely unexplored. Furthermore, circRNAs function as miRNA sponges, transcriptional regulators, RBP-binding molecules, and protein translation templates in cellular physiology50. Mature miRNAs bind specifically to target mRNAs, leading to translational repression or mRNA cleavage. Thus, circRNAs can sequester miRNAs and counteract the miRNA-mediated suppression of mRNA expression51. Our results revealed that circDcbld2 functions by sponging miR-144-3p. Notably, circDcbld2 suppression increased miR-144-3p expression in macrophages. Furthermore, miR-144-3p bound to the 3′ untranslated region of Et-1 and down-regulated Et-1 expression during liver fibrogenesis following decreased levels of circDcbld2. The impact of circDcbld2 on Et-1 was partially reversed upon miR-144-3p mimics administration.
5. Conclusions
In this study, we illuminated the first evidence for the pro-fibrogenic function of circDcbld2 in KCs during the development of HF and clarified its mechanism of action. We proved that Wtap interacts with circDcbld2, and Igf2bp2 identifies m6A-modified circDcbld2 and positively mediates the expression of circDcbld2 by enhancing its stability. In HF, circDcbld2 affected KC inflammatory factor production and HSC activation via miR-144-3p/Et-1. Moreover, circDcbld2 increased the inflammatory response and oxidative stress during the development of HF and facilitated HSC trans-differentiation into myofibroblasts, thereby accelerating disease progression (Fig. 9). However, several limitations of this study need to be addressed. For example, the patient sample size was small. Although our preliminary findings show that circDcbld2 is up-regulated in patients with HF, the value of circDcbld2 as a potential biomarker needs to be validated using more samples. Moreover, the intercellular communication between macrophages and HSCs during the experiment was of interest; however, we only briefly explored the effect of inflammatory factors and chemokines secreted by macrophages on HSC production and the underlying mechanism; these unresolved issues will be a focus of our future research. Our study indicated that circDcbld2 is a potential biomarker for HF, providing a basis for the development of novel treatment options.
Figure 9.
Wtap mediates the N6-methyladenosine (m6A) methylation of circDcbld2, Igf2bp2 recognized m6A-modified circDcbld2 and increased its stability. circDcbld2 participated in the occurrence of HF by binding miR-144-3p/Et-1 to regulate inflammatory response and oxidative stress.
Author contributions
Sai Zhu: Writing – original draft, Resources, Project administration, Data curation. Xin Chen: Funding acquisition, Formal analysis, Data curation. Lijiao Sun: Methodology, Data curation. Xiaofeng Li: Writing – original draft, Methodology, Formal analysis. Yu Chen: Software. Liangyun Li: Methodology, Data curation. Xiaoguo Suo: Methodology. Chuanhui Xu: Software, Methodology. Minglu Ji: Methodology. Jianan Wang: Data curation. Hua Wang: Project administration. Lei Zhang: Project administration. Xiaoming Meng: Methodology, Formal analysis. Cheng Huang: Software, Methodology. Jun Li: Writing – review & editing, Funding acquisition.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (U19A2001, 82370630, and 82300722); the Anhui Provincial Natural Science Foundation (No. 2308085QH248, China); the Research Fund of Anhui Institute of Translational Medicine (2021zhyx-B06 and 2022zhyx-B07, China); the China Postdoctoral Science Foundation (No. 2022M710178); the Fund of Traditional Chinese Medicine Institute of Anhui Dabie Mountain (No. TCMADM-2024-02, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2024.11.003.
Appendix A. Supporting information
The following are the Supporting information to this article:
References
- 1.Mokdad A.A., Lopez A.D., Shahraz S., Lozano R., Mokdad A.H., Stanaway J., et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo P., Liu D., Zhang Q., Yang F., Wong Y.K., Xia F., et al. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1. Acta Pharm Sin B. 2022;12:2300–2314. doi: 10.1016/j.apsb.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou G.L., Zuo S., Lu S., Hu R.H., Lu Y.Y., Yang J., et al. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-beta/Smad signaling pathway. World J Gastroenterol. 2019;25:4222–4234. doi: 10.3748/wjg.v25.i30.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 5.Liu R., Li Y., Zheng Q., Ding M., Zhou H., Li X. Epigenetic modification in liver fibrosis: promising therapeutic direction with significant challenges ahead. Acta Pharm Sin B. 2024;14:1009–1029. doi: 10.1016/j.apsb.2023.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma R.S., Harrison D.J., Kisielewski D., Cassidy D.M., McNeilly A.D., Gallagher J.R., et al. Experimental nonalcoholic steatohepatitis and liver fibrosis are ameliorated by pharmacologic activation of Nrf2 (NF-E2 p45-related factor 2) Cell Mol Gastroenterol Hepatol. 2018;5:367–398. doi: 10.1016/j.jcmgh.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma P.F., Gao C.C., Yi J., Zhao J.L., Liang S.Q., Zhao Y., et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J Hepatol. 2017;67:770–779. doi: 10.1016/j.jhep.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Li S., Zhou B., Xue M., Zhu J., Tong G., Fan J., et al. Macrophage-specific FGF12 promotes liver fibrosis progression in mice. Hepatology. 2023;77:816–833. doi: 10.1002/hep.32640. [DOI] [PubMed] [Google Scholar]
- 9.Krenkel O., Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 10.Kisseleva T., Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–166. doi: 10.1038/s41575-020-00372-7. [DOI] [PubMed] [Google Scholar]
- 11.Ge C., Tan J., Lou D., Zhu L., Zhong Z., Dai X., et al. Mulberrin confers protection against hepatic fibrosis by Trim31/Nrf2 signaling. Redox Biol. 2022;51 doi: 10.1016/j.redox.2022.102274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng F., Wang K., Aoyama T., Grivennikov S.I., Paik Y., Scholten D., et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–776. doi: 10.1053/j.gastro.2012.05.049. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong M., Dong W., Kang A., Kuai Y., Xu T., Fan Z., et al. Regulatory role and translational potential of CCL11 in liver fibrosis. Hepatology. 2023;78:120–135. doi: 10.1097/HEP.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 14.Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology. 2017;65:1039–1043. doi: 10.1002/hep.28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie X., Lv H., Liu C., Su X., Yu Z., Song S., et al. HBeAg mediates inflammatory functions of macrophages by TLR2 contributing to hepatic fibrosis. BMC Med. 2021;19:247. doi: 10.1186/s12916-021-02085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He L., Li H., Wu A., Peng Y., Shu G., Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176. doi: 10.1186/s12943-019-1109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai Y., Chang D., Ren H., Ju M., Wang Y., Chen B., et al. Engagement of N6-methyladenisine methylation of Gng4 mRNA in astrocyte dysfunction regulated by CircHECW2. Acta Pharm Sin B. 2024;14:1644–1660. doi: 10.1016/j.apsb.2024.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeck W.R., Sharpless N.E. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C., Yuan W.T., Zhou Q.B., Shao B., Guo Y.Y., Wang W.W., et al. N6-Methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics. 2021;11:4298–4315. doi: 10.7150/thno.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y., Ling Z., Cai X., Xu Y., Lv Z., Man D., et al. Activation of YAP1 by N-6-methyladenosine-modified circCPSF6 drives malignancy in hepatocellular carcinoma. Cancer Res. 2022;82:599–614. doi: 10.1158/0008-5472.CAN-21-1628. [DOI] [PubMed] [Google Scholar]
- 21.Pamudurti N.R., Bartok O., Jens M., Ashwal-Fluss R., Stottmeister C., Ruhe L., et al. Translation of circRNAs. Mol Cell. 2017;66:9–21.e7. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 23.Du W.W., Yang W.N., Liu E., Yang Z.G., Dhaliwal P., Yang B.B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y., Gao X., Zhang M., Yan S., Sun C., Xiao F., et al. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J Natl Cancer Inst. 2018;110:304–315. doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang P., Huang Z., Peng Y., Li H., Lin T., Zhao Y., et al. Circular RNA circBNC2 inhibits epithelial cell G2–M arrest to prevent fibrotic maladaptive repair. Nat Commun. 2022;13:6502. doi: 10.1038/s41467-022-34287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L., Yao X., Yao H., Ji Q., Ding G., Liu X. Exosomal miR-103-3p from LPS-activated THP-1 macrophage contributes to the activation of hepatic stellate cells. FASEB J. 2020;34:5178–5192. doi: 10.1096/fj.201902307RRR. [DOI] [PubMed] [Google Scholar]
- 27.Hu Z.Q., Zhou S.L., Li J., Zhou Z.J., Wang P.C., Xin H.Y., et al. Circular RNA sequencing identifies circASAP1 as a key regulator in hepatocellular carcinoma metastasis. Hepatology. 2020;72:906–922. doi: 10.1002/hep.31068. [DOI] [PubMed] [Google Scholar]
- 28.Chen X., Li H.D., Bu F.T., Li X.F., Chen Y., Zhu S., et al. Circular RNA circFBXW4 suppresses hepatic fibrosis via targeting the miR-18b-3p/FBXW7 axis. Theranostics. 2020;10:4851–4870. doi: 10.7150/thno.42423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bu F.T., Zhu Y., Chen X., Wang A., Zhang Y.F., You H.M., et al. Circular RNA circPSD3 alleviates hepatic fibrogenesis by regulating the miR-92b-3p/Smad7 axis. Mol Ther-Nucl Acids. 2021;23:847–862. doi: 10.1016/j.omtn.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu X.Y., Liu Y.R., Xuan W.T., Ye J., Yao H.W., Huang C., et al. Circ_1639 induces cells inflammation responses by sponging miR-122 and regulating TNFRSF13C expression in alcoholic liver disease. Toxicol Lett. 2019;314:89–97. doi: 10.1016/j.toxlet.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 31.He Y., Feng D., Li M., Gao Y., Ramirez T., Cao H., et al. Hepatic mitochondrial DNA/Toll-like receptor 9/MicroRNA-223 forms a negative feedback loop to limit neutrophil overactivation and acetaminophen hepatotoxicity in mice. Hepatology. 2017;66:220–234. doi: 10.1002/hep.29153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J.J., Tao H., Liu L.P., Hu W., Deng Z.Y., Li J. miR-200a controls hepatic stellate cell activation and fibrosis via SIRT1/Notch1 signal pathway. Inflamm Res. 2017;66:341–352. doi: 10.1007/s00011-016-1020-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhu S., Chen X., Wang J.N., Xu J.J., Wang A., Li J.J., et al. Circular RNA circUbe2k promotes hepatic fibrosis via sponging miR-149-5p/TGF-beta2 axis. FASEB J. 2021;35 doi: 10.1096/fj.202002738R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J.J., Chen X., Zhu S., Jiang L.F., Ma W.X., Chen S.Y., et al. Myc-mediated circular RNA circMcph1/miR-370-3p/Irak2 axis is a progressive regulator in hepatic fibrosis. Life Sci. 2023;312 doi: 10.1016/j.lfs.2022.121182. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y.H., Yuan B.Y., Wu Z.F., Dong Y.Y., Zhang L., Zeng Z.C. Microarray profiling of circular RNAs and the potential regulatory role of hsa_circ_0071410 in the activated human hepatic stellate cell induced by irradiation. Gene. 2017;629:35–42. doi: 10.1016/j.gene.2017.07.078. [DOI] [PubMed] [Google Scholar]
- 36.Miyoshi H., Rust C., Roberts P.J., Burgart L.J., Gores G.J. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- 37.Kang H., Seo E., Park J.M., Han N.Y., Lee H., Jun H.S. Effects of FGF21-secreting adipose-derived stem cells in thioacetamide-induced hepatic fibrosis. J Cell Mol Med. 2018;22:5165–5169. doi: 10.1111/jcmm.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X., Li X.F., Chen Y., Zhu S., Li H.D., Chen S.Y., et al. Hesperetin derivative attenuates CCl4-induced hepatic fibrosis and inflammation by Gli-1-dependent mechanisms. Int Immunopharmacol. 2019;76:105838. doi: 10.1016/j.intimp.2019.105838. [DOI] [PubMed] [Google Scholar]
- 39.Starkey Lewis P., Campana L., Aleksieva N., Cartwright J.A., Mackinnon A., O'Duibhir E., et al. Alternatively activated macrophages promote resolution of necrosis following acute liver injury. J Hepatol. 2020;73:349–360. doi: 10.1016/j.jhep.2020.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y., Wu X.Q., Li W.X., Huang H.M., Li H.D., Pan X.Y., et al. PSTPIP2 connects DNA methylation to macrophage polarization in CCl4-induced mouse model of hepatic fibrosis. Oncogene. 2018;37:6119–6135. doi: 10.1038/s41388-018-0383-0. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., Chen X., Ji Y.R., Zhu S., Bu F.T., Du X.S., et al. PLK1 regulates hepatic stellate cell activation and liver fibrosis through Wnt/beta-catenin signalling pathway. J Cell Mol Med. 2020;24:7405–7416. doi: 10.1111/jcmm.15356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen-Lefebvre A.T., Ajith A., Portik-Dobos V., Horuzsko D.D., Arbab A.S., Dzutsev A., et al. The innate immune receptor TREM-1 promotes liver injury and fibrosis. J Clin Invest. 2018;128:4870–4883. doi: 10.1172/JCI98156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristensen L.S., Andersen M.S., Stagsted L.V.W., Ebbesen K.K., Hansen T.B., Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 44.Wang J.N., Wang F., Ke J., Li Z., Xu C.H., Yang Q., et al. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating TAB3 m6A modifications via IGF2BP2-dependent mechanisms. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abk2709. [DOI] [PubMed] [Google Scholar]
- 45.Liu J., Jia G. Methylation modifications in eukaryotic messenger RNA. J Genet Genomics. 2014;41:21–33. doi: 10.1016/j.jgg.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Niu Y., Zhao X., Wu Y.S., Li M.M., Wang X.J., Yang Y.G. N6-methyl-adenosine (m6A) in RNA: an old modification with a novel epigenetic function. Genomics Proteomics Bioinformatics. 2013;11:8–17. doi: 10.1016/j.gpb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi Y.C., Chen X.Y., Zhang J., Zhu J.S. Novel insights into the interplay between m(6)A modification and noncoding RNAs in cancer. Mol Cancer. 2020;19:121. doi: 10.1186/s12943-020-01233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W., Qiao S.C., Wu X.B., Sun B., Yang J.G., Li X., et al. Circ_0008542 in osteoblast exosomes promotes osteoclast-induced bone resorption through m6A methylation. Cell Death Dis. 2021;12:628. doi: 10.1038/s41419-021-03915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramirez T., Li Y.M., Yin S., Xu M.J., Feng D., Zhou Z., et al. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J Hepatol. 2017;66:601–609. doi: 10.1016/j.jhep.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou W.Y., Cai Z.R., Liu J., Wang D.S., Ju H.Q., Nxu R.H. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. 2020;19:172. doi: 10.1186/s12943-020-01286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21:475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.