Abstract
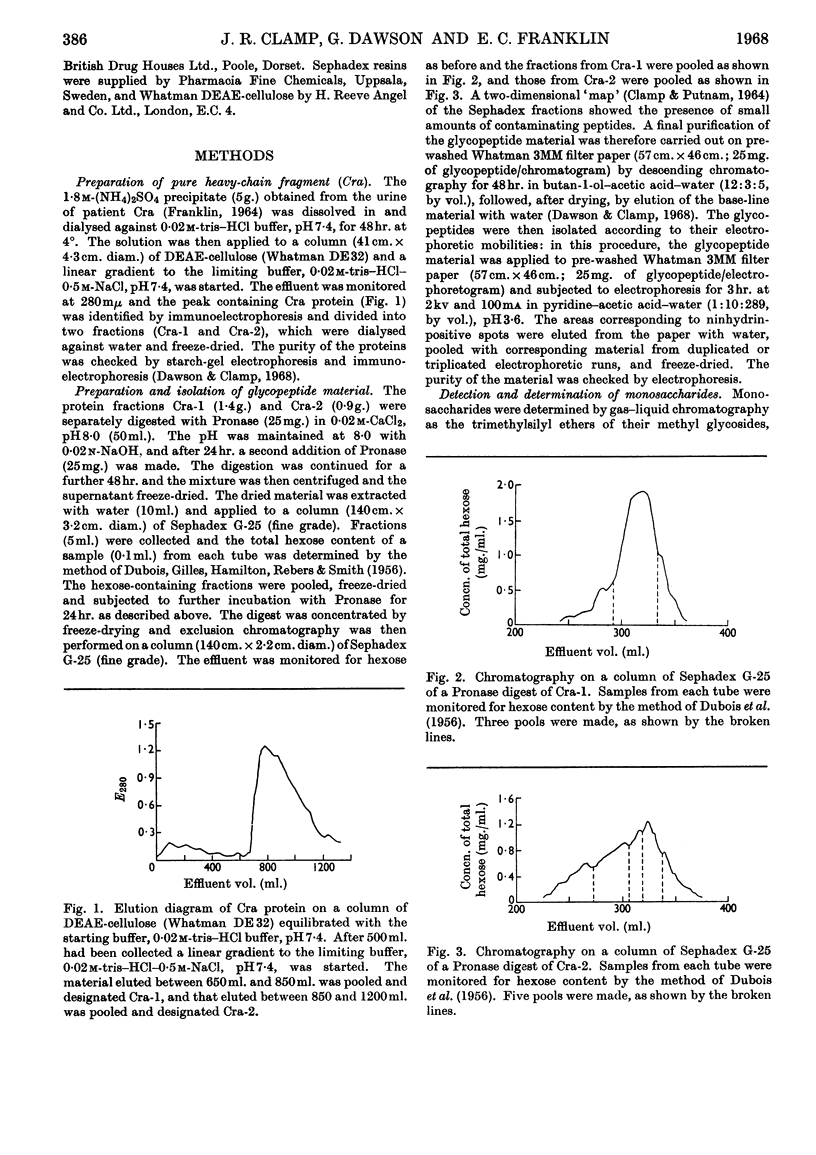
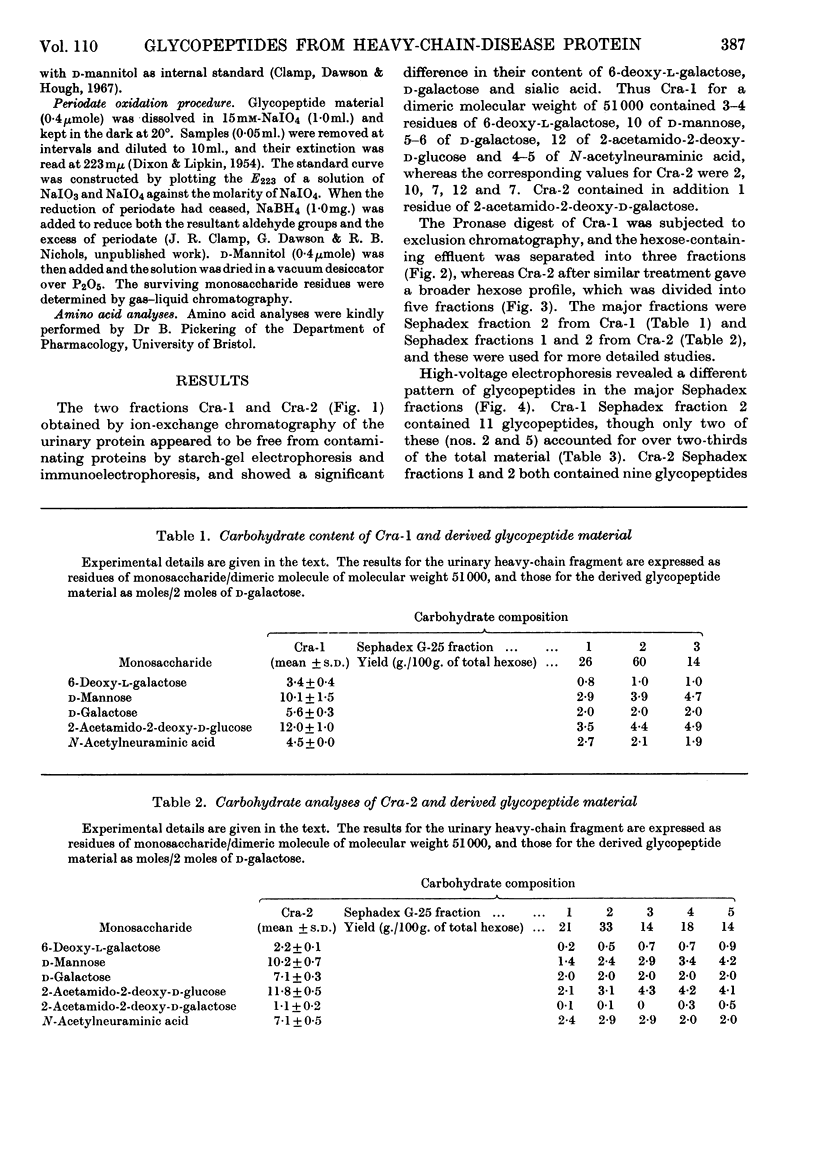
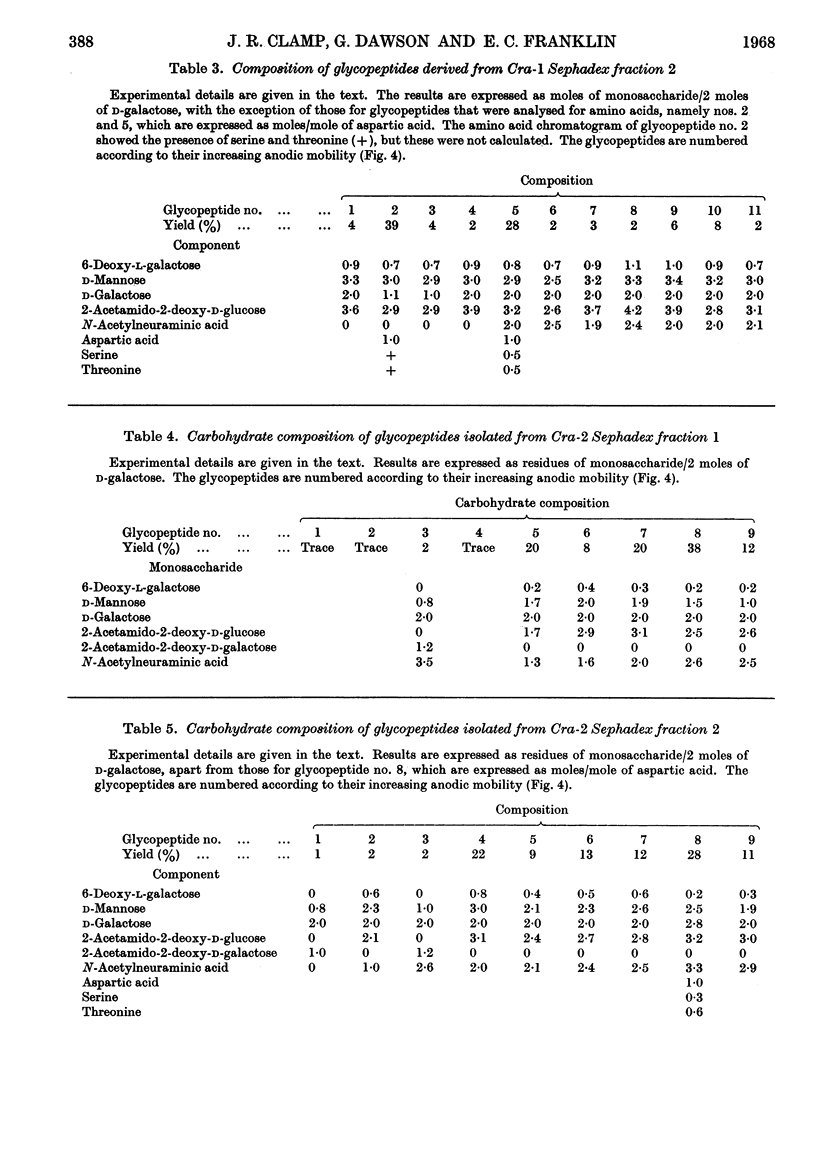
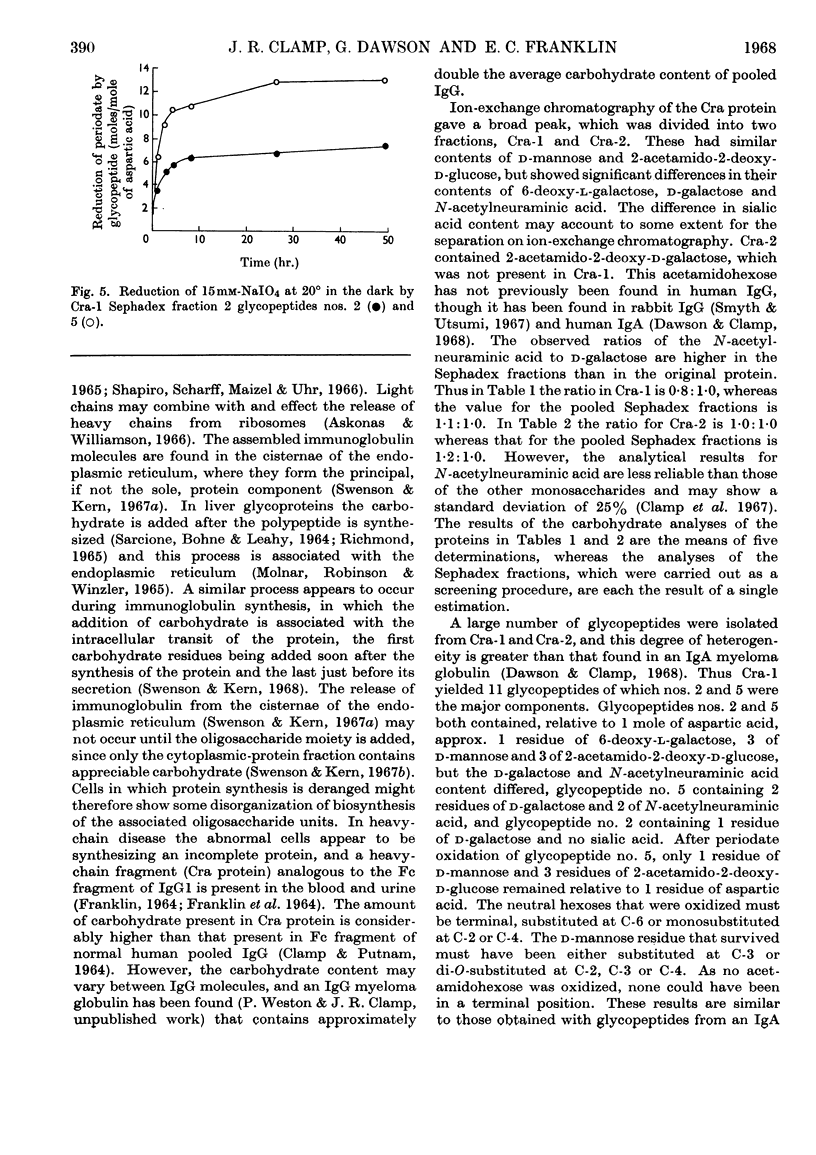
The urinary protein excreted in heavy-chain disease was separated by ion-exchange chromatography into two broad fractions designated Cra-1 and Cra-2. For a dimeric molecular weight of approx. 51000, Cra-1 contained 3–4 residues of 6-deoxy-l-galactose (l-fucose), 10 of d-mannose, 5–6 of d-galactose, 12 of 2-acetamido-2-deoxy-d-glucose (N-acetyl-d-glucosamine) and 4–5 of N-acetylneuraminic acid (sialic acid), whereas the corresponding values for Cra-2 were 2, 10, 7, 12 and 7. Cra-2 contained in addition 1 residue of 2-acetamido-2-deoxy-d-galactose (N-acetyl-d-galactosamine). Cra-1 contained an average of four oligosaccharide units, two of which contained 1 residue of 6-deoxy-l-galactose, 3 of d-mannose, 1 of d-galactose and 3 of 2-acetamido-2-deoxy-d-glucose, whereas the other two units contained the same proportions of 6-deoxy-l-galactose, d-mannose and 2-acetamido-2-deoxy-d-glucose but 2 residues of d-galactose and 2 of N-acetylneuraminic acid. Cra-2 also contained an average of four oligosaccharide units, but the range of glycopeptides was much wider, containing 0–1 residue of 6-deoxy-l-galactose, 2–3 of d-mannose, 2–3 of d-galactose, 2–3 of 2-acetamido-2-deoxy-d-glucose and 1–3 of N-acetylneuraminic acid. Possible reasons for this heterogeneity are discussed. Glycopeptides were also isolated from Cra-2 that contained 1 residue of d-mannose, 2 of d-galactose, 1 of 2-acetamido-2-deoxy-d-galactose and 0–3 of N-acetylneuraminic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Williamson A. R. Biosynthesis of immunoglobulins. Free light chain as an intermediate in the assembly of gamma G-molecules. Nature. 1966 Jul 23;211(5047):369–372. doi: 10.1038/211369a0. [DOI] [PubMed] [Google Scholar]
- Bernier G. M., Ballieux R. E., Tominaga K. T., Putnam F. W. Heavy chain subclasses of human gamma G-globulin. Serum distribution and cellular localization. J Exp Med. 1967 Feb 1;125(2):303–316. doi: 10.1084/jem.125.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPLIN H., COHEN S., PRESS E. M. PREPARATION AND PROPERTIES OF THE PEPTIDE CHAINS OF NORMAL HUMAN 19 S GAMMA-GLOBULIN (IGM). Biochem J. 1965 Apr;95:256–261. doi: 10.1042/bj0950256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAMP J. R., PUTNAM F. W. THE CARBOHYDRATE PROSTHETIC GROUP OF HUMAN GAMMA-GLOBULIN. J Biol Chem. 1964 Oct;239:3233–3240. [PubMed] [Google Scholar]
- Campbell P. N., Serck-Hanssen G., Lowe E. Studies on the protein-synthesizing activity of the ribosomes of rat liver. The activity of free polysomes. Biochem J. 1965 Nov;97(2):422–431. doi: 10.1042/bj0970422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Dawson G., Hough L. The simultaneous estimation of 6-deoxy-L-galactose (L-fucose), D-mannose, D-galactose, 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine) and N-acetylneuraminic acid (sialic acid) in glycopeptides and glycoproteins. Biochim Biophys Acta. 1967 Nov 28;148(2):342–349. doi: 10.1016/0304-4165(67)90129-8. [DOI] [PubMed] [Google Scholar]
- Dawson G., Clamp J. R. Investigations on the oligosaccharide units of an A myeloma globulin. Biochem J. 1968 Apr;107(3):341–352. doi: 10.1042/bj1070341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Polyribosomes in thin sections of 5563 plasmacytoma cells. J Mol Biol. 1967 Jan 28;23(2):215–216. doi: 10.1016/s0022-2836(67)80028-7. [DOI] [PubMed] [Google Scholar]
- Droz B. Elaboration de glycoproteines dans l'appareil de Golgi des cellules hépatiques chez le rat; étude radioautographique en microscopie électronique après injection de glactose-3H. C R Acad Sci Hebd Seances Acad Sci D. 1966 Apr 18;262(16):1766–1768. [PubMed] [Google Scholar]
- EDELMAN G. M., POULIK M. D. Studies on structural units of the gamma-globulins. J Exp Med. 1961 May 1;113:861–884. doi: 10.1084/jem.113.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C., LOWENSTEIN J., BIGELOW B., MELTZER M. HEAVY CHAIN DISEASE- A NEW DISORDER OF SERUM GAMMA-GLOBULINS : REPORT OF THE FIRST CASE. Am J Med. 1964 Sep;37:332–350. doi: 10.1016/0002-9343(64)90191-3. [DOI] [PubMed] [Google Scholar]
- FRANKLIN E. C. STRUCTURAL STUDIES OF HUMAN 7S GAMMA-GLOBULIN (G IMMUNOGLOBULIN). FURTHER OBSERVATIONS OF A NATURALLY OCCURRING PROTEIN RELATED TO THE CRYSTALLIZABLE (FAST) FRAGMENT. J Exp Med. 1964 Nov 1;120:691–709. doi: 10.1084/jem.120.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C. Structural units of human 7S gamma globulin. J Clin Invest. 1960 Dec;39:1933–1941. doi: 10.1172/JCI104218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEREMANS J. F. Immunochemical studies on protein pathology. The immunoglobulin concept. Clin Chim Acta. 1959 Sep;4:639–646. doi: 10.1016/0009-8981(59)90004-x. [DOI] [PubMed] [Google Scholar]
- Hill R. L., Delaney R., Fellows R. E., Lebovitz H. E. The evolutionary origins of the immunoglobulins. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1762–1769. doi: 10.1073/pnas.56.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUHN R. Les oligosaccharides du lait. Bull Soc Chim Biol (Paris) 1958;40(2-3):297–314. [PubMed] [Google Scholar]
- Li Y. T., Li S. C., Shetlar M. R. Isolation of glycopeptides from rat liver microsomes involved in the biosynthesis of plasma glycoprotein. J Biol Chem. 1968 Feb 10;243(3):656–665. [PubMed] [Google Scholar]
- MOLNAR J., ROBINSON G. B., WINZLER R. J. BIOSYNTHESIS OF GLYCOPROTEINS. IV. THE SUBCELLULAR SITES OF INCORPORATION OF GLUCOSAMINE-1-14-C INTO GLYCOPROTEIN RAT LIVER. J Biol Chem. 1965 May;240:1882–1888. [PubMed] [Google Scholar]
- ROSEVEAR J. W., SMITH E. L. Glycopeptides. I. Isolation and properties of glycopeptides from a fraction of human gamma-globulin. J Biol Chem. 1961 Feb;236:425–435. [PubMed] [Google Scholar]
- SARCIONE E. J., BOHNE M., LEAHY M. THE SUBECELLULAR SITE OF HEXOSAMINE INCORPORATION INTO LIVER PROTEIN. Biochemistry. 1964 Dec;3:1973–1976. doi: 10.1021/bi00900a032. [DOI] [PubMed] [Google Scholar]
- SCHARFF M. D., UHR J. W. FUNCTIONAL RIBOSOMAL UNIT OF GAMMA-GLOBULIN SYNTHESIS. Science. 1965 Apr 30;148(3670):646–648. doi: 10.1126/science.148.3670.646. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Scharff M. D., Maizel J. V., Uhr J. W. Synthesis of excess light chains of gamma globulin by rabbit lymph node cells. Nature. 1966 Jul 16;211(5046):243–245. doi: 10.1038/211243a0. [DOI] [PubMed] [Google Scholar]
- Smyth D. S., Utsumi S. Structure at the hinge region in rabbit immunoglobulin-G. Nature. 1967 Oct 28;216(5113):332–335. doi: 10.1038/216332a0. [DOI] [PubMed] [Google Scholar]
- Swenson R. M., Kern M. Synthesis and secretion of gamma-globulin by lymph node cells. II. The intracellular segregation of amino acid-labeled and carbohydrate-labeled gamma-globulin. J Biol Chem. 1967 Jul 10;242(13):3242–3244. [PubMed] [Google Scholar]
- Swenson R. M., Kern M. THE SYNTHESIS AND SECRETION OF gamma-GLOBULINS BY LYMPH NODE CELLS, I. THE MICROSOMAL COMPARTMENTALIZATION OF gamma-GLOBULINS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):417–422. doi: 10.1073/pnas.57.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. M., Kern M. The synthesis and secretion of gamma-globulin by lymph node cells. 3. The slow acquisition of the carbohydrate moiety of gamma-globulin and its relationship to secretion. Proc Natl Acad Sci U S A. 1968 Feb;59(2):546–553. doi: 10.1073/pnas.59.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]



