Abstract
Elevated glucose metabolism is a prominent characteristic of fibroblast-like synoviocytes (FLS) in rheumatoid arthritis (RA). However, the efficacy of inhibiting a single target of glucose metabolism in FLS using small molecular inhibitors is limited for RA treatment. Herein, the synergistic inhibition of FLS’ survival, proliferation, and activation by combining two glucose metabolism inhibitors, diclofenac (DC) and lonidamine (LND) was first verified. Subsequently, DC and LND were individually conjugated to cystamine-modified hyaluronic acid (HA) to prepare two polymer-prodrug conjugates. A HAP-1 peptide-modified dual polymer-prodrug conjugates-assembled nanoparticles system (HAP−1NPDC+LND) was further tailored in the optimal synergistic ratio for targeted and synergistic metabolic modulation of FLS to alleviate RA symptoms. Upon targeted uptake by FLS in inflamed joints, HAP−1NPDC+LND released DC and LND within the intracellular reductive microenvironment, where DC hinders glucose uptake and LND suppresses glycolytic enzymes to eliminate FLS synergistically. Additionally, the secretion of lactic acid and pro-inflammatory factors from FLS were reduced, thereby disrupting the crosstalk between FLS and pro-inflammatory macrophages. Finally, HAP−1NPDC+LND demonstrated promising efficacy in a mouse model of collagen-induced arthritis (CIA). Overall, this research provides valuable insights into novel therapeutic strategies for the safe and effective of treatment RA through targeted and synergistic metabolic modulation of FLS.
Key words: Dual prodrug nanoparticles, Metabolic modulation, Fibroblast-like synoviocytes, Rheumatoid arthritis, Glucose transporter member 1, Glycolytic enzyme, Lactic acid, Synovial microenvironment
Graphical abstract
A HAP-1 peptide-modified dual polymer-prodrug conjugates-assembled nanoparticles system (HAP-1NPDC+LND) is tailored for targeted and synergistic metabolic modulation of FLS, remodeling crosstalk between FLS and pro-inflammatory macrophages for effective RA treatment.

1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by synovitis as the pathological basis and can ultimately lead to joint deformity1. During the progression of RA, the typical structure of the synovium, normally consisting of 2–3 cell layers, thickens to 10–20 cell layers, which is associated with persistent proliferation and activation of fibroblast-like synoviocytes (FLS)2,3. Proliferative and activated FLS promote RA progression by releasing various factors and engaging in cell–cell crosstalk4. For instance, FLS-secreted lactic acid in the arthritic synovial microenvironment (SME) polarizes macrophages toward pro-inflammatory M1-type5, 6, 7. In turn, these macrophages produce pro-inflammatory factors that stimulate FLS to release pro-inflammatory cytokines, chemokines, and tissue-damaging factors8. Additionally, FLS participate in recruiting inflammatory cells, inducing synovial neovascularization, promoting osteoclast formation and cartilage degradation9. However, current US Food and Drug Administration (FDA)-approved medications for RA mainly target the hyperactive immune response and resulting inflammation without directly addressing FLS’ proliferation and activation10,11. This limitation frequently results in a high relapse rate after discontinuing treatment for RA. Recently, several investigated delivery systems for RA treatment involve killing FLS through local photodynamic or photothermal therapy12,13. However, these methods often cause damage to surrounding healthy tissues or exacerbate the inflammation due to excessive reactive oxygen species or high temperature. Furthermore, locally treating small joints in various body parts poses challenges as well12,13. Therefore, there is an urgent need to develop an effective and safe FLS-targeted modulation strategy for RA treatment.
Recently, conducted studies have demonstrated that elevated glucose metabolism is a prominent characteristic of FLS in RA14, 15, 16, 17. This metabolic alteration involves the up-regulation of glucose transporter member 1 (GLUT-1) expression or enhancement of the activity of rate-limiting glycolytic enzymes15, 16, 17, 18, resulting in the abnormal accumulation of various metabolites within FLS. These metabolites further promote FLS’ survival, proliferation, and activation, thereby contributing to chronic inflammation and joint damage in RA15,19. Nanocarrier has recently been utilized for delivering glucose oxidase to consume glucose for FLS elimination12. However, the constant supply of glucose from the body may weaken this effect while also exacerbating SME acidity due to gluconic acid production20. Several small molecular inhibitors targeting glucose metabolism have shown potential in inhibiting FLS survival and inducing apoptosis, as well as modulating FLS-mediated inflammation18,21,22. Nevertheless, FLS exhibit compensatory mechanisms when a single target within glucose metabolism is inhibited23,24. Furthermore, the therapeutic efficacy of small molecular inhibitors is limited by their poor water solubility, targeting capabilities, and short half-life25. To date, no attempts have been made to develop a delivery system for these glycolytic blockers that can achieve effective RA therapy through targeted modulation of FLS metabolism.
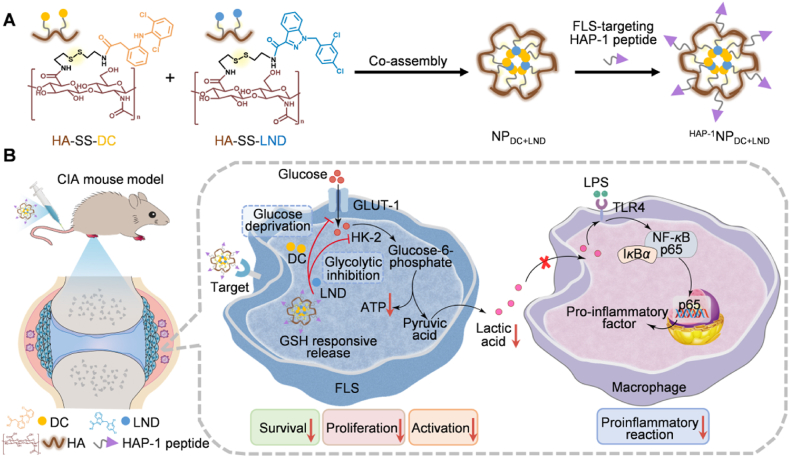
Hence, we developed a polymeric dual prodrug nanoparticle system to achieve targeted and synergistic metabolic modulation of FLS for enhanced RA treatment (Scheme 1). Diclofenac (DC) and lonidamine (LND), highly selective small molecular inhibitors targeting GLUT-1 and hexokinase 2 (HK-2), respectively, have been extensively investigated for their potential as glycolytic inhibitors to impede proliferation and induce apoptosis of tumor cells26,27. We initially verified the optimal synergistic effect of DC and LND against FLS at a molar ratio of 2:1. Subsequently, DC and LND were covalently conjugated to hyaluronic acid (HA) through disulfide bond linker, respectively. These two polymer-prodrug conjugates were then co-assembled into dual prodrug nanoparticles (NPDC+LND) at the optimal synergistic ratio, followed by modification with the FLS-targeting peptide (HAP-1) to obtain HAP−1NPDC+LND28. Upon intravenous (i.v.) injection, HAP−1NPDC+LND specifically targeted FLS in arthritic joints. After recognition and endocytosis by FLS, DC and LND are released from HAP−1NPDC+LND in response to the intracellular reductive microenvironment. The released DC hindered glucose uptake while LND inhibited glycolytic enzymes, thereby synergistically impeding FLS’ survival, proliferation, and activation. Furthermore, this targeted and synergistic metabolic modulation of FLS reduced the secretion of pro-inflammatory factors and lactic acid levels which subsequently decreased macrophage activation while regulating SME. Ultimately, this polymeric dual prodrug nanoparticles system demonstrated excellent efficacy in eliminating FLS and reducing inflammation in mouse model of RA.
Scheme 1.
Schematic illustration of synergistic metabolic modulation of FLS with targeted dual prodrug nanoparticles (HAP−1NPDC+LND) to mitigate RA. (A) Schematic design of the HAP-1 peptide-modified polymeric dual prodrug nanoparticles (HAP−1NPDC+LND); (B) Upon i.v. injection, HAP−1NPDC+LND would target the FLS in inflamed joints, triggering the release of DC and LND in response to the high GSH microenvironment within FLS. The released DC blocks glucose uptake, while LND inhibits glycolytic enzymes, thereby synergistically suppressing the survival, proliferation and activation of FLS. In addition, targeted metabolic regulation of FLS can reduce the secretion of pro-inflammatory factors and lactic acid, thereby mitigating macrophage activation and regulating SME.
2. Materials and methods
2.1. Preparation and characterization of HAP−1NPDC+LND
2.1.1. Synthesis of HA–SS–NH2
1000 mg of HA was dissolved in 50 mL phosphate-buffered saline (PBS) (0.01 mol/L, pH = 7.4), and EDC (0.505 g, 2.636 mmol) and NHS (0.303 g, 2.636 mmol) were subsequently added into the above solution under magnetic stirring for 2 h. Following this, cystamine dihydrochloride (1.782 g, 7.909 mmol) was introduced into the above solution and stirred for 24 h at room temperature. Then, the resulting mixture solution was dialyzed against deionized water for 48 h to obtain HA–SS–NH2 through freeze-drying29.
2.1.2. Synthesis of HA–SS–DC and HA–SS–LND
Hydrophobic modification of HA was achieved by establishing an amide linkage between HA–SS–NH2 and DC or LND29. Firstly, 40 mg of HA–SS–NH2 was dissolved in 10 mL methanamide at 60 °C. Subsequently, 40 mg of LND or DC was dissolved in dimethylformamide (DMF) and added dropwise to the above solution. The resulting mixture was stirred for 48 h. Following this, the solution was dialyzed against deionized water for 48 h and then underwent freeze-drying. The chemical structures of HA–SS–NH2, HA–SS–LND, and HA–SS–DC conjugate were determined by 1H NMR spectroscopy (Bruker 400 MHz, Bruker BioSpin, Rheinstetten, Germany).
2.1.3. Preparation of NPDC+LND and HAP−1NPDC+LND
HA–SS–LND and HA–SS–DC were dissolved in 2 mL methanamide at 60 °C, followed by dropwise addition into PBS (0.01 mol/L, pH = 7.4). Subsequently, the solution was dialyzed against deionized water for 48 h and then freeze-dried. The mixture of the two polymers was analyzed using UV‒Vis spectra (Shimadzu Corporation, Kyoto, Japan). The peptide sequences were as follows: SFHQFARATLAS28. The peptide was synthesized by solid-phase synthesis30. HAP-1 peptide was conjugated to the free amino group of HA–SS–NH2 by amide reaction after magnetic stirring with equimolar amounts of EDC and NHS for 2 h, followed by dialysis with deionized water for 48 h and freeze-drying. The morphology of the nanoparticles was characterized by transmission electron microscopy (TEM, JEOL JEM-2100, Tokyo, Japan). The size distribution and zeta potential of different nanoparticles were measured using Zetasizer Nano ZS (Malvern, UK). The stability of the nanoparticles over a period of 7 days at pH 7.4 in PBS and their responsiveness under GSH conditions were evaluated by TEM and dynamic light scattering (DLS).
2.2. In vitro cytotoxicity assessment
FLS were seeded in 96-well cell culture plates at the density of 5 × 103 cells per well and incubated with various concentrations of free DC, LND, and nanoparticles for 24 h at 37 °C in a humidified atmosphere containing 5% CO2. Subsequently, the cell viability was measured using a standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay on a microplate reader (BioTek, Winooski, VT, USA).
2.3. Cellular uptake of RhB-labeled NPDC+LND and HAP−1NPDC+LND
FLS were seeded in a confocal dish, and incubated medium containing RhB-labeled NPDC+LND and HAP−1NPDC+LND for 12 h. After being cultured for 12 h, the cells were washed with PBS, and stained by Hoechst 33342. Subsequently, the cells were immediately observed using confocal laser scanning microscopy (CLSM, Nikon, Japan). Similarly, the cells were collected, and the mean fluorescence intensity was detected by flow cytometry (BD FACSCelesta, San Diego, CA, USA).
2.4. Glucose content, lactic acid, and intracellular ATP levels assay
FLS at a density of 1 × 105 per well were plated in a 6-well plate and grown for 24 h. Subsequently, the cells were treated with tumor necrosis factor α (TNF-α) (20 ng/mL) along with varying concentrations of DC, LND, and nanoparticles for an additional 24 h. The levels of extracellular glucose, extracellular lactic acid, and intracellular ATP content were quantified using respective assay kits following the provided instructions.
2.5. Measurements of cytokines level
FLS were pre-treated with different drugs and co-cultured with RAW264.7 in a transwell system with a 0.4 μm thick microporous membrane. After being cultivated for 24 h, the supernatant was collected from culture media and subjected to enzyme-linked immunosorbent assay (ELISA) analysis for the secretion of iNOS, TNF-α, or IL-1β.
2.6. Western blot analyses
To investigate the expression levels of related proteins after various treatments, cellular lysis was performed using a lysis buffer. The protein concentration was determined by the BCA protein assay kit. Subsequently, the protein sample was mixed with loading buffer and incubated for 10 min at 95 °C. It was then subjected to SDS-PAGE loading buffer gel electrophoresis followed by transfer to a polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was subsequently blocked with Tris Buffered Saline with Tween-20 (TBST) containing 5% skim milk for 2 h. Following this, PVDF membranes were incubated overnight at 4 °C with various primary antibodies, including GLUT-1, HK-2, MMP-2, p65, IκBα, p-IκBα, β-Tubulin and GAPDH. Then, PVDF membranes were washed with TBST and incubated with the secondary antibodies at room temperature for 2 h. Finally, visualization of protein detection was achieved using an enhanced chemiluminescence (ECL) kit.
2.7. Evaluation of macrophage phenotype
FLS were seeded in Transwells with a 0.4 μm thick microporous membrane and pre-treated with different drugs. RAW264.7 macrophages were spread on the cell crawl sheet at the base of the 24-well plate, and the Transwells were placed in the 24-well plate for 24 h. Subsequently, the cells were fixed with 4% paraformaldehyde (PFA) for 10 min, permeated with 0.1% Triton X-100 at room temperature for 20 min, incubated with anti-FITC-iNOS at room temperature for 40 min, and finally stained with Hoechst 33342 for 15 min. Fluorescence imaging was conducted using confocal microscopy.
2.8. Collagen-induced arthritis (CIA) mouse model and treatment protocols
Male DBA/1 J mice (6–8 weeks old) were obtained from Guangzhou Qingle Life Science Co., Ltd. All animal experiment procedures were ethically approved by the Institutional Animal Care and Use Committee (IACUC) of Zhujiang Hospital (LAEC-2023-151), Southern Medical University (Guangzhou, China), in accordance with international guidelines.
A CIA model was established according to our previously reported method31. Briefly, male DBA/1 J mice aged 6–8 weeks with similar body weight were selected to establish the mouse model of CIA. An appropriate amount of bovine type II collagen (CII) was emulsified with a complete Freund's adjuvant and injected into the base of each mouse's tail. On Day 21, mice received 100 μL of CII emulsified in incomplete Freund's adjuvant to boost the immune response. To monitor arthritis progression, ankle thickness was measured every three days by a researcher using a vernier caliper. Besides, clinical scores were assessed every three days after disease onset based on the criteria32.
To investigate the therapeutic effect of CIA mice, treatment was started on Day 30 after the initial immunization. On Day 30, the mice were randomly assigned to 6 treatment groups (n = 5 per group), including, saline, DC+LND, NPDC, NPLND, NPDC+LND, and HAP−1NPDC+LND. A group of healthy mice was also designed as a negative control. The medication was administered intravenously every three days for a total of five doses.
2.9. In vivo fluorescent imaging
For in vivo fluorescence imaging, Cy5-labeled NPDC+LND and HAP−1NPDC+LND were tail i.v. injected into CIA model mice. Subsequently, the mice were anesthetized and placed on an animal plate heated to 37 °C. The time-dependent biodistribution in mice was imaged using an IVIS Spectrum in vivo imaging system (PerkinElmer; Living Image Software) at 12, 24, 48, and 72 h after injection with the excitation wavelength set at 640 nm. At 24 h post-injection, the major organs (heart, liver, spleen, lung, and kidneys) and inflamed paws were harvested from these mice to quantitatively determine the ex vivo fluorescence intensity of nanoparticles.
2.10. Micro-CT analysis
The hind ankle joints were collected on Day 3 after the final treatment, fixed in 4% PFA and subjected to scanning with the assistance of Wuhan Doctor Biological Technology Co., Ltd. (Wuhan, China). Subsequently, micro-CT imaging was performed using a Skyscan 1276 scanner (Bruker BioSpin, Rheinstetten, Germany) to acquire high-resolution three-dimensional reconstructions of the hind ankle joints.
2.11. Histological and immunohistochemical studies
The hind ankle joints were harvested on Day 3 after the final treatment and fixed with 4% PFA. Subsequently, the fixed hind ankle joints underwent decalcification by changing 10% (w/v) tetrasodium EDTA solution daily for 2 weeks. Following decalcification, the hind ankle joints were paraffin-embedded and sectioned for subsequent staining with hematoxylin and eosin (H&E), safranin-O, toluidine blue, GLUT-1, HK-2, and FAP-α staining.
2.12. Statistical analysis
All results were presented as mean ± standard deviation (SD). Statistical significance between groups was analyzed using the Student's t-test or ANOVA of GraphPad Prism 8.0 software.
3. Result and discussion
3.1. DC and LND inhibit glucose metabolism in FLS synergistically
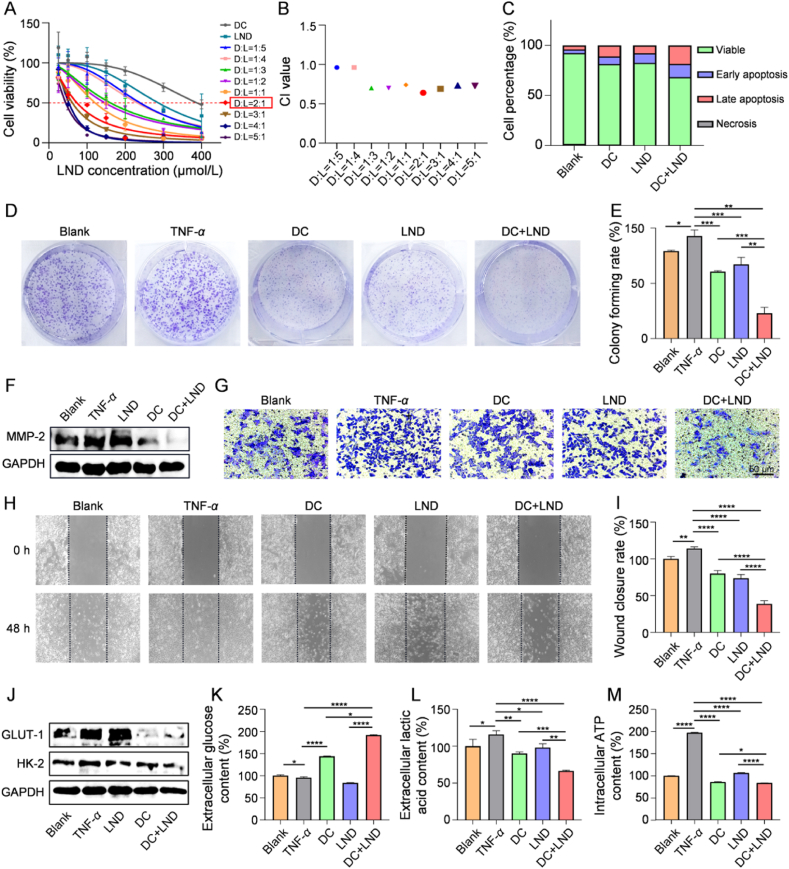
DC and LND are commonly utilized inhibitors of glucose metabolism that impede cellular glucose uptake and inhibit intracellular glycolytic enzyme activity by targeting GLUT-1 and HK-2, respectively18,33. However, targeting a single component in the glucose metabolism pathway may have limited efficacy. Therefore, we combined DC with LND to explore their potential synergistic effect on inhibiting survival, proliferation, and activation of FLS through metabolic modulation. Firstly, the inhibitory effect of the combination treatment on FLS’ survival was evaluated at different molar ratios after 24 h (Fig. 1A). As shown in Supporting Information Table S1, the combination treatment exhibited significantly lower IC50 values compared to individual drug treatments. Moreover, the combination index (CI) values below 1 across various DC/LND ratios from 1:5 to 5:1 indicated synergy between the two drugs (Fig. 1B). The most pronounced synergistic effect was observed at a molar ratio of 2:1 for DC/LND, with a CI of 0.64. Notably, at this ratio, the IC50 values for DC and LND decreased to 147.1 and 73.6 μmol/L, respectively, compared to when each drug was administered alone (384.7 μmol/L for DC and 286.7 μmol/L for LND) (Fig. 1A and Table S1). Consequently, apoptosis of FLS increased to 31.6% with this combination ratio as opposed to only achieving rates of 18.8% for DC or 17.5% for LND alone (Fig. 1C). These results confirm the synergistic inhibition effect achieved by combining DC and LND, particularly at a ratio of 2:1 on FLS’ survival.
Figure 1.
DC and LND inhibit glucose metabolism in FLS synergistically. (A) Cytotoxicity study and (B) corresponding combination index treated with dual drugs at different molar ratios of concentration; (C) Flow cytometric analysis of apoptosis in FLS treated with DC, LND, and dual drugs; (D) Colony forming and (E) quantification of TNF-α-stimulated FLS treated with DC, LND, and dual drugs; (F) Western blot of MMP-2 in TNF-α-stimulated FLS treated with DC, LND, and dual drugs; (G) Transwell assays of the invasion abilities of TNF-α-stimulated FLS treated with DC, LND, and dual drugs; (H) The scratch assays and (I) quantitative analysis of the migration abilities of TNF-α-stimulated FLS treated with DC, LND, and dual drugs; (J) Western blot of GLUT-1 and HK2 expression levels in FLS; (K) Extracellular glucose level, (L) extracellular lactic acid level, and (M) intracellular ATP level of TNF-α-stimulated FLS after incubation with DC, LND, and dual drugs for 24 h. Data are shown as mean ± SD (n = 3). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
The synergistic effect of the two drugs at the optimal ratio on inhibiting FLS’ proliferation and activation was subsequently evaluated. Colony formation assays demonstrated a significant inhibition of colony-forming ability in TNF-α stimulated FLS (activated FLS) treated with DC+LND (22.8%) compared to single-drug treatments (60.6% for DC, 67.1% for LND) (Fig. 1D and E). Activated FLS are known to express and secrete metallomatrix proteinases (MMPs) in inflamed joints of RA, leading to the degradation of the basement membrane and extracellular matrix, thereby promoting the invasion and migration of FLS34. Western blot analysis showed a more significant decrease in MMP-2 expression in DC+LND treatment compared to DC or LND group (Fig. 1F and Supporting Information Fig. S1). Consistent with these findings, DC+LND treatment exhibited the best inhibition effect on FLS invasion (Fig. 1G). Additionally, scratch assay results indicated that the suppression of FLS migration was most pronounced after DC+LND treatment (Fig. 1H and I). Therefore, the combination of the two drugs exerts a synergistic effect on suppressing survival, proliferation, and activation of FLS.
Subsequently, we combine DC and LND to investigate their potential inhibitory effects on FLS survival, proliferation, and activation by regulating glucose metabolism. Firstly, the expression levels of GLUT-1 and HK-2 proteins were examined through Western blot analysis. Treatment with DC or LND alone resulted in reduced expression of GLUT-1 and HK-2 in activated FLS, respectively. In contrast, administration of DC+LND resulted in a simultaneous down-regulation of GLUT-1 and HK-2 proteins, with their expressions being 45.5% and 53.3% compared to the TNF-α group (Fig. 1J and Supporting Information Fig. S2). Notably, the glucose uptake by FLS in DC and DC+LND groups was decreased due to the GLUT-1 inhibition. However, in LND group, there was an increase in glucose uptake attributed to the compensatory mechanisms aimed at meeting cellular energy demands following glycolytic enzyme inhibition23,24 (Fig. 1K). Furthermore, the generation of lactic acid and ATP from FLS as downstream products of glucose metabolism was diminished in both the DC (accounting for 77.8% and 43.5% of TNF-α group) and LND (accounting for 84.7% and 53.9% of TNF-α group) groups. Particularly, in DC+LND group, the extracellular concentration of lactic acid and intracellular level of ATP was further decreased to 57.5% and 42.4% of TNF-α group, respectively (Fig. 1L and M). In summary, the combination of the two drugs can comprehensively intervene in glucose uptake and subsequent glycolysis, thus inhibiting the glucose metabolism level of FLS to suppress survival, proliferation, and activation.
3.2. Preparation and characterization of dual prodrug nanoparticles (HAP−1NPDC+LND)
Both DC and LND are characterized as hydrophobic drugs, which exhibit rapid elimination from the body. Although the combination of two drugs can achieve dual-target therapy, their in vivo distribution and pharmacokinetics differ, potentially limiting the synergistic effect35. To efficiently deliver drugs to inflamed joints at the above optimal ratio, the dual prodrug co-delivery polymeric nanoparticles were engineered. Firstly, HA was modified by cystamine to obtain HA–SS–NH2 (Supporting Information Fig. S3). Subsequently, a condensation reaction between DC or LND with carboxyl groups on HA–SS–NH2 resulted in the synthesis of polymer-prodrug conjugates, namely HA–SS–DC or HA–SS–LND. 1H NMR spectra showed the characteristic peak of DC or LND in HA–SS–DC or HA–SS–LND respectively (Supporting Information Fig. S4), indicating the successful preparation of the polymer-prodrug conjugates. The drug coupling efficiencies of HA–SS–DC and HA–SS–LND were measured by high performance liquid chromatography (HPLC) to be 9.3 ± 0.2% and 11.9 ± 0.4%, and the drug contents of HA–SS–DC and HA–SS–LND were 8.5 ± 0.2% and 10.6 ± 0.3%, respectively.
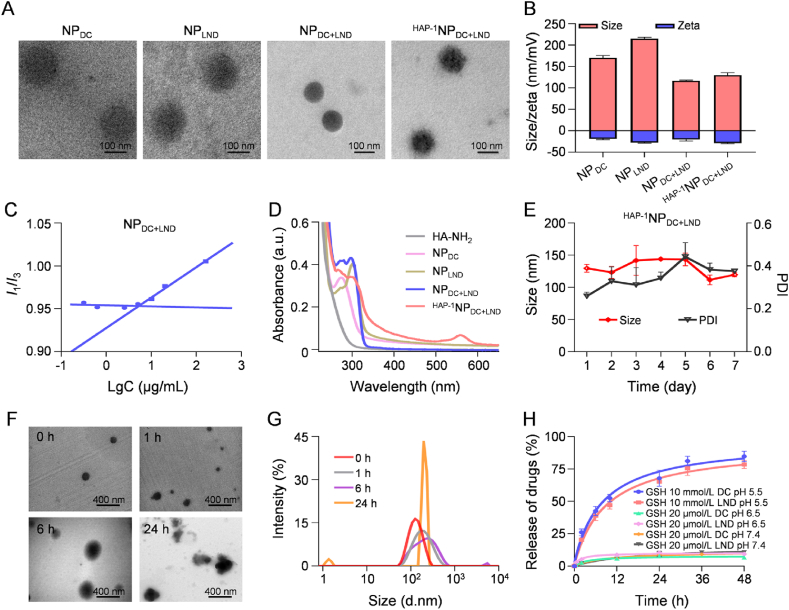
The self-assembly of amphiphilic HA–SS–DC and HA–SS–LND conjugates into NPDC and NPLND was achieved using the emulsion-solvent evaporation method. Subsequently, according to the above synergistic effect of DC/LND at different ratios, the co-assembly of HA–SS–DC and HA–SS–LND into NPDC+LND was fixed at a molar ratio of 2:1. TEM results exhibited that the conjugates formed spherical nanoparticles (Fig. 2A). DLS analysis showed that the average sizes of HA–SS–DC self-assembled nanoparticles (NPDC), HA–SS–LND self-assembled nanoparticles (NPLND), and NPDC+LND were 214.9 ± 3.6, 170.2 ± 5.5, 116.5 ± 1.72 nm, respectively (Fig. 2B and Supporting Information Fig. S5), consistent with the TEM observations (Fig. 2A). The critical aggregation concentration (CAC) values of NPDC, NPLND, and NPDC+LND were measured to be 22.6, 7.8, and 5.2 μg/mL, respectively, ensuring the stability of the self-assembly nanoparticles at low concentration (Fig. 2C and Supporting Information Fig. S6). The UV‒Vis spectra of NPDC and NPLND showed the characteristic absorption peaks of DC and LND at 280 and 300 nm respectively, further validating the conjugation of DC and LND within the nanoparticles. Besides, the UV‒Vis spectrum of NPDC+LND showed the simultaneous appearance of characteristic absorption peaks of DC and LND, affirming the co-assembly of HA–SS–DC and HA–SS–LND conjugates (Fig. 2D). The drug contents of DC and LND in NPDC+LND were 5.94 ± 0.1% and 3.19 ± 0.4%, respectively.
Figure 2.
Preparation and characterization of dual prodrug nanoparticles (HAP−1NPDC+LND). (A) TEM of NPDC, NPLND, NPDC+LND, and HAP−1NPDC+LND. Scale bars = 100 nm; (B) Hydrodynamic size and zeta potential determined of NPDC, NPLND, NPDC+LND, and HAP−1NPDC+LND; (C) The CAC of NPDC+LND in PBS; (D) UV-Vis spectra of HA–SS–NH2, NPDC, NPLND, NPDC+LND, and HAP−1NPDC+LND; (E) The stability of HAP−1NPDC+LND in PBS 7.4 at 37 °C for 7 days; (F) TEM and (G) DLS analysis of the dissociation of HAP−1NPDC+LND in response to 10 mmol/L GSH for 0, 1, 6, and 24 h. (H) Cumulative drug release of HAP−1NPDC+LND at different conditions; Data are presented as the mean ± SD (n = 3).
To facilitate enhanced accumulation of NPDC+LND in the FLS within inflamed joints, the HAP-1 peptide, known for its specific binding effect on FLS28, was synthesized (Supporting Information Fig. S7) and subsequently conjugated onto the nanoparticle surface. The morphology of HAP-1-conjugated NPDC+LND (HAP−1NPDC+LND) retained the spherical shape, with a slight increase in particle size and a reduction in zeta potential compared to NPDC+LND (Fig. 2A and B). After labeling HAP-1 with isothiocyanate-Rhodamine B (RhB), the UV‒Vis spectrum of HAP−1NPDC+LND showed the characteristic absorption peak of RhB at 560 nm, confirming successful modification of HAP-1 on the nanoparticles (Fig. 2D). Both NPDC+LND and HAP−1NPDC+LND exhibited robust stability in pH 7.4 PBS at 37 °C for 7 days, ensuring their safety for application purposes (Fig. 2E and Supporting Information Fig. S8).
The inflammatory environment in the inflamed joints induces a heightened intracellular redox state in FLS36. Consequently, disulfide compounds, susceptible to cleavage in a highly intracellular reductive microenvironment, were selected as the linker for drug conjugation. Firstly, the disassembly process of HAP−1NPDC+LND in a reductive environment was observed through TEM. HAP−1NPDC+LND gradually disintegrated into smaller fragments and aggregated into larger particles over 24 h (Fig. 2F) in the presence of 10 mmol/L GSH. This process could be further supported by the change of the DLS signal of the original HAP−1NPDC+LND at a single peak to dual peaks (Fig. 2G). Meanwhile, the drug release kinetics of HAP−1NPDC+LND showed that less than 11.0% of DC and LND were released within 48 h in PBS in the presence of 20 μmol/L GSH at pH 7.4 and 6.5 (pH 7.4: simulate physiological conditions; pH 6.5: simulate inflammatory microenvironment), whereas 78.5% of LND and 84.7% of DC were released within the same time frame in PBS containing 10 mmol/L GSH at pH 5.5 (simulate the intracellular microenvironment of FLS), respectively (Fig. 2H). These results indicated that HAP−1NPDC+LND exhibited robust stability under physiological conditions and inflammatory microenvironment37,38, while facilitating prompted drug release specifically within articular FLS, thus ensuring both the safety and efficacy of drug administration. Besides, none of the four kinds of nanoparticles induce any hemolysis of erythrocytes, demonstrating good biosafety in vivo (Supporting Information Fig. S9).
3.3. HAP−1NPDC+LND modulate glucose metabolism in FLS in vitro
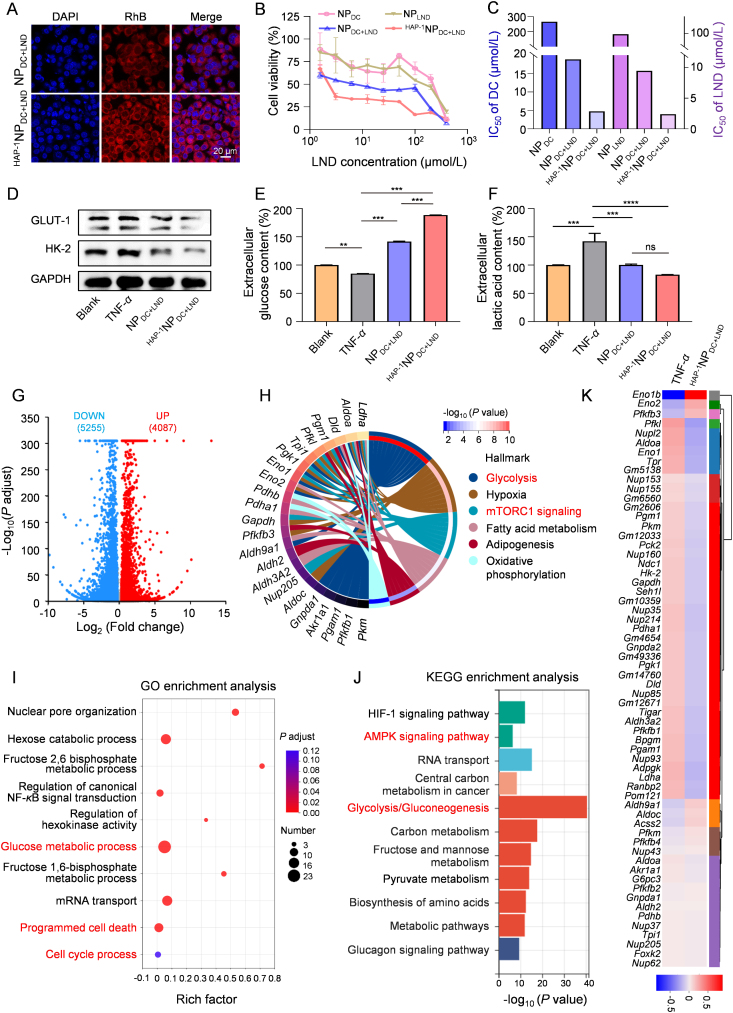
The recognition and endocytosis of nanoparticles by FLS can be mediated by HA through the CD44 receptor, resulting in a targeted effect on FLS39. The modification of HAP-1 on NPDC+LND further enables the targeted recognition and adhesion of nanoparticles to FLS, thereby improving drug uptake. Both NPDC+LND and HAP−1NPDC+LND were labeled with RhB and co-incubated with FLS. RhB-labeled HAP−1NPDC+LND exhibited superior cellular uptake efficiency compared with RhB-labeled NPDC+LND in FLS, as confirmed by CLSM imaging (Fig. 3A and Supporting Information Fig. S10) and flow cytometry (Supporting Information Fig. S11). This increased internalization is anticipated to enhance the therapeutic efficacy. Consequently, HAP−1NPDC+LND demonstrated enhanced inhibition efficacy against FLS compared to NPDC+LND and outperformed NPLND and NPDC notably (Fig. 3B). The IC50 values of DC and LND in HAP−1NPDC+LND against FLS for 24 h were measured to be 4.8 μmol/L and 2.4 μmol/L respectively, which are significantly lower than those of NPDC (266.6 μmol/L) and NPLND (97.6 μmol/L) alone, as well as NPDC+LND (18.9 μmol/L for DC and 9.4 μmol/L for LND) (Fig. 3C).
Figure 3.
HAP−1NPDC+LND modulate glucose metabolism in FLS in vitro. (A) CLSM images of FLS uptake after incubation with NPDC+LND and HAP−1NPDC+LND for 12 h; Scale bar = 20 μm; (B) Cytotoxicity and (C) IC50 of NPDC, NPLND, NPDC+LND, and HAP−1NPDC+LND; (D) Western blot of GLUT-1 and HK-2 expression levels in TNF-α-stimulated FLS after incubation with NPDC+LND and HAP−1NPDC+LND for 24 h; (E) Extracellular glucose level and (F) extracellular lactic acid level of TNF-α-stimulated FLS after incubation with NPDC+LND and HAP−1NPDC+LND for 24 h; (G) Volcano plot showing DEGs in TNF-α group and HAP−1NPDC+LND-treated groups; (H) Hallmark analysis of the DEGs associated with glucose metabolism; (I) GO enrichment analysis of glucose metabolism-associated genes; (J) KEGG enrichment analysis of glucose metabolism-associated genes; (K) Heat map of up-regulated and down-regulated genes associated with glucose metabolism in FLS after HAP−1NPDC+LND treatment; Data are shown as mean ± SD (n = 3). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. ns, not significant.
The notable inhibitory effect of HAP−1NPDC+LND on FLS may be attributed to its targeted suppression of glucose metabolism. Western blot analysis revealed that the dual prodrug nanoparticles effectively decreased the expression levels of GLUT-1 and HK-2 simultaneously. Particularly, the protein expression of GLUT-1 and HK-2 in HAP−1NPDC+LND group was only 28.1% and 54.1% in that of TNF-α group, respectively (Fig. 3D and Supporting Information Fig. S12). Consequently, HAP−1NPDC+LND demonstrated a more pronounced reduction in the glucose metabolism activity of FLS compared to NPDC+LND, leading to the diminished uptake of glucose (extracellular glucose content was increased by 222.0% compared with TNF-α group) (Fig. 3E) and secretion of lactic acid (accounting for 58.4% of TNF-α group) (Fig. 3F).
To further confirm the targeted modulation of glucose metabolism of FLS to suppress survival, proliferation, and activation by HAP−1NPDC+LND, RNA sequencing analysis on the FLS after different treatments was conducted. A volcano plot was generated to describe the differential expressed genes (DEGs) between the HAP−1NPDC+LND group and TNF-α group (Fig. 3G). The DEGs were filtered based on an absolute fold change >1.2 and a P adjust <0.05 criteria. Venn diagram analysis revealed 62 glucose metabolism-related hub genes (42 up-regulated and 20 down-regulated) among the DEGs following HAP−1NPDC+LND treatment (Supporting Information Fig. S13). To explore the pathways and mechanisms involved in these hub genes, enrichment analysis including Hallmark, Gene ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) were performed (Fig. 3H‒J). As a result, these hub genes were found to be enriched in glycolysis, mTORC1 signaling, programmed cell death, cell cycle process, and AMPK signaling pathway. These pathways are involved in mechanisms that affect cellular energy metabolism, apoptosis, proliferation, and migration13,40, 41, 42, 43, 44. Moreover, the heatmap depicting the expression pattern of these hub genes after different treatments was presented (Fig. 3K). In particular, Hk-216, Pkm45, Ldha46, and other critical glycolytic enzyme genes showed significant down-regulation.
Overall, the above analyses further substantiate that the regulation of glucose metabolism exerts inhibitory effects on the survival, proliferation, and activation of FLS.
3.3. HAP−1NPDC+LND remodel the crosstalk between FLS and macrophages in vitro
FLS can engage in crosstalk with macrophages to amplify inflammation in SME of RA. Over-proliferated and activated FLS contribute to elevated levels of lactic acid in the SME due to elevated glucose metabolism. Lactic acid not only contributes to the acidification of the SME but also promotes macrophage activation, leading to the production of pro-inflammatory factors5,47. Consequently, these pro-inflammatory factors further activate FLS to produce additional cytokines, chemokines, and tissue-destroying factors4.
Therefore, to investigate the effect of modulating glucose metabolism in FLS on the crosstalk with macrophages, FLS were pre-treated with nanoparticles (600 μg/mL) and co-cultured with RAW264.7 cells in Transwells for 24 h (Fig. 4A). Previous studies have shown that lactic acid secreted from FLS can boost the activation of Toll-like receptor 4 (TLR4) in macrophages and the expression of inflammatory genes dependent on the NF-κB signaling pathway47. Upon phosphorylation and degradation of IκB, cytosol NF-κB translocates into the nucleus and binds to the κB motifs in gene promoters, resulting in the upregulation of inflammation-related genes (Fig. 4A)48, 49, 50. Western blot analysis (Fig. 4B and Supporting Information Fig. S14) along with heatmap quantification (Fig. 4C) revealed reduced expression levels of cytosolic NF-κB subunit p65 and IκBα, as well as increased phosphorylation of IκB in macrophages of TNF-α group, indicating activation of NF-κB signaling pathway. In NPDC+LND and HAP−1NPDC+LND groups, levels of cytosolic NF-κB subunit p65, IκBα and phosphorylation IκB returned essentially to those observed in the control group. These results demonstrated that the synergistic modulation of glucose metabolism in FLS by NPDC+LND and HAP−1NPDC+LND could inhibit the activation of NF-κB pathway in macrophages. Inhibition of the NF-κB signaling pathway would attenuate macrophage polarization to pro-inflammatory M1 phenotype49,50. CLSM revealed a notable reduction in inducible nitric oxide synthase (iNOS) expression in macrophages after HAP−1NPDC+LND treatment (Fig. 4D and Supporting Information Fig. S15). ELISA analysis also showed a significant decrease in iNOS expression on macrophages in both NPDC+LND (71.7%) and HAP−1NPDC+LND (44.3%) groups compared with TNF-α group (Fig. 4E). iNOS is known to promote the release of inflammatory factors51. Consequently, HAP−1NPDC+LND treatment resulted in reduced TNF-α production, which was only 84.2% of NPDC+LND treatment group (Fig. 4F). Besides, HAP−1NPDC+LND treatment also led to a reduction in the production of IL-1β, accounting for only 48.5% of that seen in the NPDC+LND group (Fig. 4G), further proving the targeted regulation effect of HAP−1NPDC+LND. FLS treated with a lower dose of nanoparticles (480 μg/mL) showed similar trends (Supporting Information Fig. S16). These results indicated that HAP−1NPDC+LND can remodel the crosstalk between FLS and macrophages through targeted modulation of glucose metabolism in FLS, further alleviating inflammation.
Figure 4.
HAP−1NPDC+LND remodel the crosstalk between FLS and macrophages in vitro. (A) Schematic illustration of FLS with various treatments affect macrophage phenotype assay; (B) Western blot and (C) heatmap quantification of NF-κB pathway in macrophages after various treatments; (D) CLSM images of macrophage phenotypes after various treatments; Scale bar = 20 μm; The expression level of (E) iNOS, (F) TNF-α, and (G) IL-1β after various treatments. Data are shown as mean ± SD (n = 3). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. ns, not significant.
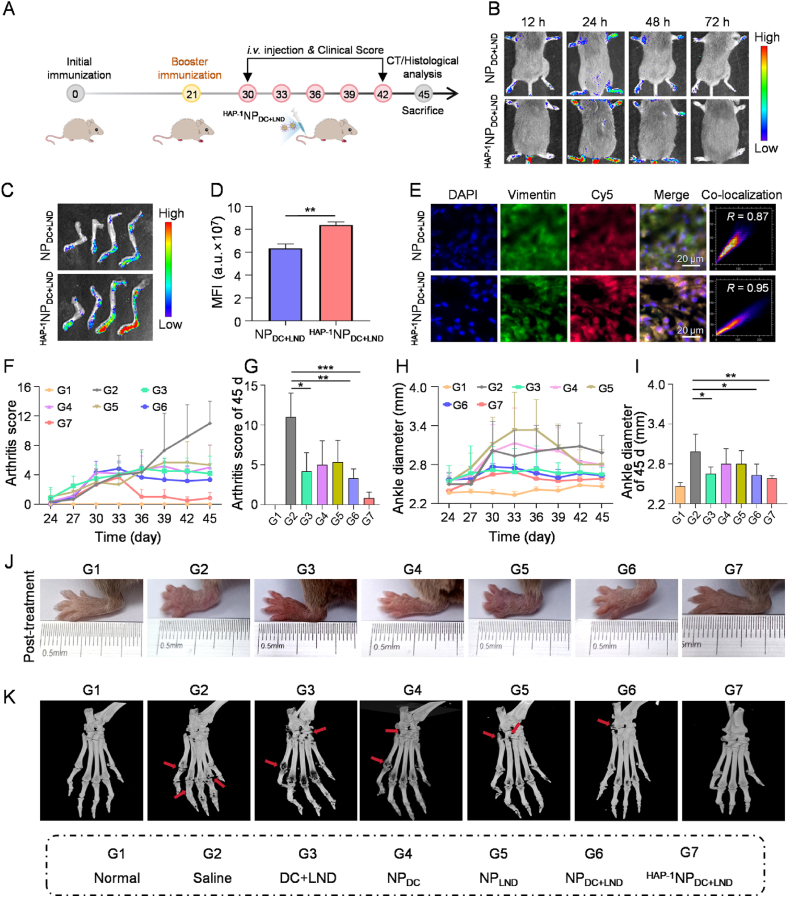
3.5. HAP-1NPDC+LND target FLS and alleviate arthritic progression in CIA mice
The effective accumulation of drugs in the inflamed joints, particularly in FLS, is crucial for enhancing drug efficacy in RA therapy in vivo. Hence, a CIA mice model was established (Fig. 5A). Then, NPDC+LND and HAP−1NPDC+LND were labeled with Cy5, and their distribution in the CIA model was evaluated through fluorescence imaging. Normal mice with minimal FLS presence in the joints were used as controls. As shown in Fig. 5B, RA model mice exhibited prominent Cy5 fluorescence in their paws even after 48 h post-administration of NPDC+LND and HAP−1NPDC+LND, while negligible fluorescence signal was detected in paws of normal mice after i.v. administration of HAP−1NPDC+LND (Supporting Information Fig. S17). To some extent, this shows the targeting ability of nanoparticles to FLS in RA. Moreover, model mice treated with HAP−1NPDC+LND exhibited a significantly higher Cy5 fluorescence in the inflamed joints compared to those treated with NPDC+LND, indicating an enhanced accumulation of HAP−1NPDC+LND in these regions (Fig. 5B‒D). Meanwhile, while both nanoparticles accumulated in the liver and kidney after 24 h, HAP−1NPDC+LND showed reduced accumulation in these organs and increased accumulation specifically within the inflamed joints compared to NPDC+LND (Supporting Information Fig. S18). To further explore the targeting capacity of HAP−1NPDC+LND towards FLS, anti-vimentin antibody-labeled FLS (green fluorescent) was visualized using an immunofluorescence microscope to observe the distribution of nanoparticles in the inflamed joints. As shown in Fig. 5E, Cy5-labeled HAP−1NPDC+LND displayed a more pronounced overlap with green fluorescence of FLS compared to Cy5-labeled NPDC+LND. Fluorescence analysis further revealed a significant co-localization of Cy5-labeled HAP−1NPDC+LND with FLS (Pearson's R = 0.95), surpassing the co-localization of Cy5-labeled NPDC+LND with FLS (Pearson's R = 0.87) (Fig. 5E). The presence of HA in NPDC+LND facilitated its targeting ability towards FLS by mediating nanoparticle recognition and endocytosis through CD44 receptors within inflamed joints39. Moreover, the specific binding interaction between HAP-1 augmented both nanoparticle targeting efficiency and their association with FLS. Overall, these results suggested that due to the specific binding interaction of HAP-1 with FLS, HAP−1NPDC+LND possesses targeting capability towards FLS in the inflamed joints and may enhance the therapeutics' effectiveness for RA.
Figure 5.
HAP−1NPDC+LND target FLS and alleviate arthritic progression in CIA mice. (A) The schematic illustration of the therapeutic timeline in a CIA mouse model; (B) In vivo fluorescence images of CIA model mice at different time points after administration with Cy5-labeled NPDC+LND and Cy5-labeled HAP−1NPDC+LND; (C) Ex vivo fluorescence images and (D) quantitative analysis of mean fluorescence intensity of paws 24 h after administration with NPDC+LND and HAP−1NPDC+LND; (E) Representative immunofluorescence image of NPDC+LND and HAP−1NPDC+LND distribution in FLS of the inflamed joints; Scale bar = 20 μm; (F) The clinical scores from arthritis onset to the end of therapy in CIA mice; (G) The clinical scores of CIA mice on Day 45; (H) Hind ankle thickness from arthritis onset to the end of therapy in CIA mice; (I) Hind ankle thickness of CIA mice on Day 45; (J) Representative images of inflamed paws at different treatment endpoints; (K) Computed tomography (CT) images of paws after different therapy; Data are presented as mean values ± SD (n = 3). ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001.
The therapeutic effectiveness of HAP−1NPDC+LND for RA treatment was subsequently evaluated on the CIA animal model (Fig. 5A). The treatment groups included normal, saline, DC+LND, NPDC, NPLND, NPDC+LND, and HAP−1NPDC+LND. In vivo imaging experiments revealed that nanoparticles were cleared from the site of inflammation within about 72 h, so the treatments were administered every three days for a total of five sessions. No significant alterations in body weight were observed during the treatment period, suggesting the safety profile of nanoparticles (Supporting Information Fig. S19). Besides, H&E staining of the vital organs (including the heart, liver, spleen, lungs, and kidneys) showed no abnormalities in appearance, color, and size (Supporting Information Fig. S20). Ankle diameters were measured in mice, and clinical scoring was performed based on established criteria to evaluate the treatment efficacy32,52. The clinical index (CI) of HAP−1NPDC+LND group decreased to approximately 1 by the end of the experiment, approaching that of the normal group and surpassing those of DC+LND (CI = 4.16), NPDC (CI = 5), NPLND (CI = 5.33), and NPDC+LND (CI = 3.33) groups (Fig. 5F and G). Furthermore, mice treated with NPDC+LND and HAP−1NPDC+LND exhibited significantly reduced ankle thickness compared to DC+LND, NPDC, and NPLND groups (Fig. 5H and I). Notably, HAP−1NPDC+LND treatment exhibited the smallest paw thickness, consistent with the CI data. Additionally, macroscopic evaluation of the total hind ankle joints morphology confirmed these findings (Fig. 5J). These results indicate that HAP−1NPDC+LND demonstrates superior therapeutic efficacy in RA compared to the other treatment groups.
Subsequently, the hind ankle joints structure was evaluated for disease severity in the CIA model using computed tomography (CT) imaging. As shown in Fig. 5K, the saline group exhibited evident characteristics of RA, including joint space narrowing, osteoporosis, and bone degradation surrounding the joints. Both free DC+LND and single prodrug nanoparticle treatments showed only slight alleviation of these RA symptoms. In addition, NPDC+LND exhibited slight bone erosion. Remarkably, no indications of osteoporosis were observed in HAP−1NPDC+LND group, with smooth bone surfaces observed in the joints and heels, indicating the absence of bone erosion. The CT results further demonstrated that HAP−1NPDC+LND effectively prevented bone destruction in RA, providing a compelling rationale for the synergistic and targeted therapeutic outcomes mentioned above.
3.6. HAP−1NPDC+LND improve arthritic inflammation and cartilage damage by FLS-targeted synergistic metabolic modulation
To obtain a more comprehensive assessment of the synovial inflammatory infiltration and cartilage injury post-treatment, the hind ankle joints of CIA mice were dissected for histological analysis, including H&E, toluidine blue, and safranin-O staining analyses. As shown in Fig. 6A, the saline group displayed pronounced synovial hyperplasia and inflammatory infiltration in stark contrast to the normal group, as evidenced by H&E staining. The therapeutic effects of DC+LND, NPDC, and NPLND treatments were relatively modest. In contrast, both NPDC+LND and HAP−1NPDC+LND exhibited excellent inhibition of synovial hyperplasia and inflammatory infiltration, attributed to their synergistic suppression of glucose metabolism in FLS, thereby supporting anti-inflammatory and anti-proliferative effects. Notably, the therapeutic effect of HAP−1NPDC+LND is more prominent than that of NPDC+LND possibly because HAP-1 targeting enhances FLS modulation. When comparing the cartilage tissues with those from the normal group, RA-afflicted mice in saline group displayed extensive erosion with severely compromised structure by defects and attenuation. Key components such as chondrocytes and glycosaminoglycan within cartilage were significantly depleted, which are the hallmark features of RA (Fig. 6B)53, 54, 55. Treatment with DC+LND, NPDC, and NPLND did not obviously ameliorate these pathological changes in RA cartilage. Compared with NPDC+LND, HAP−1NPDC+LND intervention reversed these pathological alterations more effectively, restoring the cartilage's structural integrity and component composition to a state similar to that observed in normal mice. These histological findings collectively underscored the remarkable therapeutic outcome of HAP−1NPDC+LND treatment, closely aligning with the aforementioned observational results.
Figure 6.
HAP−1NPDC+LND improve the arthritic inflammation and cartilage damage by FLS-targeted synergistic metabolic modulation. (A) Histological analysis with H&E, (B) Toluidine blue, and safranin-O staining of a section of the hind ankle joints after various treatments; Scale bar = 100 μm; (C) The expression of GLUT-1, and HK-2 in synovial tissue of the hind ankle joints were detected by immunohistochemical staining; Scale bar = 100 μm; (D) The expression of FAP-α in synovial tissue of the hind ankle joints were detected by immunohistochemical staining; Scale bar = 100 μm; (E) the CLSM images of M1-macrophage in synovial tissue; Scale bar = 40 μm.
To elucidate the mechanism of the improved arthritic inflammation and cartilage damage by HAP−1NPDC+LND treatment, immunohistochemical stainings were performed on hind ankle joint tissues. As shown in Fig. 6C, compared to normal mice, the expression levels of GLUT-1 and HK-2 proteins in the synovium of hind ankle joints were significantly increased in the saline group, confirming the elevated glucose metabolism in RA models. Notably, there was a significant reduction in GLUT-1 and HK-2 protein expressions observed in NPDC+LND group, while DC+LND, NPDC and NPLND treatments only showed a slight decrease. As expected, the expressions of GLUT-1 and HK-2 proteins in the synovial membrane in HAP−1NPDC+LND group resulted in lower levels of GLUT-1 and HK-2 proteins within the synovial membrane compared to NPDC+LND. Moreover, immunohistochemical staining using FAP-α as a biomarker revealed an abundance of activated FLS within inflamed joints56. As shown in Fig. 6D, HAP−1NPDC+LND treatment led to a significant reduction in activated FLS count when compared with other groups, indicating that the targeted and synergistic inhibition of glucose metabolism can deplete FLS effectively. Furthermore, the effects of nanoparticles on macrophage phenotypes within the SME were further explored. M1 macrophages were identified with FITC-CD86 (green fluorescence) labeling. By comparing the different treatments, it was observed that the expression level of CD86 was lowest in the HAP−1NPDC+LND group, proving the reduced infiltration of pro-inflammatory macrophages in the SME (Fig. 6E). Therefore, HAP−1NPDC+LND not only regulate glucose metabolism of FLS to eliminate FLS but also remodel arthritic SME for the prevention of synovitis.
4. Conclusions
We initially confirmed the synergistic inhibitory effect on FLS’ survival, proliferation, and activation by combining two inhibitors of glucose metabolism (DC and LND), particularly at a ratio of 2:1. Subsequently, a HAP-1 peptide-modified polymeric dual prodrug nanoparticles system (HAP−1NPDC+LND) was tailored at the optimal synergistic ratio for targeted and synergistic modulation of glucose metabolism in FLS to mitigate RA. The HAP−1NPDC+LND facilitated the specific release of DC and LND within FLS in inflamed joints through HAP-1-mediated targeting mechanism, capitalizing on the reductive microenvironment present within FLS. The combination of DC and LND effectively deprived the glucose source of FLS while disrupting its glycolytic enzymes, leading to a pronounced synergic inhibition of glucose metabolism that ultimately inhibited FLS survival, proliferation and activation. Furthermore, HAP−1NPDC+LND reduced the secretion levels of inflammatory factors and lactic acid from FLS in vitro, weakening the activation of pro-inflammatory macrophages. The in vivo results demonstrated that HAP−1NPDC+LND achieved excellent targeted and synergistic metabolic modulation specifically in FLS by reducing the infiltration of pro-inflammatory macrophages and preventing cartilage damage in the CIA model. This study spotlighted the promising potential of HAP−1NPDC+LND as a targeted approach for synergistically modulating metabolism in FLS, providing new insights for designing RA therapy based on metabolic intervention.
Author contributions
Shaobing Li: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Juntao Lin: Methodology, Investigation, Formal analysis, Data curation. Chengxinqiao Wang: Validation, Methodology. Junhan Liu: Methodology, Validation. Yupeng Wang: Writing – review & editing, Supervision. Yan Chen: Writing – review & editing, Supervision, Funding acquisition. Dongfang Zhou: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization.
Conflicts of interest
The authors declare no competing financial interest.
Acknowledgments
This work is supported by grants from the National Key Research and Development Program of China (2022YFC3601900/2022YFC3601902), the National Natural Science Foundation of China (22275081, 82372117), the Guangdong Basic and Applied Basic Research Foundation (2024A1515011462, 2022A1515011292 and 2024A1515010464), and the China Postdoctoral Science Foundation (2022M711532 and 2022T150302).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting Information to this article can be found online at https://doi.org/10.1016/j.apsb.2024.11.007.
Contributor Information
Yupeng Wang, Email: wangyupeng5@i.smu.edu.cn.
Yan Chen, Email: smu_chen@163.com.
Dongfang Zhou, Email: dfzhou@smu.edu.cn.
Appendix A. Supporting information
The following is the Supporting Information to this article:
References
- 1.Culemann S., Gruneboom A., Nicolas-Avila J.A., Weidner D., Lammle K.F., Rothe T., et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature. 2019;572:670–675. doi: 10.1038/s41586-019-1471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo P.P., Jiang J., Chu R., He F., Ge M.L., Fang R.H., et al. GRK2 mediated degradation of SAV1 initiates hyperplasia of fibroblast-like synoviocytes in rheumatoid arthritis. Acta Pharm Sin B. 2024;14:1222–1240. doi: 10.1016/j.apsb.2023.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masoumi M., Mehrabzadeh M., Mahmoudzehi S., Mousavi M.J., Jamalzehi S., Sahebkar A., et al. Role of glucose metabolism in aggressive phenotype of fibroblast-like synoviocytes: latest evidence and therapeutic approaches in rheumatoid arthritis. Int Immunopharmacol. 2020;89 doi: 10.1016/j.intimp.2020.107064. [DOI] [PubMed] [Google Scholar]
- 4.Noss E.H., Brenner M.B. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223:252–270. doi: 10.1111/j.1600-065X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao B., Zeng L.T., Chen D.Y., Xie S.Q., Jin Z.K., Li G.L., et al. NIR-photocatalytic regulation of arthritic synovial microenvironment. Sci Adv. 2022;8 doi: 10.1126/sciadv.abq0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q.L., Asenso J., Xiao N., Gao J.Z., Xiao F., Kuai J.J., et al. Lactic acid regulation: a potential therapeutic option in rheumatoid arthritis. J Immunol Res. 2022;2022:1–11. doi: 10.1155/2022/2280973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanlon M.M., Canavan M., Barker B.E., Fearon U. Metabolites as drivers and targets in rheumatoid arthritis. Clin Exp Immunol. 2022;208:167–180. doi: 10.1093/cei/uxab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee A., Qiao Y., Grigoriev G., Chen J., Park-Min K.H., Park S.H., et al. Tumor necrosis factor α induces sustained signaling and a prolonged and unremitting inflammatory response in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2013;65:928–938. doi: 10.1002/art.37853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nygaard G., Firestein G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol. 2020;16:316–333. doi: 10.1038/s41584-020-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McInnes I.B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–2337. doi: 10.1016/S0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 11.Tsaltskan V., Firestein G.S. Targeting fibroblast-like synoviocytes in rheumatoid arthritis. Curr Opin Pharmacol. 2022;67 doi: 10.1016/j.coph.2022.102304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu S., Wu X.N., Li Z., Xu X.Y., Wang J., Du Y.W., et al. A smart nanoreactor based on an O2-economized dual energy inhibition strategy armed with dual multi-stimuli-responsive “doorkeepers” for enhanced CDT/PTT of rheumatoid arthritis. ACS Nano. 2022;16 doi: 10.1021/acsnano.2c07338. [DOI] [PubMed] [Google Scholar]
- 13.Huang R.Q., Zhang C.Y., Bu Y.Y., Li Z., Zheng X., Qiu S., et al. A multifunctional nano-therapeutic platform based on octahedral yolk-shell Au NR@CuS: photothermal/photodynamic and targeted drug delivery tri-combined therapy for rheumatoid arthritis. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121088. [DOI] [PubMed] [Google Scholar]
- 14.Fearon U., Hanlon M.M., Floudas A., Veale D.J. Cellular metabolic adaptations in rheumatoid arthritis and their therapeutic implications. Nat Rev Rheumatol. 2022;18:398–414. doi: 10.1038/s41584-022-00771-x. [DOI] [PubMed] [Google Scholar]
- 15.De Oliveira P.G., Farinon M., Sanchez-Lopez E., Miyamoto S., Guma M. Fibroblast-like synoviocytes glucose metabolism as a therapeutic target in rheumatoid arthritis. Front Immunol. 2019;10:1743. doi: 10.3389/fimmu.2019.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bustamante M.F., Oliveira P.G., Garcia-Carbonell R., Croft A.P., Smith J.M., Serrano R.L., et al. Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann Rheum Dis. 2018;77:1636–1643. doi: 10.1136/annrheumdis-2018-213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Carbonell R., Divakaruni A.S., Lodi A., Vicente-Suarez I., Saha A., Cheroutre H., et al. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 2016;68:1614–1626. doi: 10.1002/art.39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song G.H., Lu Q.Q., Fan H., Zhang X.M., Ge L.N., Tian R.S., et al. Inhibition of hexokinases holds potential as treatment strategy for rheumatoid arthritis. Arthritis Res Ther. 2019;21:87. doi: 10.1186/s13075-019-1865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biniecka M., Canavan M., McGarry T., Gao W., McCormick J., Cregan S., et al. Dysregulated bioenergetics: a key regulator of joint inflammation. Ann Rheum Dis. 2016;75:2192–2200. doi: 10.1136/annrheumdis-2015-208476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M., Wang D.M., Chen Q., Li C.X., Li Z.Q., Lin J. Recent advances in glucose-oxidase-based nanocomposites for tumor therapy. Small. 2019;15 doi: 10.1002/smll.201903895. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Zheng J.Y., Liu J.Q., Yang J., Liu Y., Wang C., et al. Succinate/NLRP3 inflammasome induces synovial fibroblast activation: therapeutical effects of clematichinenoside AR on arthritis. Front Immunol. 2016;7:532. doi: 10.3389/fimmu.2016.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Y.Y., Zeng S., Huang M.C., Qiu Q., Xiao Y.J., Shi M.H., et al. Inhibition of 6-phosphofructo-2-kinase suppresses fibroblast-like synoviocytes-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Br J Pharmacol. 2017;174:893–908. doi: 10.1111/bph.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Y., Li Y.M., Huang Z.J., Li X.Y., Wang Y., Hou J.W., et al. A nanounit strategy disrupts energy metabolism and alleviates immunosuppression for cancer therapy. Nano Lett. 2022;22:6418–6427. doi: 10.1021/acs.nanolett.2c02475. [DOI] [PubMed] [Google Scholar]
- 24.Wu S.X., Zhang K.X., Liang Y., Wei Y.B., An J.Y., Wang Y.F., et al. Nano-enabled tumor systematic energy exhaustion via Zinc (II) interference mediated glycolysis inhibition and specific glut1 depletion. Adv Sci. 2022;9 doi: 10.1002/advs.202103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dou Y., Li C.W., Li L.L., Guo J.W., Zhang J.X. Bioresponsive drug delivery systems for the treatment of inflammatory diseases. J Control Release. 2020;327:641–666. doi: 10.1016/j.jconrel.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou N., Liu Q.Y., Wang X., He L.X., Zhang T., Zhou H., et al. The combination of hydroxychloroquine and 2-deoxyglucose enhances apoptosis in breast cancer cells by blocking protective autophagy and sustaining endoplasmic reticulum stress. Cell Death Discov. 2022;8:286. doi: 10.1038/s41420-022-01074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W.H., Luo G.F., Lei Q., Hong S., Qiu W.X., Liu L.H., et al. Overcoming the heat endurance of tumor cells by interfering with the anaerobic glycolysis metabolism for improved photothermal therapy. ACS Nano. 2017;11:1419–1431. doi: 10.1021/acsnano.6b06658. [DOI] [PubMed] [Google Scholar]
- 28.Mi Z.B., Lu X.L., Mai J.C., Ng B.G., Wang G.Q., Lechman E.R., et al. Identification of a synovial fibroblast-specific protein transduction domain for delivery of apoptotic agents to hyperplastic synovium. Mol Ther. 2003;8:295–305. doi: 10.1016/s1525-0016(03)00181-3. [DOI] [PubMed] [Google Scholar]
- 29.Yan K., Feng Y.C., Gao K., Shi X.J., Zhao X.B. Fabrication of hyaluronic acid-based micelles with glutathione-responsiveness for targeted anticancer drug delivery. J Colloid Interf Sci. 2022;606:1586–1596. doi: 10.1016/j.jcis.2021.08.129. [DOI] [PubMed] [Google Scholar]
- 30.Behrendt R., White P., Offer J. Advances in Fmoc solid-phase peptide synthesis. J Pept Sci. 2016;22:4–27. doi: 10.1002/psc.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand D.D., Latham K.A., Rosloniec E.F. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Rao P.S., Qian H.Y., Shi Y.S., Chen S.J., Lan J.Y., et al. Regulatory fibroblast-like synoviocytes cell membrane coated nanoparticles: a novel targeted therapy for rheumatoid arthritis. Adv Sci. 2023;10 doi: 10.1002/advs.202204998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu H.T., Zhou H., Zhang W.J., Jin P., Shi Q.Q., Miao Z.H., et al. Three birds with one stone: co-encapsulation of diclofenac and DL-menthol for realizing enhanced energy deposition, glycolysis inhibition and anti-inflammation in HIFU surgery. J Nanobiotechnology. 2022;20:215. doi: 10.1186/s12951-022-01437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X.R., Li S.F., Mei W.Y., Liu X.D., Zhou R.B. Isorhamnetin downregulates MMP2 and MMP9 to inhibit development of rheumatoid arthritis through SRC/ERK/CREB pathway. Chin J Integr Med. 2024;30:299–310. doi: 10.1007/s11655-023-3753-6. [DOI] [PubMed] [Google Scholar]
- 35.Singh S., Tiwary N., Sharma N., Behl T., Antil A., Anwer M., et al. Integrating nanotechnological advancements of disease-modifying anti-rheumatic drugs into rheumatoid arthritis management. Pharmaceuticals. 2024;17:248. doi: 10.3390/ph17020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J., Feng Z., Chen L., Li Y., Bian H.J., Geng J.J., et al. TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat Commun. 2022;13:676. doi: 10.1038/s41467-021-27948-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J.C., Yang B.W., Shi J.L. A nanomedicine-enabled ion-exchange strategy for enhancing curcumin-based rheumatoid arthritis therapy. Angew Chem Int Ed Engl. 2023;62 doi: 10.1002/anie.202310061. [DOI] [PubMed] [Google Scholar]
- 38.Treuhaft P.S., Mccarty D.J. Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 1971;14:475–484. doi: 10.1002/art.1780140407. [DOI] [PubMed] [Google Scholar]
- 39.Gorantla S., Gorantla G., Saha R.N., Singhvi G. CD44 receptor-targeted novel drug delivery strategies for rheumatoid arthritis therapy. Expert Opin Drug Deliv. 2021;18:1553–1557. doi: 10.1080/17425247.2021.1950686. [DOI] [PubMed] [Google Scholar]
- 40.Wang J.W., Chen Q.W., Luo G.F., Ji P., Han Z.Y., Song W.F., et al. Interference of glucose bioavailability of tumor by engineered biohybrids for potentiating targeting and uptake of antitumor nanodrugs. Nano Lett. 2022;22:8735–8743. doi: 10.1021/acs.nanolett.2c03608. [DOI] [PubMed] [Google Scholar]
- 41.Lai L., Shin G.Y., Qiu H.Y. The role of cell cycle regulators in cell survival-dual functions of cyclin-dependent kinase 20 and p21Cip1/Waf1. Int J Mol Sci. 2020;21:8504. doi: 10.3390/ijms21228504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y.H., Gao P., Wang Y.Q., Xu L.Z., Zeng K.W., Tu P.F. Small-molecule targeting PKM2 provides a molecular basis of lactylation-dependent fibroblast-like synoviocytes proliferation inhibition against rheumatoid arthritis. Eur J Pharmacol. 2024;972 doi: 10.1016/j.ejphar.2024.176551. [DOI] [PubMed] [Google Scholar]
- 43.Yang H.X., Liu C.Z., Lin X.J., Li X., Zeng S., Gong Z.H., et al. Wogonin inhibits the migration and invasion of fibroblast-like synoviocytes by targeting PI3K/AKT/NF-κB pathway in rheumatoid arthritis. Arch Biochem Biophys. 2024;755 doi: 10.1016/j.abb.2024.109965. [DOI] [PubMed] [Google Scholar]
- 44.Barker B.E., Hanlon M.M., Marzaioli V., Smith C.M., Cunningham C.C., Fletcher J.M., et al. The mammalian target of rapamycin contributes to synovial fibroblast pathogenicity in rheumatoid arthritis. Front Med. 2023;10 doi: 10.3389/fmed.2023.1029021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu D.Y., Liang J.Y., Lin J., Yu C.H. PKM2: a potential regulator of rheumatoid arthritis via glycolytic and non-glycolytic pathways. Front Immunol. 2019;10:2919. doi: 10.3389/fimmu.2019.02919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H.M., Guo H.L., Xu C., Liu L., Hu S.Y., Hu Z.H., et al. Inhibition of glycolysis by targeting lactate dehydrogenase a facilitates hyaluronan synthase 2 synthesis in synovial fibroblasts of temporomandibular joint osteoarthritis. Bone. 2020;141 doi: 10.1016/j.bone.2020.115584. [DOI] [PubMed] [Google Scholar]
- 47.Samuvel D.J., Sundararaj K.P., Nareika A., Lopes-Virella M.F., Huang Y. Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J Immunol. 2009;182:2476–2484. doi: 10.4049/jimmunol.0802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simmonds R.E., Foxwell B.M. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology. 2008;47:584–590. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 49.Zhou F., Mei J.T., Han X.G., Li H.J., Yang S.B., Wang M.Q., et al. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-κB/MAPK signaling and protecting chondrocytes. Acta Pharm Sin B. 2019;9:973–985. doi: 10.1016/j.apsb.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon S.B., Hong H., Lim H.J., Choi J.H., Choi Y.P., Seo S.W., et al. A novel IRAK4/PIM1 inhibitor ameliorates rheumatoid arthritis and lymphoid malignancy by blocking the TLR/MYD88-mediated NF-κB pathway. Acta Pharm Sin B. 2023;13:1093–1109. doi: 10.1016/j.apsb.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song W.X., Li D.Y., Tao L., Luo Q., Chen L.G. Solute carrier transporters: the metabolic gatekeepers of immune cells. Acta Pharm Sin B. 2020;10:61–78. doi: 10.1016/j.apsb.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y., Mu J.Q., Xu Z.K., Zhong H.P., Chen Z.Q., Ni Q.K., et al. Modular acid-activatable acetone-based ketal-linked nanomedicine by dexamethasone prodrugs for enhanced anti-rheumatoid arthritis with low side effects. Nano Lett. 2020;20:2558–2568. doi: 10.1021/acs.nanolett.9b05340. [DOI] [PubMed] [Google Scholar]
- 53.Yu B., Sun W., Lin J.T., Fan C.Y., Wang C.X.Q., Zhang Z.S., et al. Using Cu-based metal-organic framework as a comprehensive and powerful antioxidant nanozyme for efficient osteoarthritis treatment. Adv Sci. 2024;11 doi: 10.1002/advs.202307798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong Q., Liu Z.X., Liang H.F., Wu D.G., Chen Y., Yu B. Inhibition of HOXD11 promotes cartilage degradation and induces osteoarthritis development. J Orthop Surg Res. 2024;19:111. doi: 10.1186/s13018-024-04573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y., Luo S., Peng X., Zhao T., He Q., Wu M., et al. An intra-articular injectable phospholipids-based gel for the treatment of rheumatoid arthritis. Asian J Pharm Sci. 2023;18 doi: 10.1016/j.ajps.2023.100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dorst D.N., Rijpkema M., Buitinga M., Walgreen B., Helsen M.M.A., Brennan E., et al. Targeting of fibroblast activation protein in rheumatoid arthritis patients: imaging and ex vivo photodynamic therapy. Rheumatology. 2022;61:2999–3009. doi: 10.1093/rheumatology/keab664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.