Abstract
Dry eye disease (DED) is a prevalent and intractable ocular disease induced by a variety of causes. Elevated sphingomyelin (SM) levels and pro-inflammatory cytokines were detected on the ocular surface of DED patients, particularly in the meibomian glands. Sphingomyelin synthase 2 (SMS2), one of the proteins involved in SM synthesis, would light a novel way of developing a DED therapy strategy. Herein, we report the design and optimization of a series of novel thiophene carboxamide derivatives to afford 14l with an improved highly potent inhibitory activity on SM synthesis (IC50, SMS2 = 28 nmol/L). Moreover, 14l exhibited a notable protective effect of anti-inflammation and anti-apoptosis on human corneal epithelial cells (HCEC) under TNF-α-hyperosmotic stress conditions in vitro, with an acceptable ocular specific distribution (corneas and meibomian glands) and pharmacokinetics (PK) profiles (t1/2, cornea = 1.11 h; t1/2, meibomian glands = 4.32 h) in rats. Furthermore, 14l alleviated the dry eye symptoms including corneal fluorescein staining scores and tear secretion in a dose-dependent manner in mice. Mechanically, 14l reduced the mRNA expression of Tnf-α, Il-1β and Mmp-9 in corneas, as well as the proportion of very long chain SM in meibomian glands. Our findings provide a new strategy for DED therapy based on selective SMS2 inhibitors.
Key words: Sphingomyelin synthase 2, Thiophene carboxamide, SMS2 inhibitor, Dry eye disease, Tumor necrosis factor receptor 1, Human corneal epithelial cells, Sphingomyelin, Meibomian glands
Graphical abstract
This study elucidated a structure–activity relationship-based design and optimization of novel SMS2 inhibitors exhibiting less toxic effects and highly effective therapeutic effects on a murine dry eye disease model.

1. Introduction
Dry eye disease (DED) is a multifactorial illness that is associated with ocular surface inflammation and increased tear film osmolarity1. The normal tear film is mainly composed of lipids, electrolyte aqueous solution, mucins and vitamins2. In terms of DED patients, the lack of tear secretion and/or the instability of the tear film lipids layer (TFLL) caused faster evaporation of the tear to induce a higher osmolarity, inflammation and even ocular surface injury.
Considering the complexity of DED pathology, many drugs have been developed to ameliorate DED over the past decades (Fig. 1). For example, cyclosporine A (CsA) is a natural cyclopeptide consisting of 11 deduced amino acids which could inhibit the inflammatory response via immunosuppressive effects3. A small molecular drug, Lifitegrast, blocks the interaction between lymphocyte function-associated antigen 1 (LFA-1) and intercellular cell adhesion molecule 1 (ICAM-1) to relieve the T-cell-mediated inflammation in DED4. In addition, several drugs such as diquasofol (a P2Y2 receptor agonist) were applied for DED treatment by stimulating tear secretion from ocular epithelial cells and goblet cells5,6. However, poor patient compliance calls for more novel and effective candidates to overcome the limitations of present drugs7,8.
Figure 1.
Representative anti-DED drugs and selective SMS2 inhibitors.
The presence of corneal and conjunctival inflammation results in an increased patients' DED symptomatology9. In a hyperosmolarity-induced corneal epithelial cells model, an increased level of sphingomyelin (SM), which plays a crucial role in cell death and inflammation process10, was observed. Moreover, an elevated SM concentration of corneas and/or tear was also observed in several animal DED models11,12. Clinically, meibomian gland dysfunction (MGD) is another main driving factor of evaporative DED but its mechanism is still not fully elucidated13. As a result of elevated concentration of SM in MGD patients’ meibomian glands and tear, SM would be considered as one of the prevalent biomarker molecules of DED14,15. Actually, SM plays a crucial role in the composition of membrane components and inflammation-associated signaling transduction16, 17, 18, 19, 20. Hence, suppressing the level of SM concentration might be a promising approach for DED treatment.
In mammals, SM was synthesized by sphingomyelin synthase (SMS) family proteins21 including SMS1 and SMS2. Especially, it has been proved that the gene knock-out of Sgms2 was of benefit to the prevention and treatment of several diseases, such as atherosclerosis22, insulin resistance23, tumor diseases24,25 and inflammation26, 27, 28, and had fewer safety risks29, 30, 31, 32. Therefore, SMS2 might be a potential new-generation drug target for DED treatment. Although several SMS2 inhibitors (Fig. 1) such as a quinolone analogue33 19, a benzyloxybenzo[d]isoxazole derivertive34 15w and a salicylamide derivative35 ly93 were reported and exhibited preliminary inhibitory efficiency against SMS2, there was no highly effective one used for DED treatment still. Herein, we discovered a novel thiophene carboxamide derivative with highly potent SMS2 inhibitory activity and selectivity through a structure‒activity relationship (SAR)-based optimization. Furthermore, the SM synthesis inhibitory efficiency, protective effects under inflammatory-hyperosmotic stress conditions in human corneal epithelial cells (HCEC), ocular pharmacokinetics and pharmacodynamics effects in a murine DED model were evaluated for the preferential compound 14l.
2. Results and discussion
2.1. General synthesis procedures
The organic synthesis routes of thiophene carboxamide analogues are shown in Scheme 1, Scheme 2. On the one hand, the intermediate 2 was synthesized by different initial (2-hydroxyl)aromaticformates through an O-alkylation reaction (Scheme 1). The compound 2 was hydrolyzed with sodium hydrate to give intermediate 3. In order to obtain the amide compounds 5a‒5f, 6 and 7a‒7k, a condensation reaction catalyzed by HBTU was performed using carboxylic acid compound 3 and corresponding aromatic amides, while several inertial amides were reacted with the intermediate 4 to afford the final products. On the other hand, toluene derivatives 11 were used for the synthesis of haloalkane analogue 13 through a 12-mediated nucleophilic substitution reaction and a free radical-induced bromination reaction (Scheme 2). The key intermediate 8 was obtained by deprotection of 5a to react with different haloalkane analogs to give final compounds 9a‒9e, 10 and 14a‒14r.
Scheme 1.
Preparation of compounds 5a‒5f, 6, 7a‒7ka. aReagents and conditions: (a) BnBr, K2CO3, KI, CH3CN, r.t., overnight; (b) NaOH, THF: H2O = 1: 1, reflux, 12 h; (c) Ar-NH2, HBTU, DIPEA, DMF, r.t., overnight; (d) (COCl)2, DMF, DCM, r.t., overnight; (e) Ar-NH2, DIPEA, DCM, r.t., overnight.
Scheme 2.
Preparation of compounds 9a‒9e, 10, 14a‒14ra. aReagents and conditions: (a) BBr3, DCM, r.t., overnight; (b) R1X (X = Cl, Br or I), K2CO3, DMF, r.t., overnight; (c) R2I, K2CO3, CH3CN, 60 °C, overnight; (d) NBS, AIBN, CCl4, 70 °C, 12 h.
2.2. Core moiety design and SAR analysis
In previous works24,34,35, a benzo[d]isoxazole derivative 1b was discovered as a novel SMS2 inhibitor by a scaffold hopping strategy from compound 1a. As the three-dimensional structure of SMS2 has not been resolved, it is worth noting that the scaffold hopping strategy of 1b uncovered an intramolecular hydrogen bond (H-bond)-keeping active conformation which exhibited a higher ClogP36 property and less improvement of SMS2 inhibitory activity34. Moreover, 1b exhibited a much lower Papp value (<2.0 × 10−6 cm/s) and higher efflux ratio than 1a in a Caco-2 cell-based permeability assay and is probably a P-glycoprotein (Pgp) substrate (Fig. 2), which could account for the less improvement of SMS inhibitory efficiency of 1b and 1a analogues in cells24,35. To obtain highly potent SMS2 inhibitors, we designed a thiophene carboxamide derivative 5a as an ideal lead compound containing a conservative intramolecular H-bond and a non-covalent 1,4–O...S interaction37 (Fig. 2).
Figure 2.
Structures and Caco-2 cell permeability of lead compounds. aResults are expressed as the mean of duplicates. bResults are predicted using SwissADME.
Results of enzymatic inhibitory measurement showed that 5a has a better SMS2 inhibitory activity and a higher selectivity ratio than lead compound 1a (Table 1). However, 5b lost both SMS2 and SMS1 inhibitory activity drastically. When several heavy substitute groups and/or a nitrogen atom were introduced into the core thiophene ring of compounds such as 5c‒5f, their inhibitory activities were decreased apparently. Besides, replacing the hydrogen atom of amide with a methyl group interrupts the intramolecular H-bond to give 6 and also leads to the reduction of SMS2 inhibitory activity.
Table 1.
SAR exploration of the core moiety.
 | ||||||||
| Compd. | Core | SMS2 |
SMS1 |
Selectivity ratioa | ClogPb | LLEc | ||
| Inhibition @ 1 μmol/L (%)a | IC50 (nmol/L)a | Inhibition @ 100 μmol/L (%)a | IC50 (μmol/L)a | |||||
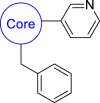
| 1a | 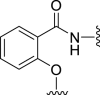 |
29 | 3942 | 35 | 191 | 48 | 3.02 | 2.38 |
| 5a | 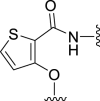 |
66 | 400 | 20 | 219 | 547 | 3.07 | 3.33 |
| 5b | 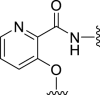 |
‒16 | NT | −18 | – | – | 2.38 | – |
| 5c | 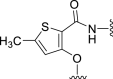 |
34 | NT | −14 | – | – | 3.48 | – |
| 5d | 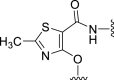 |
‒8 | NT | 10 | – | – | 3.01 | – |
| 5e | 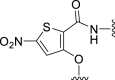 |
‒11 | NT | −4 | – | – | 2.52 | – |
| 5f | 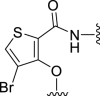 |
42 | NT | 14 | – | – | 3.70 | – |
| 6 | 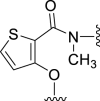 |
28 | NT | 20 | – | – | 3.31 | – |
SMS activity is measured by purified SMS enzyme inhibitory assay in vitro. The results are expressed as the mean of duplicates. Selectivity ratio is calculated by a formula of “IC50, SMS1/IC50, SMS2”.
ClogP is predicted by SwissADME.
LLE is calculated by a formula of “pIC50, SMS2 – ClogP”. —, not applicable.
At first, to understand the highly improved SMS2 inhibitory activity of 5a, computer-aided theoretical studies were performed. Results of torsion energy scanning showed that 1a and 5a determined a preference for the conformation (Fig. 3A) with a Ψ value of 0 and 360. As a result of the intramolecular interaction between the low-lying σ∗ orbitals of the C–S bond and the oxygen atom in the C O bond37, 5a exhibited a higher torsion energy barrier (Ψ = 90° and 270°) of approximately 4 kcal/mol than 1a when they changed the conformation from preferential states (Ψ = 0 and 360°) to an inferior one (Ψ = 180°). It is worth noting that the single crystal X-ray structure study revealed the actual preferential conformation of 5a (Fig. 3B and Supporting Information Table S1) which was much similar to the above simulated preferential conformations (Ψ = 0 and 360°). Conversely, 5b prefers to keep a different stable conformation (Ψ = 180°) which was probably caused by the loss of non-covalent 1,4–O…S interaction and the repulsion interaction between pyridine's nitrogen atom and the oxygen atom at C O bond.
Figure 3.
(A) Conformational energy comparison of 1a, 5a and 5b based on different Ψ values. 1a: X = “C C”, Y = Z = “C”; 5a: X = “S”, Y = Z = “C”; 5b: X = “N”, Y = “C C”, Z = “C”. Ψ refers to the “X–C–C O” dihedral angle shown in formula structures. All calculations are performed at the B3LYP/6-31+G(d,p) level of theory, and the scanned dihedral is depicted in red and bold. (B) X-ray single crystal structure of 5a.
Next, the results of enzymatic activity measurement showed a tight space tolerance of the pyridyl group in 5a. Neither electron withdrawing groups (7a‒7d) nor electron donating groups (7e‒7h) could increase the inhibitory activities (Table 2). Besides, when the pyridyl group was replaced with pyridazinyl (7i and 7j) or phenyl group (7k), the activities were also reduced, which demonstrated the much higher conservation of the pyridyl group in these inhibitors. As the three-dimensional structure of SMS2 was still unrevealed, to understand the SAR of the pyridyl group at 5a analogues, we introduced a novel docking model using 5a and a putative human SMS2 model (UniProt Q8NHU3) predicted by Alphafold238. In this model, 5a lies in the catalytic pocket of SMS2 (Fig. 4). The core thiophene ring formed a π–π stacking interaction with the HIS229 (a crucial catalytic residue of SMS239) while the pyridine moiety formed a π–π stacking interaction with TYR167 and a key H-bond interaction with ARG170. As a result of the existence of non-covalent O…S interaction and the intra-molecular H-bond in 5a, a conjugated aromatic plane consisting of the core moiety, the pyridine moiety and the amide linker played a significant role in the binding between 5a and SMS2.
Table 2.
SAR exploration of the pyridine moiety.
 | ||||||||
| Compd. | Ar | SMS2 |
SMS1 |
Selectivity ratio | ClogPb | LLEc | ||
| Inhibition @ 1 μmol/L (%)a | IC50 (nmol/L)a | Inhibition @ 100 μmol/L (%)a | IC50 (μmol/L)a | |||||
| 5a | 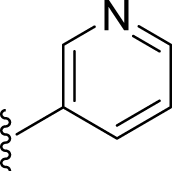 |
66 | 400 | 20 | 219 | 547 | 3.07 | 3.33 |
| 7a |  |
41 | 1793 | 53 | – | – | 3.44 | 2.31 |
| 7b |  |
7 | – | −39 | – | – | 3.60 | – |
| 7c |  |
56 | – | 52 | – | – | 3.43 | – |
| 7d | 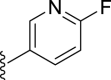 |
12 | – | 57 | – | – | 3.43 | – |
| 7e |  |
3 | – | −20 | – | – | 3.45 | – |
| 7f |  |
−4 | – | −17 | – | – | 3.40 | – |
| 7g | 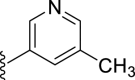 |
12 | – | 28 | – | – | 3.47 | – |
| 7h | 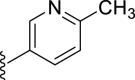 |
−3 | – | 22 | – | – | 3.46 | – |
| 7i | 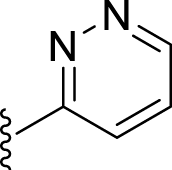 |
25 | – | 22 | – | – | 2.69 | – |
| 7j |  |
−16 | – | 5 | – | – | 2.55 | – |
| 7k |  |
3 | – | 60 | – | – | 3.79 | – |
SMS activity is measured by purified SMS enzyme inhibitory assay in vitro. The results are expressed as the mean of duplicates. Selectivity ratio is calculated by a formula of “IC50, SMS1/IC50, SMS2”.
ClogP is predicted by SwissADME.
LLE is calculated by a formula of “pIC50, SMS2 – ClogP”. —, not applicable.
Figure 4.
Molecular docking simulation of 5a (green stick) and putative human SMS2 (UniProt Q8NHU3, blue ribbons, red surface and purple wires). The π–π stacking interaction is shown as dark red dotted line. H-bonds are shown as yellow dotted lines.
Furthermore, although some efforts have been made before, the SAR of the benzyloxyl group has not been fully illustrated. According to the molecular docking model shown in Fig. 4, the benzyl moiety keeps a vertical conformation compared to the horizontal core moiety. The 2- and 3-sites at benzyl moiety were parallel to the trans-membrane region of SMS2, while the 4-site was burned into a narrow hydrophobic pocket (Fig. 4). Thus we replaced the benzyl group with different aromatic rings such as naphthalenes (9a, 9b), anthracene (9c) and quinolone (9d) and alkyl chains such as n-heptane (9e). Both 9a and 9c exhibited higher inhibitory activities than other compounds, which illustrated that rigid aromatic rings with appropriate volume were favorable to improving the inhibitory activity (Table 3). However, a benzene sulfonate derivative 10 lost the inhibitory activity entirely by virtue of its lower ClogP value. Thus, the optimization of modest space and hydrophobicity in benzyl moiety of 5a was needed for the inhibitory activity elevation.
Table 3.
SAR exploration of the benzyl group moiety.
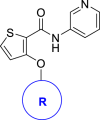 | ||||
| Compd. | R | Inhibition against SMS2 @ 1 μmol/L (%)a | Inhibition against SMS1 @ 100 μmol/L (%)a | ClogPb |
| 5a |  |
66 | 20 | 3.07 |
| 9a | 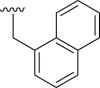 |
86 | 39 | 4.01 |
| 9b | 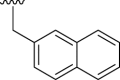 |
36 | 42 | 4.00 |
| 9c |  |
79 | 5 | 4.79 |
| 9d |  |
45 | 21 | 3.37 |
| 9e |  |
−11 | 67 | 4.32 |
| 10 | 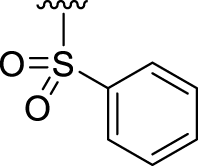 |
−3 | ‒16 | 2.71 |
SMS activity is measured by purified SMS enzyme inhibitory assay in vitro. The results are expressed as the mean of duplicates.
ClogP is predicted by SwissADME.
Finally, we performed a systematic optimization over the benzyl group. Chlorine atom was introduced to the 2-, 3- and 4-sites at the benzene ring of 5a to obtain compounds 14a‒14c respectively (Table 4). Consistent with the activities of 9a and 9b, 14a and 14b exhibited better inhibitory activities than 14c, which revealed a high spatial tolerance at the 2- and 3-sites of the benzene ring. A 2-chloro-5-florobenzen derivative 14d also has an improved inhibitory activity and selectivity against SMS2. Considering the hydrophobicity of the binding pocket of benzyl group (Fig. 4), a series of alkoxyl groups was introduced into the 2- site of the benzene ring to get compounds 14e‒14p. Interestingly, the SMS2 inhibitory activities of these compounds were increased significantly as the carbon number was raised from 1 to 8 in the alkyl side chain, while the inhibitory activities were reduced slightly as the carbon number raised from 8 to 12 in turn. It should be noted that high selectivity ratios of these compounds on SMS2 and SMS1 were maintained at a range of 100- to 2000-fold approximately. Considering the highly improved inhibitory activities of 14b and 14l, we designed two hybrid compounds 14q and 14r. Exhilaratingly, 14q exhibited an optimal SMS2 inhibitory activity with an IC50 value of 15 nmol/L while 14r lost much activity. Considering the SMS2 inhibitory activities, selectivity and LLE properties of the above compounds, we choose 14l as a preferential one for further biological activity studies.
Table 4.
Optimization of the substituent group at benzyl group moiety.
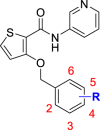 | ||||||||
| Compd. | R | SMS2 |
SMS1 |
Selectivity ratio | ClogPb | LLEc | ||
| Inhibition @ 1 μmol/L (%)a | IC50 (nmol/L)a | Inhibition @ 100 μmol/L (%)a | IC50 (μmol/L)a | |||||
| 5a | H | 66 | 400 | 20 | 219 | 547 | 3.07 | 3.33 |
| 14a | 2-Cl | 64 | 307 | 56 | – | – | 3.61 | 2.90 |
| 14b | 3-Cl | 68 | 199 | 60 | – | – | 3.68 | 3.02 |
| 14c | 4-Cl | 26 | 38792 | 52 | – | – | 3.66 | 0.75 |
| 14d | 2-Cl, 5-F | 79 | 70 | 53 | 102 | 1460 | 3.93 | 3.23 |
| 14e | 2-OCH3 | 82 | 70 | 33 | 160 | 2295 | 3.12 | 4.04 |
| 14f | 2-O(n-C2H5) | 83 | 78 | 56 | 78 | 1004 | 3.44 | 3.67 |
| 14g | 2-O(n-C3H7) | 78 | 103 | 74 | 49 | 477 | 3.81 | 3.18 |
| 14h | 2-O(n-C4H9) | 85 | 65 | 81 | 20 | 313 | 4.05 | 3.14 |
| 14i | 2-O(n-C5H11) | 86 | 74 | 88 | 10 | 142 | 4.51 | 2.62 |
| 14j | 2-O(n-C6H13) | 88 | 67 | 93 | 6 | 85 | 4.66 | 2.52 |
| 14k | 2-O(n-C7H15) | 91 | 29 | 90 | 13 | 459 | 5.17 | 2.37 |
| 14l | 2-O(n-C8H17) | 95 | 28 | 87 | 16 | 589 | 5.34 | 2.21 |
| 14m | 2-O(n-C9H19) | 91 | 35 | 86 | 13 | 360 | 5.88 | 1.58 |
| 14n | 2-O(n-C10H21) | 92 | 31 | 87 | 13 | 409 | 6.12 | 1.39 |
| 14o | 2-O(n-C11H23) | 95 | 29 | 90 | 13 | 445 | 6.56 | 0.98 |
| 14p | 2-O(n-C12H25) | 93 | 34 | 81 | 18 | 540 | 6.83 | 0.64 |
| 14q | 2-O(n-C8H17), 3-Cl | 98 | 15 | 92 | 10 | 649 | 5.99 | 1.83 |
| 14r | 2-O(n-C8H17), 5-Cl | 91 | 111 | 85 | 8 | 70 | 5.95 | 1.01 |
SMS activity is measured by purified SMS enzyme inhibitory assay in vitro. The results are expressed as the mean of duplicates. Selectivity ratio is calculated by a formula of “IC50, SMS1/IC50, SMS2”.
ClogP is predicted by SwissADME.
LLE is calculated by a formula of “pIC50, SMS2 – ClogP”. —, not applicable.
2.3. Molecular docking simulation
Given that 14l has excellent inhibitory activity and selectivity against SMS2, in order to gain insight into the possible interactions between 14l and SMS2, a molecular docking study was performed based on a putative human SMS2 (UniProt Q8NHU3) and SMS1 (UniProt Q86VZ5) protein structures predicted by Alphafold238 (Fig. 5). Apparently, many similar interactions between 5a and SMS2 was also observed between 14l and SMS2. Besides, the aliphatic chain at 2-site of benzene moiety in 14l binds to a long and open hydrophobic pocket consisting of VAL139, ILE143, TRP142, TRP146, PHE159, PHE83 and ILE163 residues in SMS2. Interestingly, possibly due to the spatial restriction of TRP146 and PHE83, the optimal 8-carbon aliphatic chain brought a better SMS2 inhibitory activity of 14l. As for the high selectivity of 14l over SMS2 and SMS1, a bulky MET288 residue in SMS1 was observed which might block the binding of 14l and SMS1. Conversely, VAL219, a smaller residue in SMS1, increased a bit of spatial tolerance of the 4- site of benzyl moiety, which could account for the same SMS1 inhibitory activities of 14a, 14b and 14c which were substituted at different sites of the benzyl moiety.
Figure 5.
Molecular docking simulation of 14l (green stick) and putative human SMS2 (UniProt Q8NHU3, blue ribbons, red surface and purple wires) and human SMS1 (UniProt Q86VZ5, blue sticks). The π–π stacking interaction is shown as dark red dotted line. H-bonds are shown as yellow dotted lines.
2.4. Inhibitory activity on cellular SM synthesis and protective effect on cell viability of 14l under inflammatory-hyperosmotic stress conditions
Considering the potent SMS2 inhibitory activity of 14l, the human corneal epithelial cell (HCEC) line, a useful tool in DED studies40,41, was used to evaluate the cellular toxicity, the cellular SM synthesis inhibitory activity and protective effect on cell viability under inflammation-hyperosmotic stress conditions of 14l. Results of cell viability test showed that 14l had less influence on the cell viability within 25 μmol/L, while a commonly approved anti-DED drug, CsA, exhibited an apparent toxicity at the same concentration in HCEC (Fig. 6A). Consistent with the enzymatic activity measurement results, 14l strongly inhibits the SM synthesis in HCEC with an EC50 value of 300 nmol/L, while a known control compound ly93 exhibited moderate EC50 value of 3988 nmol/L (Fig. 6B). Besides, CsA had no significant effect on the SMS activity of HCEC at 1 μmol/L while 14l exhibited efficient SMS inhibitory activities at the same concentration (Supporting Information Fig. S2).
Figure 6.
The effects of 14l on the viability, SM synthesis activity and TNF-α-induced injury of HCEC. (A) Effects of 14l and CsA on HCEC viability determined by MTT assay for 48 h under indicated concentrations (n = 3). (B) Determination of SM synthesis activity inhibitory EC50 in HCEC treated with different compounds for 6 h (n = 3). The effects of TNF-α on the viability on HCEC under homotonic (C) and hypertonic (D) stress condition for 24 h (n = 3). The concentration- (E) (n = 6) and time- (F) (n = 3) dependent protective manner of 14l (1 μmol/L) on TNF-α (10 ng/mL)-induced HCEC damage under hyperosmotic stress condition (550 mOsm). (G) 14l maintains HCEC integrity under TNF-α-hyperosmotic stress condition. HCEC were incubated with 14l (1/3 and 1 μmol/L) or CsA (1 μmol/L) and TNF-α (10 ng/mL) under hyperosmotic stress condition (HO, 550 mOsm) for 5 h. Data are expressed as mean ± SEM. ns: no significance, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
According to previous work1, there are mainly two pathological processes involved in DED, namely ocular surface inflammation and increased tear film osmolarity. Pro-inflammation cytokines such as tumor necrosis factor alpha (TNF-α) and sodium chloride-induced hyperosmotic conditions were used for preclinical study of DED in vitro42,43 and in vivo41. In this study, we found that TNF-α could apparently induce HCEC death under hyperosmotic conditions but not under isosmotic conditions within 20 ng/mL concentration (Fig. 6C and D). After incubation with 14l and CsA under TNF-α-hyperosmotic stress conditions for 24 h, a concentration dependent protective effect on HCEC viability with an EC50 value of 611 nmol/L and an Emax value of 30% was observed in 14l treatment group but not in CsA treatment group (Fig. 6E). Furthermore, 14l exhibited an apparent and durative protective effect in HCEC under the above stress conditions after incubation for 3 h (Fig. 6F). Intuitively, a higher integrity of HCEC population and a less early apoptosis proportion were observed in 14l group but not in CsA treatment group under the above stress conditions for 5 h (Fig. 6G and Supporting Information Fig. S3). Previous studies showed that SMS2 was an important mediator of inflammation-associated signaling transduction26,44. Consistent with previous study41, an elevated protein expression level of TNF-α was observed in HCEC under hyperosmotic stress conditions (Fig. 7A and B). Notably, both 14l and CsA inhibited TNF-α protein expression under hyperosmotic stress conditions. Besides, 14l could effectively prevent TNF-α-induced TNFR1-Caspase3 activation, which possibly accounted for the above fast and durative protection of 14l on HCEC viability under TNF-α-hyperosmotic stress conditions (Fig. 7C‒E). The above results demonstrated that 14l exhibited potent SM synthesis inhibitory activity, low toxicity and fast and effective protection effects on HCEC viability under inflammatory-hyperosmotic stress conditions, encouraging us to carry out a further study on the pharmacokinetics and pharmacodynamics properties of 14l in vivo.
Figure 7.
Anti-inflammation and anti-apoptosis effects of 14l in HCEC under hyperosmotic stress condition. (A, B) TNF-α protein expression in HCEC treated with 14l (1 μmol/L) and CsA (1 μmol/L) under hyperosmotic (HO, 550 mOsm) stress condition (n = 3). (C–E) TNFR1, caspase3 (CASP3) and cleaved-caspase3 (cleaved-CASP3) protein expression in HCEC treated with 14l (14l-L: 1/3 μmol/L; 14l-H: 1 μmol/L) and CsA (1 μmol/L) under TNF-α (10 ng/mL)-hyperosmotic (550 mOsm) stress condition (n = 4). Data are expressed as mean ± SEM. ns: no significance, ∗P < 0.05, ∗∗P < 0.01.
2.5. Ocular distribution and plasma pharmacokinetics profiles of 14l
To study the ocular distribution and plasma pharmacokinetics profiles of 14l, the male SD rats were used for PK assay with single drop administration. Considering the challenges of drug solubility, permeability and retention in ocular surface, (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD)45,46 was used to afford a high concentration and appropriate distribution of 14l. No adverse effect of 14l was observed during the PK study. 14l distributed mainly in the meibomian glands, conjunctiva and cornea (Supporting Information Fig. S4 and Table 5) where concentrations were above the EC50 value of 300 nmol/L (131 ng/mL) (Supporting Information Fig. S4). It is noteworthy that 14l was not detected in the blood and retina, which indicated a well systematic safety in vivo. Notably, 14l maintained a concentration above the EC50 value over 8 h in meibomian glands but within 4 h in the cornea, which suggested that a higher administration frequency may be needed in further pharmacodynamics studies. Thus, 14l had good tissue specificity distribution and acceptable ocular and plasma PK profiles for further pharmacodynamics evaluation.
Table 5.
Mean pharmacokinetic parameters of 14l in SD rats after single drop administration of 14l eye drops at a dose of 16 μg/eye (n = 3).
| PK parameter | Cmax (ng/g) | Tmax (h) | T1/2 (h) | Tlast (h) | AUC0‒t (h·ng/g) | AUC0‒∞ (h·ng/g) |
|---|---|---|---|---|---|---|
| Meibomian glands | 2732.66 | 1.00 | 4.32 | 24.00 | 14379.61 | 14670.67 |
| Cornea | 3052.49 | 1.00 | 1.11 | 4.00 | 4339.41 | 4567.46 |
| Conjunctiva | 358.02 | 0.25 | 1.30 | 4.00 | 489.73 | 564.99 |
| Retina | NDa | NDa | NDa | NDa | NDa | NDa |
| Blood | NDa | NDa | NDa | NDa | NDa | NDa |
ND: not detected.
2.6. Alleviation of 14l on dry eye symptoms in a murine DED model
To evaluate the effects of 14l on the DED symptoms, the fluorescein staining assay and Schirmer's test were performed in a murine DED model induced by an intelligently controlled environmental system47. Compared with the normal control group, increased corneal fluorescein staining scores were observed in the DED control group (Fig. 8), whereas CsA and 14l decreased the corneal fluorescein scores significantly. There was no significance of the corneal fluorescein scores between 14l high dose and CsA treatment group. The 14l low dose group's scores were higher than the two other treatment groups but lower than the DED control group. As for tear secretion evaluation tested by a Schirmer's test, the tear volume was improved significantly in 14l high dose and CsA treatment group but not in 14l low dose group. Therefore, 14l could significantly alleviate the DED symptoms including corneal fluorescein staining scores and tear secretion in a dose-dependent way.
Figure 8.
Effects of 14l on the corneal fluorescein staining score and tear secretion. (A) Representative images of corneal fluorescein staining and Schirmer's test. (B) Comparison of corneal fluorescein staining scores in different groups (n = 11). (C) Comparison of tear volume in different groups (n = 11). Mice are treated with 5 μL of eye drops of the vehicle (50% HP-β-CD in normal saline; g/g), CsA (a commercial cyclosporine A eye drops; Zirun®; 0.05%; g/mL), and 14l (low dose: 0.001%; high dose: 0.025%; g/g) twice a day for 7 days. Con: normal mice control group; DED: dry eye mice control group; CsA: cyclosporine A eye drops; 14l-L: low dose of 14l group; 14l-H: high dose of 14l group. Data are expressed as mean ± SD. ns: no significance, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.0001.
2.7. Reduction of 14l on mRNA expression of pro-inflammation cytokines and mmp9 in cornea and modulation SM content in meibomian glands
In mechanism, 14l and CsA could decrease the mRNA expression of pro-inflammation cytokines including Tnf-α, interleukin 1β (Il-1β) and matrix metalloproteinase 9 (Mmp-9) in mouse corneas investigated by a real-time PCR assay (Fig. 9A‒C). Among them, the expression of Tnf-α and Mmp-9 were decreased significantly in all treatment groups, while the expression of Il-1β was reduced only in 14l high dose group and CsA treatment group. To investigate the ability of 14l to modulate SM content in the meibum, an LC‒MS/MS-based lipidomic analysis assay was performed for murine meibomian gland tissue. Lipidomic analysis results showed that 14l reduced the proportion of very long chain SM species which might influence the rigid and melting temperature of meibum (Fig. 9D). Moreover, we found that high dosage of 14l did not induce an extreme increase and accumulation for ceramide (Supporting Information Fig. S5A). It is worth noting that the levels of main non-polar lipids including triglycerides (TG) and cholesterol esters (ChE) were not impacted in all treatment groups (Supporting Information Fig. S5B and C).
Figure 9.
Effects of 14l on mRNA expression levels of pro-inflammation cytokines and Mmp-9 in cornea and on SM content in meibomian glands. (A–C) The mRNA expression level of pro-inflammatory cytokines including Tnf-α, Il-1β, and Mmp-9 (n = 6) in cornea. (D) Sphingomyelin lipidomic analysis of meibomian glands (n = 6). Mice are treated with 5 μL of eye drops of the vehicle (50% HP-β-CD in normal saline; g/g), CsA (a commercial eye cyclosporine A drops; Zirun®; 0.05%; g/mL), and 14l (low dose: 0.001%; high dose: 0.025%; g/g) twice a day for 7 days. Con: normal mice control group; DED: dry eye mice control group; CsA: cyclosporine A eye drops; 14l-L: low dose of 14l group; 14l-H: high dose of 14l group. Data are expressed as mean ± SD. ns: no significance, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
3. Experimental
3.1. Synthetic materials and methods
All commercially available solvents and reagents were used without further purification. Reactions were monitored by thin-layer chromatography (TLC) using pre-coated TLC Silica gel (Yantai Jiangyou Co., Ltd., 0.4–0.5 mm thickness, Yantai, China). Column chromatography was carried out by MPLC (Teledyne ISCO, Inc., CombiFlashRF®, Lincoln NE, USA) using silica gel (Yantai Jiangyou Co., Ltd., 300–400 mesh, Yantai, China). Low-resolution mass spectra were determined on an Agilent liquid-chromatography mass spectrometer system consisted of an Agilent 1260 infinity LC coupled to an Agilent 6120 Quadrupole mass spectrometer (Agilent Technologies Inc., Agilent 6120 Quadrupole, Santa Clara, CA, USA). High-resolution mass spectra was conducted on a triple TOF 5600+ MS/MS system (AB Sciex LLC., Framingham, MA, USA) in the positive ESI mode. The purity (Supporting Information Fig. S1) of final compounds were determined by HPLC (Shimadzu Scientific Instruments, Inc., Shimadzu LC-20A, Kyoto, Japan) with a column (Nacalai Tesque, Inc., COSMOSIL 5C18-MS-II, 4.6 mm × 250 mm, 40 °C, UV 254 nm, flow rate = 1.80 mL/min, Kyoto, Japan), eluting with mixtures of water/methanol (containing 0.1% TFA, Thermo Fisher Scientific Inc., HPLC grade, Waltham, MA, USA). The ratio of mobile methanol was increased linearly from 40% to 95% over 4 min and then maintained at 95% over the next 5 min, and the methanol was decreased linearly from 95% to 40% over 3 min, followed by re-equilibration at 40% methanol for 3 min. All the assayed compounds possess ≥95% purity. 1H NMR and 13C NMR spectra were recorded on a Bruker AC400 or a Bruker AC600 NMR spectrometer (Bruker Daltonics, Inc., Billerica, MA, USA) using tetramethylsilane as an internal reference. The melting point data was determined using a GM70 automatic melting point instrument (Shanghai Zhuoguang Instrument Technology Co., Ltd., GM70, Shanghai, China).
3-((2-(Octyloxy)benzyl)oxy)-N-(pyridin-3-yl)thiophene-2-carboxamide (14l)
14l was synthesized with 8 and 1-(bromomethyl)-2-(octyloxy)benzene through general procedure A (see Supporting Information) as a yellow solid (PE:EA = 65:35). Yield: 67%. 1H NMR (600 MHz, CDCl3) δ 9.37 (s, 1H), 8.31–8.21 (m, 2H), 8.19–8.14 (m, 1H), 7.50 (d, J = 5.5 Hz, 1H), 7.47–7.39 (m, 2H), 7.25–7.19 (m, 1H), 7.07–6.96 (m, 3H), 5.31 (s, 2H), 3.96 (t, J = 6.5 Hz, 2H), 1.69–1.63 (m, 2H), 1.34–1.16 (m, 10H), 0.86 (t, J = 7.3 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 160.20, 157.21, 156.22, 144.43, 140.54, 135.20, 130.90, 130.14, 130.07, 126.40, 123.53, 123.30, 120.57, 117.47, 116.65, 111.74, 70.50, 68.27, 31.61, 29.11, 29.02, 28.97, 25.84, 22.48, 13.96. HRMS (ESI): m/z [M+H]+ calcd for C25H30N2O3S: 439.2050, found: 439.2053; mp: 52.86–54.50 °C; HPLC purity 99.4%.
The synthesis and characterization data of other compounds are provided in the Supporting Information.
3.2. Enzymatic SM synthesis inhibitory assay
The inhibitory activity against human SMS2 and SMS1 was tested using a reported protocol34. Specifically, 5 nmol/L of SMS2 and 30 nmol/L of SMS1 were used to evaluate the inhibitory activity of compounds respectively. The enzyme was allowed to incubate with different compounds at room temperature for 5 min before adding the substrate mixture of DMPC (Sigma–Aldrich, Inc., CAS: 18194-24-6, Milwaukee, WI, USA) and C6-NBD-Ceramide (Santa Cruz Biotechnology, Inc., CAS: 94885-02-6, Helena, MT, USA). The mixture was incubated at 37 °C for 30 min, and the reaction was quenched by adding EtOH. Finally, the C6-NBD-SM signaling was detected by HPLC (Shimadzu). The data was analyzed using Graphpad Prism 8 software (GraphPad Software, LLC., version 8.3.0, BOSTON, MA, USA). LLE values are calculated by a formula of “pIC50, SMS2 – ClogP” described as before48.
3.3. Torsion energy scanning
All calculations of torsion energy scanning were performed are performed at the B3LYP/6-31+G(d,p) level of theory with Guassian 09 software (Gaussian, Inc., Gaussian 09, Revision A.02, Wallingford, CT, USA). The conformations containing intra-molecular hydrogen bonds were chosen as the initial conformations. The intervals of dihedral angle were set to 30°.
3.4. Molecular docking simulation
The three-dimensional structures of human SMS1 and SMS2 proteins predicted by Alphafold238 were used as receptor structures. A key catalytic residue HIS229 of SMS2 was chosen as the core residue of the docking pocket with “Induced-fit docking” tools of Schrödinger software (Schrödinger, LLC., Maestro Version 11.2.014 Release 2017-2, New York, NY, USA). All docking results were visualized and analyzed using UCSF Chimera software (the Resource for Biocomputing, Visualization, and Informatics at the University of California, UCSF Chimera, alpha version 1.15, San Francisco, CA, USA).
3.5. Caco-2 monolayers permeability assay
The permeability assay was performed with Caco-2 cells monolayers (see Supporting Information, Hefei PreceDo Medical Laboratory Co., Ltd., Hefei, China). The trans-epithelial electrical resistance (TEER) values were tested to evaluate the influence of different compounds on the Caco-2 monolayer integrity. No significant changes of TEER value were observed in this study (Supporting Information Table S2).
3.6. Cell viability and toxicity assay
To evaluate the viability and cytotoxicity of 14l and CsA (Bide Pharmatech Ltd., CAS: 59865-13-3, Shanghai, China) in corneal epithelial cells, HCEC (Shandong Eye Hospital, a kind gift of Professor Weiyun Shi, Ji'nan, China) were seeded at 104 cells per well in 96-well plates and allowed to grow for 24 h. Then cells were treated with fresh DMEM-F12 (Shanghai Titan Scientific Co., Ltd., C8015-500 mL, Shanghai, China) containing different concentrations of compounds. After incubation for an indicated period of time, the cells were washed with PBS and incubated with fresh culture medium containing 0.5 mg/mL MTT (Bide Pharmatech Ltd., CAS: 298-93-1, Shanghai, China) for another 4 h. After removing the culture medium slowly, the formazan crystal was resolved using DMSO. The cell viability and cytotoxicity were determined by reading the absorbance of formazan DMSO solution at 490 nm by BioTek Cytation5 microplate reader (BioTek Instruments, Inc., Cytation 5, VT, USA).
3.7. Cellular SM synthesis inhibitory assay
To evaluate the SM synthesis inhibitory activity of 14l and ly93 in cells, HCEC were seeded at 104 cells per well in 96-well plates and allowed to grow for 24 h. Then cells were pretreated with fresh DMEM-F12 (Shanghai Titan Scientific Co., Ltd.) containing different concentration compounds for 1 h and incubated with 2 μmol/L C6-NBD-ceramide (Santa Cruz Biotechnology, Inc.) for another 5 h. Then methanol was added to the above mixture to quench the reaction. The cells were grinded with pipette tips and collected into eppendorf tubes for ultrasonication for 5 min and centrifugation at 12,000 rpm (Beckman Coulter, Inc., Microfuge 20/20R, CA, USA) for 20 min. The supernatant was analyzed by HPLC (Shimadzu Scientific Instruments, Inc.). Data was analyzed using GraphPad Prism 8 software (GraphPad Software LLC.).
3.8. HCEC injury and rescue assay
The HCEC were seeded at 104 cells per well in 96-well plates and allowed to grow for 24 h. Then cells were pretreated with fresh DMEM-F12 (Shanghai Titan Scientific Co., Ltd.) containing different concentration recombinant human soluble TNF-α (Beyotime Biotechnology, Inc., P5320-10 μg, Shanghai, China) and/or 120 mmol/L NaCl (for 550 mOsm, Shanghai Titan Scientific Co., Ltd., CAS: 7647-14-5, Shanghai, China) and DMSO or compounds for indicated time. After incubation, the cell viability was determined using MTT assay described above. Data was analyzed using GraphPad Prism 8 software (GraphPad Software LLC.).
3.9. Cell morphology and flow cytometric analysis
HCEC were seeded at 3 × 105 cells per well in 6-well plates and allowed to grow for 24 h. After incubation with fresh DMEM-F12 (Shanghai Titan Scientific Co., Ltd.) containing 10 ng/mL recombinant human soluble TNF-α (Beyotime Biotechnology, Inc.) and/or 120 mmol/L NaCl (for 550 mOsm, Shanghai Titan Scientific Co., Ltd.) and DMSO or compounds for 5 h. After incubation and washing with PBS (Dalian Meilun Biotech Co., Ltd., MA0015-1, Dalian, China), the cell morphology was analyzed with BioTek Cytation5 imager (BioTek Instruments, Inc.). The apoptosis proportion was tested using Annexin V-FITC/PI Cell Apoptosis Detection Kit (Dalian Meilun Biotech Co., Ltd., MA0220-2, Dalian, China) with flow cytometer (Beckman Coulter, Inc., CytoFlex S, CA, USA) and analyzed using CytExpert software (Beckman Coulter, Inc., version 2.4.0.28, CA, USA).
3.10. Western blot assay
To determine the influence of 14l and CsA (Bide Pharmatech Ltd.) on the protein expression of TNF-α,HCEC were seeded at 3 × 105 cells per well in 6-well plates and allowed to grow for 24 h. After incubating with fresh DMEM-F12 (Shanghai Titan Scientific Co., Ltd.) containing 10 ng/mL recombinant human soluble TNF-α (Beyotime Biotechnology Inc.) and/or 120 mmol/L NaCl (for 550 mOsm, Shanghai Titan Scientific Co., Ltd.) and DMSO or compounds for 6 h. Cells were washed with PBS and lysed using RIPA lysis buffer (Shanghai Epizyme Biomedical Technology Co., Ltd., PC101, Shanghai, China). The total protein concentration was determined using Bicinchoninic acid (BCA) kit (Dalian Meilun Biotech Co., Ltd., MA0082, Dalian, China). After adjustment to the same protein concentration and addition with loading buffer (Dalian Meilun Biotech Co., Ltd., MA0003, Dalian, China), the samples were boiled for 10 min and then centrifuged at 12000 rpm (Beckman Coulter, Inc.) for 10 min. Next, the samples were separated with SDS-PAGE gel and transferred to PVDF membrane. After blockage and wash of membranes, the membranes were allowed to incubate with the primary antibody at 4 °C overnight and then with the secondary antibody at room temperature for 2 h. Finally, the membranes were imaged using ECL luminescence reagent (Dalian Meilun Biotech Co., Ltd., MA0186-2, Dalian, China) with Bio-rad ChemiDoc Imaging System (Bio-Rad Laboratories Co., Ltd., ChemiDoc™ Touch, Hercules, CA, USA). The antibodies used include anti-TNF-α (ABclonal Technology Co., Ltd., A11534, Wuhan, China), anti-TNFR1 (ABclonal Technology Co., Ltd., A1540, Wuhan, China), anti-Caspase3 and cleaved-Caspase3 (Beyotime Biotechnology, Inc., AC030, Shanghai, China), HRP anti-β-actin (ABclonal Technology Co., Ltd., AC028, Wuhan, China) and HRP-goat-anti-rabbit IgG (ABclonal Technology Co., Ltd., AS014, Wuhan, China). Data was analyzed using ImageJ software (National Institutes of Health, ImageJ 1.53e, Bethesda, MD, USA) and GraphPad Prism 8 software (GraphPad Software LLC.).
3.11. Animal
Male C57BL/6 mice (6–8 weeks of age) were purchased from Shanghai JieSiJie Laboratory Animal Co., Ltd. Male SD rats (200 g body weight per mouse) were purchased from Shanghai BiKaiKeYi Biotechnology Co., Ltd. All animal experiments were approved by the Animal Care and Use Committee, Fudan University (approval NO. 2023-06-YH-ZL-72).
3.12. Pharmacokinetics and ocular tissue distribution study
To evaluate the pharmacokinetics and ocular tissue distribution properties of 14l, 14l was monitored in plasma and ocular tissues within 24 h after single topical instillation administration at 30 μL/eye (16 μg/eye) in male SD rats. 14l was dissolved in 50% HP-β-CD in normal saline to give 14l eye drops. The plasma, cornea, conjunctiva, retina and meibomian glands samples were collected at 0.25, 0.5, 1, 2, 4, 8 and 24 h post-dosage. The 14l of plasma supernatant and tissue homogenate samples were extracted with 80% (v/v) methanol in H2O and determined by a liquid chromatography−tandem mass spectrometry (LC‒MS/MS) system (AB Sciex LLC., AB 4000 Q-TRAP, Framingham, MA, USA). The concentration of 14l was subjected to a non-compartmental pharmacokinetic analysis by using the Phoenix WinNonlin software (Pharsight Corporation, Phoenix WinNonlin version 6.4, New York, NY, USA).
3.13. Dry eye disease model and administration of eye drops
DED model was established by housing the C57BL/6 mice in a controlled environment chamber (CEC, Alisn Instrument (Shanghai) Co., Ltd., THA 250, Shanghai, China) which maintained the humidity at 22.5 ± 4.5% and the temperature at 25 ± 1 °C. The CEC was set to simulate a regular light/dark (12 h/12 h) cycle. Mice were placed in the CEC for 14 days for induction of DED. After modeling, mice were returned to normal environment and randomly divided into five groups and received different treatments respectively. Drugs were applied topically in the form of eye drops. Each eye of the mice received 5 μL of eye drops twice per day for 7 days.
3.14. Tear volume measurement (Schirmer's test)
All mice were anesthetized using 1% pentobarbital sodium (provided by EYE & ENT Hospital of Fudan University) and a phenol red thread (Jingming New Technological Development Co., Ltd., Tianjin, China) was placed in the temporal bulbar conjunctiva. The thread was removed from the conjunctiva after 30 s and the wetted length was measured and recorded. Data was analyzed using GraphPad Prism 8 software (GraphPad Software LLC.).
3.15. Corneal fluorescein staining
All mice were anesthetized as previously described. Under anesthesia, 1 μL of 2% fluorescein sodium (MedChemExpress LLC., HY-D0208, Monmouth Junction, NJ, USA) was applied to the cornea. After 90 s, the fluorescein sodium was carefully removed without disturbing the ocular surface. The corneal epithelium was then evaluated under cobalt blue illumination referring to the standard National Eye Institute grading system. Briefly, the cornea was divided into five regions each graded from 0 to 3 according to the severity of ocular surface staining. The scores of the five regions were added up to represent the overall cornea damage. Data was analyzed using GraphPad Prism 8 software (GraphPad Software LLC.).
3.16. Quantitative PCR analysis
Total RNA was extracted from the harvested corneas using universal RNA purification kit (EZBioscience, EZB-RN4, Roseville, CA, USA) following the attached protocol. The concentration of the extracted RNA was measured using Nanodrop (Thermo Fisher, Nanodrop One, Waltham, MA, USA), and cDNA was prepared using color reverse transcription kit (EZBioscience, A0010CGQ, Roseville, CA, USA) according to the manufacturer's protocol. Thereafter, quantitative real-time PCR was performed using color SYBR green qPCR master mix (EZBioscience, A0012-R1, Roseville, CA, USA) and 7300Plus Real-Time PCR Instrument (Applied Biosystems, 7300Plus, Waltham, MA, USA). Target gene expression was normalized to the expression of the housekeeping gene β-actin.
The primer sequences used are listed as follows: β-actin (forward 5′ TTCGTTGCCGGTCCACACCC 3′, reverse 5′ GCTTTGCACATGCCGGAGCC 3′); Il-1β (forward 5′ GTACAAGGAGAACCAAGCAAC 3′, reverse 5′ CCGTCTTTCATTACACAGGA 3′); Tnf-α (forward 5′ GCCTCCCTCTCATCAGTTCT 3′, reverse 5′ ACTTGGTGGTTTGCTACGAC 3′); Mmp9 (forward 5′ GCAGAGGCATACTTGTACCG 3′, reverse 5′ TGATGTTATGATGGTCCCACTTG 3′).
3.17. Lipidomic analysis
To determine the SM content in meibomian glands, the lipid samples were extracted from the meibomian gland tissues (from one eye per mouse) by adding 100 μL PBS solution and then grinding to a homogeneous mixture at −20 °C. The samples were vortexed in a clean 15 mL glass centrifuge tube together with 1.5 mL of methanol for 1 min followed by 5 mL of MTBE for 1 min. Once homogenized, samples were rocked on a shaker at room temperature for 1 h. Then, samples were vortexed after adding 1.25 mL of water for 1 min. Once finished, samples were placed at −20 °C for 1 h and then centrifuged at 1000×g (Beckman Coulter, Inc.) for 10 min at 4 °C. Two-phase layers could be observed within the glass centrifuge tube. A total of 4 mL of the supernatant was collected and dried under a stream of nitrogen. The extracted lipid samples were stored at −80 °C before LC-MS/MS analysis. Lipids were eluted via C30 by using a 3 μm, 2.1 mm × 150 mm column (Waters Corporatio, Cambridge, MA, USA) with a flow rate of 260 μL/min using buffer A (10 mmol/L ammonium formate at a 60:40 ratio with acetonitrile:water) and buffer B (10 mmol/L ammonium formate at a 90:10 ratio with isopropanol: acetonitrile). Gradients were held in 32% buffer B for 1.5 min and run from 32% buffer B to 45% buffer B at 1.5–4 min; from 45% buffer B to 52% buffer B at 4–5 min; from 52% buffer B to 58% buffer B at 5–8 min; from 58% buffer B to 66% buffer B at 8–11 min; from 66% buffer B to 70% buffer B at 11–14 min; from 70% buffer B to 75% buffer B at 14–18 min; from 75% buffer B to 97% buffer B at 18–21 min; 97% buffer B was held from 21 to 25 min; from 97% buffer B to 32% buffer B at 25–25.01 min; and 32% buffer B was held for 8 min. All the ions were acquired by non-targeted MRM transitions associated with their predicted retention time in a positive and negative mode switching fashion. ESI voltage was +5500 and −4500 V in positive or negative mode, respectively.
4. Conclusions
In summary, we designed and synthesized a series of thiophene carboxamide derivatives as potent and selective SMS2 inhibitors. Among them, 14l exhibited a highly potent SMS2 enzymatic inhibitory activity with an IC50 value of 28 nmol/L and a selectivity ratio of 589-fold compared to SMS1. In a TNF-α-hyperosmotic stress condition-induced HCEC apoptosis model, 14l exhibited a fast and durative protection effect on the HCEC viability. Furthermore, 14l exhibited acceptable ocular PK profiles, low toxicity and alleviation of dry eye symptoms in a murine DED model. Our data demonstrated that inhibition of SMS2 activity could be a novel strategy for DED therapy due to its well anti-inflammatory and SM modulation effects.
Author contributions
Lu Zhou, Jiaxu Hong and Yu Cao conceived and directed this project. Jintong Yang designed and synthesized compounds, performed the computer-assisted drug design (CADD) experiments, contributed to the enzyme, cell and animal experiments and wrote the manuscript. Yiteng Lu contributed to the cell and animal experiments and PCR assay and revision of the manuscript. Kexin Hu contributed to the expression and purification of SMS proteins. Xinchen Zhang contributed to the PK evaluation experiment. Wei Wang and Xin Xiao contributed to the synthesis of compounds. Xichen Wan and Yuqing Wu contributed to the animal experiments. Shuxian Zhang and He Huang contributed to the lipidomics analysis. Mingguang Mo, Deyong Ye, Zhibei Qu and Yimin Hu reviewed and revised the paper. All authors provided critical feedback and have approved the final version of the manuscript.
Conflicts of interest
Lu Zhou, Jiaxu Hong, Jintong Yang, Wei Wang, Xinchen Zhang and Deyong Ye are named inventors of the pending patent application (CN 202310875923.0) related to the work described.
Acknowledgments
This study was supported by the National Key R&D Program of China (2023YFC3603303 and 2023YFA0915000), the National Natural Science Foundation of China (22077019 and 82171102), Shanghai Municipal Committee of Science and Technology (21TQ016 and 21XD1420600, China), the Shanghai Medical Innovation Research Program (22Y21900900, China), the Shanghai Key Clinical Research Program (SHDC2020CR3052B, China) and the Class IV Peak Disciplines (Shanghai Institute of Precision Medicine) from the Shanghai Municipal Education Commission. The authors would like to thank Professor Weiyun Shi (Shandong Eye Hospital, Ji'nan, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2024.10.005.
Contributor Information
Yu Cao, Email: yu.cao@shsmu.edu.cn.
Jiaxu Hong, Email: Jiaxu.hong@fdeent.org.
Lu Zhou, Email: zhoulu@fudan.edu.cn.
Appendix A. Supporting information
The following is the Supporting Information to this article:
References
- 1.Lemp M.A., Baudouin C., Baum J., Dogru M., Foulks G.N., Kinoshita S., et al. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Mondal H., Kim H.J., Mohanto N., Jee J.P. A review on dry eye disease treatment: recent progress, diagnostics, and future perspectives. Pharmaceutics. 2023;15 doi: 10.3390/pharmaceutics15030990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuda S., Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 4.Donnenfeld E.D., Perry H.D., Nattis A.S., Rosenberg E.D. Lifitegrast for the treatment of dry eye disease in adults. Expert Opin Pharmacother. 2017;18:1517–1524. doi: 10.1080/14656566.2017.1372748. [DOI] [PubMed] [Google Scholar]
- 5.Endo K.I., Sakamoto A., Fujisawa K. Diquafosol tetrasodium elicits total cholesterol release from rabbit meibomian gland cells via P2Y(2) purinergic receptor signalling. Sci Rep. 2021;11:6989. doi: 10.1038/s41598-021-86433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura M., Imanaka T., Sakamoto A. Diquafosol ophthalmic solution for dry eye treatment. Adv Ther. 2012;29:579–589. doi: 10.1007/s12325-012-0033-9. [DOI] [PubMed] [Google Scholar]
- 7.Choi S.W., Kim J. Therapeutic contact lenses with polymeric vehicles for ocular drug delivery: a review. Materials. 2018;11 doi: 10.3390/ma11071125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramadan A.A., Eladawy S.A., Abu El-Enin A.S.M., Hussein Z.M. Development and investigation of timolol maleate niosomal formulations for the treatment of glaucoma. J Pharm Invest. 2020;50:59–70. [Google Scholar]
- 9.Rao S.K., Mohan R., Gokhale N., Matalia H., Mehta P. Inflammation and dry eye disease-where are we? Int J Ophthalmol. 2022;15:820–827. doi: 10.18240/ijo.2022.05.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magny R., Kessal K., Regazzetti A., Ben Yedder A., Baudouin C., Melik Parsadaniantz S., et al. Lipidomic analysis of epithelial corneal cells following hyperosmolarity and benzalkonium chloride exposure: new insights in dry eye disease. Biochim Biophys Acta Mol Cel Biol Lipids. 2020;1865 doi: 10.1016/j.bbalip.2020.158728. [DOI] [PubMed] [Google Scholar]
- 11.Ham B.M., Cole R.B., Jacob J.T. Identification and comparison of the polar phospholipids in normal and dry eye rabbit tears by MALDI-TOF mass spectrometry. Invest Ophthalmol Vis Sci. 2006;47:3330–3338. doi: 10.1167/iovs.05-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X., Zhang C., Tian L., Wu L., Jie Y., Wang N., et al. In situ metabolic profile and spatial distribution of ocular tissues: new insights into dry eye disease. Ocul Surf. 2022;24:51–63. doi: 10.1016/j.jtos.2021.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Baudouin C., Messmer E.M., Aragona P., Geerling G., Akova Y.A., Benitez-del-Castillo J., et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100:300–306. doi: 10.1136/bjophthalmol-2015-307415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galor A., Sanchez V., Jensen A., Burton M., Maus K., Stephenson D., et al. Meibum sphingolipid composition is altered in individuals with meibomian gland dysfunction-a side by side comparison of meibum and tear sphingolipids. Ocul Surf. 2022;23:87–95. doi: 10.1016/j.jtos.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paranjpe V., Tan J., Nguyen J., Lee J., Allegood J., Galor A., et al. Clinical signs of meibomian gland dysfunction (MGD) are associated with changes in meibum sphingolipid composition. Ocul Surf. 2019;17:318–326. doi: 10.1016/j.jtos.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Angelo G., Moorthi S., Luberto C. Role and function of sphingomyelin biosynthesis in the development of cancer. Adv Cancer Res. 2018;140:61–96. doi: 10.1016/bs.acr.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Gewaid H., Aoyagi H., Arita M., Watashi K., Suzuki R., Sakai S., et al. Sphingomyelin is essential for the structure and function of the double-membrane vesicles in hepatitis C virus RNA replication factories. J Virol. 2020;94 doi: 10.1128/JVI.01080-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulders A.C., Peters S.L., Michel M.C. Sphingomyelin metabolism and endothelial cell function. Eur Heart J. 2007;28:777–779. doi: 10.1093/eurheartj/ehm025. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Beamonte R., Lou-Bonafonte J.M., Martinez-Gracia M.V., Osada J. Sphingomyelin in high-density lipoproteins: structural role and biological function. Int J Mol Sci. 2013;14:7716–7741. doi: 10.3390/ijms14047716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honma N., Hatta I., Okazaki T., Tokudome Y. Modulation of function and structure of stratum corneum in sphingomyelin synthase 2-deficient mice. Chem Phys Lipids. 2022;249 doi: 10.1016/j.chemphyslip.2022.105255. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Cao Y. The sphingomyelin synthase family: proteins, diseases, and inhibitors. Biol Chem. 2017;398:1319–1325. doi: 10.1515/hsz-2017-0148. [DOI] [PubMed] [Google Scholar]
- 22.Liu J., Huan C., Chakraborty M., Zhang H., Lu D., Kuo M.S., et al. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ Res. 2009;105:295–303. doi: 10.1161/CIRCRESAHA.109.194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y.J., Greimel P., Hirabayashi Y. GPRC5B-mediated sphingomyelin synthase 2 phosphorylation plays a critical role in insulin resistance. iScience. 2018;8:250–266. doi: 10.1016/j.isci.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng Y., Hu J.C., He S.H., Lou B., Ding T.B., Yang J.T., et al. Sphingomyelin synthase 2 facilitates M2-like macrophage polarization and tumor progression in a mouse model of triple-negative breast cancer. Acta Pharmacol Sin. 2021;42:149–159. doi: 10.1038/s41401-020-0419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng K., Chen Z., Feng H., Chen Y., Zhang C., Yu J., et al. Sphingomyelin synthase 2 promotes an aggressive breast cancer phenotype by disrupting the homoeostasis of ceramide and sphingomyelin. Cell Death Dis. 2019;10:157. doi: 10.1038/s41419-019-1303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prymas K., Swiatkowska A., Traczyk G., Ziemlinska E., Dziewulska A., Ciesielska A., et al. Sphingomyelin synthase activity affects TRIF-dependent signaling of toll-like receptor 4 in cells stimulated with lipopolysaccharide. Biochim Biophys Acta Mol Cel Biol Lipids. 2020;1865 doi: 10.1016/j.bbalip.2019.158549. [DOI] [PubMed] [Google Scholar]
- 27.Gowda S., Yeang C., Wadgaonkar S., Anjum F., Grinkina N., Cutaia M., et al. Sphingomyelin synthase 2 (SMS2) deficiency attenuates LPS-induced lung injury. Am J Physiol Lung Cel Mol Physiol. 2011;300:L430–L440. doi: 10.1152/ajplung.00208.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y., Hu F., Yang G., Meng Q. Lack of sphingomyelin synthase 2 reduces cerebral ischemia/reperfusion injury by inhibiting microglial inflammation in mice. Exp Ther Med. 2020;20:241. doi: 10.3892/etm.2020.9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z., Chiang Y.P., He M., Worgall T.S., Zhou H., Jiang X.C. Liver sphingomyelin synthase 1 deficiency causes steatosis, steatohepatitis, fibrosis, and tumorigenesis: an effect of glucosylceramide accumulation. iScience. 2021;24 doi: 10.1016/j.isci.2021.103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu M.H., Takemoto M., Watanabe K., Luo H., Nishimura M., Yano M., et al. Deficiency of sphingomyelin synthase-1 but not sphingomyelin synthase-2 causes hearing impairments in mice. J Physiol. 2012;590:4029–4044. doi: 10.1113/jphysiol.2012.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yano M., Yamamoto T., Nishimura N., Gotoh T., Watanabe K., Ikeda K., et al. Increased oxidative stress impairs adipose tissue function in sphingomyelin synthase 1 null mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano M., Watanabe K., Yamamoto T., Ikeda K., Senokuchi T., Lu M., et al. Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J Biol Chem. 2011;286:3992–4002. doi: 10.1074/jbc.M110.179176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yukawa T., Nakahata T., Okamoto R., Ishichi Y., Miyamoto Y., Nishimura S., et al. Discovery of 1,8-naphthyridin-2-one derivative as a potent and selective sphingomyelin synthase 2 inhibitor. Bioorg Med Chem. 2020;28 doi: 10.1016/j.bmc.2020.115376. [DOI] [PubMed] [Google Scholar]
- 34.Mo M., Yang J., Jiang X.C., Cao Y., Fei J., Chen Y., et al. Discovery of 4-benzyloxybenzo[d]isoxazole-3-amine derivatives as highly selective and orally efficacious human sphingomyelin synthase 2 inhibitors that reduce chronic inflammation in db/db mice. J Med Chem. 2018;61:8241–8254. doi: 10.1021/acs.jmedchem.8b00727. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Huang T., Lou B., Ye D., Qi X., Li X., et al. Discovery, synthesis and anti-atherosclerotic activities of a novel selective sphingomyelin synthase 2 inhibitor. Eur J Med Chem. 2019;163:864–882. doi: 10.1016/j.ejmech.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 36.Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7 doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beno B.R., Yeung K.S., Bartberger M.D., Pennington L.D., Meanwell N.A. A survey of the role of noncovalent sulfur interactions in drug design. J Med Chem. 2015;58:4383–4438. doi: 10.1021/jm501853m. [DOI] [PubMed] [Google Scholar]
- 38.Alphafold home page. Available from: https://alphafold.ebi.ac.uk/.
- 39.Yeang C., Varshney S., Wang R., Zhang Y., Ye D., Jiang X.C. The domain responsible for sphingomyelin synthase (SMS) activity. Biochim Biophys Acta. 2008;1781:610–617. doi: 10.1016/j.bbalip.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren Y., Lu H., Reinach P.S., Zheng Q., Li J., Tan Q., et al. Hyperosmolarity-induced AQP5 upregulation promotes inflammation and cell death via JNK1/2 activation in human corneal epithelial cells. Sci Rep. 2017;7:4727. doi: 10.1038/s41598-017-05145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., Li J., Hou C., Li J., Peng H., Wang Q. The effect of astaxanthin on inflammation in hyperosmolarity of experimental dry eye model in vitro and in vivo. Exp Eye Res. 2020;197 doi: 10.1016/j.exer.2020.108113. [DOI] [PubMed] [Google Scholar]
- 42.Nagaarudkumaran N., Mirzapour P., McCanna D., Ngo W. Temporal change in pro-inflammatory cytokine expression from immortalized human corneal epithelial cells exposed to hyperosmotic stress. Curr Eye Res. 2022;47:1488–1495. doi: 10.1080/02713683.2022.2125531. [DOI] [PubMed] [Google Scholar]
- 43.Kimura K., Teranishi S., Fukuda K., Kawamoto K., Nishida T. Delayed disruption of barrier function in cultured human corneal epithelial cells induced by tumor necrosis factor-alpha in a manner dependent on NF-kappaB. Invest Ophthalmol Vis Sci. 2008;49:565–571. doi: 10.1167/iovs.07-0419. [DOI] [PubMed] [Google Scholar]
- 44.Hailemariam T.K., Huan C., Liu J., Li Z., Roman C., Kalbfeisch M., et al. Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation. Arterioscler Thromb Vasc Biol. 2008;28:1519–1526. doi: 10.1161/ATVBAHA.108.168682. [DOI] [PubMed] [Google Scholar]
- 45.Morrison P.W., Connon C.J., Khutoryanskiy V.V. Cyclodextrin-mediated enhancement of riboflavin solubility and corneal permeability. Mol Pharm. 2013;10:756–762. doi: 10.1021/mp3005963. [DOI] [PubMed] [Google Scholar]
- 46.Javitt J.C.J.N., Mcdonnell P., Inventor; Zelano AJ, assignee . 1994 Jan 5. Topical compositions for the eye comprising a beta-cyclodextrin derivative and a carbonic anhydrase inhibitor. [Google Scholar]
- 47.Yu M., Lee S.M., Lee H., Amouzegar A., Nakao T., Chen Y., et al. Neurokinin-1 receptor antagonism ameliorates dry eye disease by inhibiting antigen-presenting cell maturation and T helper 17 cell activation. Am J Pathol. 2020;190:125–133. doi: 10.1016/j.ajpath.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leeson P.D., Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov. 2007;6:881–890. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.













