Abstract
Disruption of the intestinal mucosal barrier caused by gut dysbiosis and metabolic imbalance is the underlying pathology of inflammatory bowel disease (IBD). Traditional Chinese medicine Wuji Wan (WJW) is commonly used to treat digestive system disorders and showed therapeutic potential for IBD. In this interdisciplinary study, we aim to investigate the pharmacological effects of WJW against experimental colitis by combining functional metabolomics and gut-microbiota sequencing techniques. Treatment with WJW altered the profile of the intestinal microbiota and notably increased the abundance of Lactobacillus, thereby facilitating the conversion of tryptophan into indole-3-acetic acid (IAA) and indoleacrylic acid (IA). These indole derivatives activated the aryl hydrocarbon receptor (AhR) pathway, which reduced colonic inflammation and restored the expression of intestinal barrier proteins. Interestingly, the beneficial effects of WJW on gut barrier function improvement and tryptophan metabolism were disappeared in the absence of gut microbiota. Finally, pre-treatment with the AhR antagonist CH-223191 confirmed the essential role of IAA-mediated AhR activation in the therapeutic effects of WJW. Overall, WJW enhanced intestinal barrier function and reduced colonic inflammation in a murine colitis model by modulating Lactobacillus–IAA–AhR signaling pathway. This study provides novel insights into colitis pathogenesis and presents an effective therapeutic and preventive approach against IBD.
Key words: Wuji Wan, Colitis, Gut microbiota, Tryptophan metabolite, Intestinal barrier, Aryl hydrocarbon receptor, Indole-3-acetic acid, Lactobacillus
Graphical abstract
The findings in this study mechanistically confirmed that Wuji Wan confers priority pharmacological efficacy against the ulcer colitis in mice by the regulation of Lactobacillus–indoles–AhR axis.

1. Introduction
The human gut harbors almost 1014 microorganisms that have co-evolved with the host for thousands of years, resulting in a complicated and mutually beneficial relationship1. The involvement role of gut microbiota in human disease has been extensively investigated in the past decade. The intestinal epithelium serves as a protective barrier, both physically and chemically, safeguarding the intestinal epithelial mucosa and nearby organs against detrimental microorganisms. The gut dysbiosis, marked by changes in the diversity, structure, and/or activities of the resident microbiota, can destroy the integrity of the intestinal epithelial barrier, resulting in heightened bacterial translocation and immune response activation2,3. This can affect the onset, advancement, and worsening of gastrointestinal disorders, such as inflammatory bowel disease (IBD)1. IBD, which includes ulcerative colitis and Crohn's disease, is characterized by persistent and recurring inflammation of the gastrointestinal tract4,5. This disease represents a significant global health concern, and its annual incidence has shown a substantial increase in parallel with shifts in diet and lifestyle patterns6. While the precise causes of IBD are multifaceted and involve a mixture of genetic and environmental factors, emerging research suggests that an impaired intestinal barrier, triggered by gut dysbiosis, precedes the development of IBD7.
The production of small-molecule metabolites by the gut microbiota is merging as a crucial factor in maintaining the integrity of the intestinal barrier2. The three primary categories of gut microbial metabolites that are currently receiving extensive research attention and have been implicated in the pathogenesis of IBD include: (1) short-chain fatty acids (SCFAs), synthesized de novo by microbes, (2) bile acids (BAs), initially produced in the liver and modified by the gut microbiota within the gut lumen before exerting their effects on the host, and (3) tryptophan (Trp) metabolites, produced from the dietary breakdown by gut microbiota8. Most of these metabolites exert their effects as signaling molecules and substrates for metabolic reactions to initiate an immune response within the gut mucosa and regulate inflammatory processes4,9. For instance, SCFAs enhance intestinal barrier function through diverse mechanisms, including the inhibition of pathogen development, reduction of intestinal inflammation, and modulation of tight junctions (TJs) structure4,10,11. Moreover, secondary BAs can stimulate the regeneration of gut epithelium and prevent the dysfunction of intestinal barrier and translocation of bacteria by activating various cell surface and nuclear receptors, including pregnane X receptor, farnesoid X receptor and G protein bile acid receptor4,12,13. Furthermore, gut microbiota can metabolize Trp into indole metabolites, which regulate the intestinal barrier function through the xenobiotic sensing pregnane X receptor and/or aryl hydrocarbon receptor (AhR). The activation of Trp–AhR pathway plays a vital role in maintaining the normal function of intestinal barrier and significantly mitigates its dysfunction induced by proinflammatory cytokines9. Therefore, targeting microbiota-modulated metabolites to restore the function of intestinal barrier might be an effective strategy for IBD therapy5,14.
Traditional Chinese medicine (TCM) has the advantages of accurate efficacy, low recurrence rate, and few side effects in treating IBD. Numerous studies have demonstrated that TCM can target and regulate the composition of gut microbiota and mitigate gut barrier dysfunction through multi-component mediation, exerting therapeutic effects on IBD14, 15, 16. Wuji Wan (WJW), a well-established TCM formula recorded in Chinese Pharmacopeia, has attracted researchers’ attention due to its beneficial effects against various gastrointestinal diseases. WJW is composed of Radix Paeoniae Alba, Fructus Evodiae Rutaecarpae, and Rhizoma Coptidis in the ratio of 6:1:617,18. Recent studies have indicated that WJW can alleviate diarrhea, improve colonic motility, and restore the intestinal microbiota and gut epithelial barrier in individuals with post-inflammatory irritable bowel syndrome17,19. Moreover, the major bioactive compounds of WJW, such as berberine, evodiamine and paeoniflorin, have been shown to rectify gut dysbiosis, restore microbial metabolites, and improve the gut barrier function in dextran sulfate sodium (DSS)-induced colitis animal models14,20,21. At the same time, these compounds have been demonstrated to exhibit lower oral bioavailability and prolonged retention in the gastrointestinal tract22,23. This indicates that the gastrointestinal tract, especially the gut microbiota, might be the site of action of WJW for IBD. Nevertheless, the regulatory effects and underlying mechanism of WJW on gut dysbiosis in IBD, especially its metabolism function, remain unexplored. Therefore, the present study systematically investigated the regulatory effects of WJW on gut dysbiosis in preventing IBD using a DSS-induced colitis mouse model, which is the most widely used and well-characterized model of IBD. This model of acute colitis displayed key features resembling human IBD, including bloody diarrhea, weight loss and mucosal ulceration24,25. Additionally, the luminal bacteria likely contribute to the progression of this form of colitis, providing a basis for exploring gut microbiota-mediated mechanisms during the therapeutic process26. Our results demonstrated that WJW might protect against intestinal barrier dysfunction in IBD by targeting the Lactobacillus–indole-3-acetic acid (IAA)–AhR axis.
2. Materials and methods
2.1. Chemicals and reagents
Magnoflorine was purchased from Beyotime Biotech Inc. (Shanghai, China), berberine, rutaecarpine, evodine were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China), jatrorrhizine, evodiamine were obtained from Meryer Co., Ltd. (Shanghai, China), albiflorion was obtained from Push bio-technology Co., Ltd. (Chengdu, China), benzoylpaeoniflorin was purchased from Standard Technology Co., Ltd. (Shanghai, China) and paeoniflorin was purchased from Baoji Fang Sheng Biological Development Co., Ltd. (Baoji, China). Dextran sulfate sodium (DSS, MW 36,000–50,000) was sourced from MP Biochemicals, while Wuji Wan (WJW; Batch No. 202001002) was acquired from Li Shizhen Pharmaceutical Co., Ltd. The antibiotics (ABX) ampicillin, neomycin sulfate, vancomycin, and metronidazole were acquired from Shanghai Macklin Biochemical Co., Ltd. The procurement of indole-3-acetic acid (IAA), indole-3-acrylate (IA), lipopolysaccharides derived from E. coli (LPS; O55:B5), CH-223191, Dulbecco's modified Eagle's medium (DMEM), TRIzol reagent, and fetal bovine serum (FBS) were all sourced from Sigma–Aldrich. Methanol (HPLC grade) and formic acid (LC/MS grade) were purchased from Macklin Biochemical Co., Ltd. Acetonitrile (HPLC grade) was purchased from Adamas-beta.
2.2. Animal experiment and handling
Male C57BL/6J mice, at the aged eight weeks, were supplied by the Experimental Animal Center of Xi'an Jiaotong University (Xi'an, China) [SCXK (Shan) 2018-001]. Mice were kept in specific pathogen-free conditions at 22 ± 1 °C on a 12-h light/dark cycle. They were provided with unlimited access to food and water and were acclimatized for a minimum of one week prior to the start of the experiments. All experimental procedures were conducted in compliance with the institutional guidelines for the care and use of laboratory animals in China and approved by the Animal Ethical Council of Xi'an Jiaotong University Health Science Center (permit NO. XJTU2019-679).
The mice were divided into five groups in a random manner: control (Ctrl), colitis (DSS), colitis plus 150 mg/kg WJW (WJW-L), colitis plus 300 mg/kg WJW (WJW-H), and colitis plus 100 mg/kg 5-aminosalicylic acid (5-ASA) groups. The onset of experimental colitis was triggered by oral administration of 2.5% (w/v) DSS in drinking water for a period of 10 consecutive days. WJW powder and 5-ASA were dissolved in water and administered to their respective groups via oral gavage throughout the 10-day duration, while the mice in Ctrl and model groups were given identical amounts of water. Details on the formulation and qualitative evaluation of WJW powder are outlined in Fig. 1 and Supporting Information Fig. S1. Daily monitoring of the mice including recording the body weight and disease activity index (DAI), which encompassed body weight reduction, stool consistency, and gross blood14. On the 10th day, the mice were euthanized, and their entire spleen and colon were weighed and subjected to photography. A 1-cm portion of the distal colon was preserved for histological and immunofluorescence analysis, while the remaining portion was rapidly frozen in liquid nitrogen and stored at −80 °C for subsequent analyses. The fecal samples were gathered to analyze the structure of intestinal microbiota and related metabolites. Serum was collected for the purpose of metabolomic analysis.
Figure 1.
Accurate identification of 9 main chemical compounds in Wuji Wan (WJW) with an LC–QTOF-MS/MS based chemical profiling method. (A) The extract ion chromatograms of 9 components: berberine, evodiamine, paeoniflorin, jatrorrhizine, benzoylpaeoniflorin, albiflorin, magnoflorine, rutaecarpine, and evodine. (B) The MS/MS spectra of 9 chemical features in WJW and the corresponding MS/MS spectra of reference compounds.
For establishing the pseudo-germ-free model, mice received ABX-dissolved drinking water (ampicillin 1 g/L, vancomycin 0.5 g/L, neomycin sulfate 1 g/L, and metronidazole 1 g/L), starting 10 days prior to colitis induction and continuing until the end of the experiment for depleting the gut microbiota15. For IAA and IA treatment, the mice daily received intragastric injections of 20 mg/kg of either IAA or IA throughout the entire duration of colitis induction27.
2.3. Preparation and qualitative analysis of Wuji Wan powder
Wuji Wan (WJW, Li Shizhen Pharmaceutical Co., Ltd., Batch No.: 202001002) powder used in this study was crude powder without any extraction process. WJW were pulverized into fine powder screening through a 300-mesh sieve for the following study. WJW powder was dissolved using distilled water to extract the WJW components into solution. The qualitative chemical profiles of WJW were analyzed by UHPLC system (Agilent 1290 Infinity, Agilent Technologies, USA) coupled to QTOF/MS (Agilent 6560 IM-QTOF, Agilent Technologies, USA). Chromatographic separation was established by ACQUITYUPLC®BEH C18 column (2.1 mm × 100 mm, 1.7 μm, Waters) with gradient change of mobile phases A and B (mobile phases A: water with 0.1% formic acid, mobile phases B: acetonitrile with 0.1% formic acid), and the gradient-elution program is as follows: 0−1 min, 2% B; 1−8 min, 2%–28% B; 8−28 min, 28%–62% B; 28−33 min, 62%–98% B. The parameter information for mass spectrometry is provided in Supporting Information Table S1. The complex components of WJW were preliminary identified by matching analysis with the compounds in the Personal Compound Database and Library (Agilent Technologies, USA), and the WJW components would be accurately identified when it has identical MS/MS spectra as the reference compounds28.
2.4. Biochemical assays
The activity of myeloperoxidase (MPO) in the colonic tissues was assessed as described previously15. The levels of tumor necrosis factor (TNF-α), interleukin (IL)-1β, IL-6, and IL-10 in the colonic tissues were measured using specific ELISA kits (eBioscience Biotechnology, CA, USA), following the guidelines provided by the manufacturer.
2.5. Histopathological assessment
Specimens from the distal colon were immersed in a 10% neutral formalin solution overnight, subsequently embedded in paraffin, sliced into sections measuring 5 μm in thickness, and stained with hematoxylin and eosin, following the established protocols. On the other hand, the partial colonic tissues were immediately fixed in Carnoy's solution during dissection for Periodic-acid Schiff (PAS) staining.
2.6. Assessment of intestinal permeability
The intestinal barrier function was examined using fluorescein isothiocyanate (FITC)-dextran (Sigma–Aldrich) as described previously29. In summary, mice were fasted for 4 h, followed by the oral administration of FITC-dextran at a dosage of 60 mg per 100 g of body weight. After 5 h, blood samples were obtained, and the concentration of FITC-dextran in the serum was measured by utilizing the standard curve method (excitation at 485 nm; emission at 525 nm; BioTek).
2.7. 16S rRNA gene sequencing and analysis
The genomic DNA of the bacteria was isolated from the fecal samples by utilizing the hexadecyltrimethylammonium bromide method and measured using agarose gel electrophoresis15. The highly variable V3–V4 region of the 16S rRNA gene was amplified using PCR, followed by sequencing the amplified products on the Illumina MiSeq platform (Illumina, San Diego, CA, USA). And the raw sequencing data underwent filtration, trimming, and subsequent clustering into identical operational taxonomic units (OTUs) with ≥97% similarity, using the Quantitative Insights into Microbial Ecology (QIIME, V1.9.1) pipeline and Uparse software (Version 7.0.1001). A representative set of sequences was generated and annotated using SILVA database based on the Mothur algorithm. The MUSCLE software (Version 3.8.31) was used for multiple sequence alignment, and the R program (Version 3.5.3) was used to calculate α-diversity indices and conduct principal coordinate analysis (PCoA) based on Bray-Curtis distance. A taxonomic cladogram was generated using linear discriminant analysis (LDA) effect size (LEfSe) with a threshold of 4 on the logarithmic LDA score.
2.8. Targeted metabolomics analysis
The precision-targeted metabolomics approach was employed to analyze the metabolomes of both serum and fecal specimens14,30. In brief, 100 μL serum samples were added in 500 μL iced ddH2O, and vortexed and ultra-sounded for 10 min, respectively. After the collection of supernatants, adding 500 μL iced methanol to EP tube for extracting with organic phase. The supernatants of the extracts of aqueous and organic phases were mixed and filtered with 0.22 μm organic phase membrane. About 100 mg fecal sample was accurately weighed and dissolved in 500 μL of ice-cold ddH2O followed by 10 min of vigorous shaking. After a 10 min sonication extraction, the sample was centrifuged at 6000 × g for 10 min at 4 °C to collect the supernatant. Then, the remaining precipitation was subjected to the same steps in 500 μL ice-cold methanol solution containing 0.001 mg/mL of the internal standard 4-chloro-d,l-phenylalanine. Finally, the aqueous and methanol extracts were mixed and centrifuged at 20,000 × g for 10 min at 4 °C and the supernatant was filtered by 0.22 μm filter membrane (organic phase) for metabolomics assay. 5 μL of the sample from each individual was combined to create a quality control sample. After analyzing every 10 samples, the quality control samples were analyzed to assess the stability and accuracy of data acquisition.
The metabolic profiles of samples were characterized by targeted metabolomics through UHPLC–TQ/MS system (Agilent 1290 Infinity LC System coupled with Agilent 6495 Series Triple Quad MS System, Agilent Technologies, USA). The parameter information for mass spectrometry is provided in Supporting Information Table S2. The ACQUITY UPLC HSS T3 column (2.1 mm i.d. × 100 mm, 1.8 μm; Waters) was used at 0.3 mL/min following gradient-elution program (A: water with 0.1% formic acid (v/v), B: acetonitrile with 0.1% formic acid (v/v); 0–2 min, 98% A; 2–10 min, 98%–65% A; 10–12 min, 65%–20% A; 12–14 min, 20%–2% A).
The MS raw data were processed using the Agilent Quantitative Analysis software (Agilent Technologies, USA), and the metabolites with peak area lower than 1000 would be manually excluded to Ensure the high quality and authenticity of the MS data. 1 μg/mL of the internal standard 4-chloro-d,l-phenylalanine were added to the sample pretreatment reagent (80% iced methanol). During the statistical analyses of metabolomics data, the peak area of metabolites was normalized using the expression levels of the internal standard and the weight of the fecal samples. Then the normalized peak area of metabolites was imported to SIMCA-P Statistical Analysis 13.0 program (Umetrics, Umeå, Sweden) for partial least squares-discriminant analysis (PLS-DA). Screening of the differential metabolites was conducted using following thresholds: variable importance in the projection (VIP) > 1 from the PLS-DA model, and the adjusted P values < 0.05 from Mann–Whitney U-test with Benjamini–Hochberg false discovery rate correction. The heatmap overview of targeted metabolomes was generated using MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/) based on the differential metabolites.
2.9. Cell culture and drug treatment
Caco-2 cell lines, sourced from the American Type Culture Collection (Rockville, MD, USA) were cultured in DMEM enriched with 20% FBS and 100 U/mL penicillin–streptomycin solution in a humidified incubator (5% CO2, 95% air, 37 °C). Cells were cultivated until 80%–90% confluence and exposed to various treatments. In brief, Caco-2 cells were planted into six-well plates at a density of 5 × 105 cells per well, and treated as follows for 24 h: (1) DMEM - control group; (2) 1 μg/mL LPS; (3) 1 μg/mL LPS plus 250 or 500 mg/L WJW; (4) 1 μg/mL LPS plus 20 μmol/L CH-223191; and (5) 1 μg/mL LPS plus 500 μmol/L IAA with or without 20 μmol/L CH-223191. All experiments were performed in triplicates. The cells were rinsed and harvested for either RNA or protein extraction. Cell viability was measured by utilizing the Cell Counting Kit-8 assay kit (Solarbio Co., Beijing, China), adhering to the instructions provide by the manufacturer.
2.10. Immunofluorescence staining
Following established procedures, Caco-2 cells and colon slices were fixed and stained using antibodies targeting zonula occludens-1 (ZO-1, 1:100, Cat #ab221547; Abcam, Cambridge, MA, USA), Occludin (1:100, Cat #ab216327; Abcam), and aryl hydrocarbon receptor (AhR, 1:150, Cat #A1451; Abclonal, Boston, MA, USA) as per standard protocols. The samples were incubated with the primary antibodies overnight at 4 °C, and then rinsed with PBS. Subsequently, they were co-stained with a fluorochrome-labeled secondary antibody at 37 °C for 1 h and counterstained with DAPI (for nucleus staining). The fluorescence images were captured using laser scanning confocal microscopy (Leica, Wetzlar, Germany).
2.11. Quantitative real-time PCR
Total RNA was extracted from the colonic tissues and Caco-2 cells employing TRIzol reagent following the guidelines provided by the manufacturer. The RNA quantity and purity were evaluated by measuring the absorbance at 260 and 280 nm. The first-strand cDNA was generated using the Superscript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). qPCR was performed using the SYBR Green reagents with total reaction volume of 20 μL on a real-time fluorescence quantitative PCR instrument (MX3005P, Agilent). The primer sequences can be found in Supporting Information Table S3, providing further details. The expression of each gene was normalized to that of GAPDH.
2.12. Western blotting
Cells and colonic tissues were lysed using the radioimmunoprecipitation assay lysis buffer supplemented with protease and phosphatase inhibitors. The protein content was quantitated by a BCA protein assay kit. Western blotting was performed as previously described, utilizing the following antibodies: ZO-1 (1:1000, Cat #ab276131; Abcam, Cambridge, MA, USA), Occludin (1:1000, Cat #ab216327; Abcam), Claudin-2 (1:1000, Cat # A14085; Abclonal), Mucin-2 (1:1000, Cat #A14659; Abclonal), AhR (1:1000, Cat #A1451; Abclonal), Cytochrome P450 1A1 (CYP1A1, 1:1000, Cat #A2159; Abclonal), myosin light chain kinase (MLCK, 1:1000, Cat #A3835; Abclonal), Phospho-MLC (p-MLC, 1:1000, Cat #3671; CST, USA), and GAPDH (1:5000, Cat #ab8245; Abcam). The protein bands were visualized using enhanced chemiluminescent reagent and then quantified using Image J software.
2.13. Statistical analyses
Statistical analysis was conducted using GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA). Data are presented as the mean ± Standard error of mean (SEM). Significant differences between two groups were evaluated using a two-tailed unpaired Student's t-test, whereas the differences among multiple groups were analyzed using one-way or two-way analysis of variance (ANOVA) followed by Dunnett's post hoc multiple comparison's test. A two-tailed Wilcoxon rank-sum test was performed using the R software to analyze the gut microbiota sequencing data. All results were considered statistically significant at P < 0.05.
3. Results
3.1. Accurate identification of WJW components by UPLC–QTOF-MS analysis
To identify the main components of WJW, a chemical profiling method with UHPLC–QTOF MS was developed. This method enabled the profiling of core chemicals in the WJW extract, which is crucial for accurately verifying the identities of WJW chemical compounds by comparing them with reference compound MS/MS spectra. By conducting matching analysis with compounds in the Personal Compound Database and Library database and manual screening, components with matching retention time and daughter ions, along with high abundance in the WJW extract, were selected for further precise identification. Subsequently, through comparative analysis of MS/MS spectra between the reference compounds and WJW chemical features, nine core chemicals of WJW were accurately identified. These included magnoflorine, berberine, and jatrorrhizine (Rhizoma Coptidis); evodiamine, rutaecarpine, and evodine (Fructus Evodiae); and albiflorin, benzoylpaeoniflorin, and paeoniflorin (Radix Paeoniae Alba) (Fig. 1A). As depicted in Fig. 1B, these nine components were consistently detected in the WJW extract, indicating their presence in higher concentrations in WJW. The details of the chemical structures of these nine components in WJW are illustrated in Fig. S1. Basically, berberine, paeoniflorin, and evodiamine, despite their low oral bioavailability, have demonstrated significant anti-colitis pharmacological activities without adverse effects. This is attributed to their regulation of gut microbiota. Therefore, it is suggested that modulating gut microbiota may be a critical mechanism by which WJW exerts its therapeutic effects in the treatment of ulcerative colitis.
3.2. WJW alleviated colitis symptoms and colonic inflammation in a mouse model
This study established a DSS-induced acute colitis mouse model, which simulated several common phenotypes of human colitis, such as prostration, diarrhea, and bloody stools14. WJW (150 or 300 mg/kg) or 5-ASA (100 mg/kg) was orally administered to the mice every day during the whole period of colitis induction. Significant body weight loss and increased DAI scores were observed in the DSS-treated mice compared to the Ctrl group, starting from Day 4 and Day 2 of induction, respectively (Fig. 2A), indicating that the acute colitis model was successfully established. WJW administration significantly reversed the DSS-induced body weight loss and DAI scores in a dose-dependent manner (Fig. 2A). The DSS-induced colitis mice also exhibited colon shortening and spleen enlargement, which are recognized as crucial indicators of colonic and systemic inflammation, respectively15. However, WJW treatment decreased the colon weight to length and spleen weight to body weight ratios when compared to the untreated model group (Fig. 2B).
Figure 2.
WJW improved the pathological phenotype of dextran sulfate sodium (DSS)-induced colitis and reduced colonic inflammation. Colitis was induced by administering 2.5% DSS dissolved in drinking water of mice for consecutive 10 days. WJW at dosage of 150 mg/kg (L) and 300 mg/kg (H), 5-aminosalicylic acid (5-ASA) (100 mg/kg) were orally administered daily in indicated group during colitis process. (A) Percentage body weight and disease activity index (DAI) scores change during 10 consecutive days with DSS treatment (n = 10). (B) Macro observation of colon and spleen, colon weight to length ratio and spleen weight to body weight ratio in indicated group (n = 10). (C) Representative hematoxylin and eosin stained pictures of the colon (scale bars, 100 μm) and histological scores of different groups (n = 3). (D) MPO activity and inflammatory cytokine concentration (IL-6, IL-1β, TNF-α and IL-10) in colon tissues (n = 3). Data are presented as the mean ± SEM. Statistical significance was determined using one-way or two-way ANOVA with Dunnett's t test for multiple-group comparisons. ∗∗∗P < 0.001 vs. Ctrl group, #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. colitis group, &P < 0.05, &&P < 0.01 and &&&P < 0.001 vs. WJW-H group.
WJW supplementation also inhibited the MPO activity, an indicator of neutrophil accumulation14, in the mice with DSS-induced colitis (Fig. 2D). Furthermore, WJW decreased the levels of the pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, and significantly increased the levels of anti-inflammatory cytokines, such as IL-10. This suggested that WJW could prevent the infiltration of inflammatory cells into the colon and mitigate active inflammation (Fig. 2D). Histological examination of the distal colon specimens showed that WJW repaired mucosal injury, restored crypt structure, and decreased the infiltration of inflammatory cells (Fig. 2C). Furthermore, high-dose WJW was more effective compared to 5-ASA. Taken together, WJW alleviated the symptoms associated with DSS-induced colitis and reduced inflammation in colon tissue.
3.3. WJW attenuated gut dysbiosis in the DSS-induced colitis model
Considering the significant contribution of gut dysbiosis to the development of IBD, the effects of WJW on the diversity and composition of the intestinal microbiota were also evaluated in DSS-induced colitis model using 16S rRNA gene sequencing. As shown in Fig. 3A, WJW administration mitigated the DSS-induced decrease in bacterial diversity and richness, as indicated by the Shannon index of α-diversity and OTUs. A distinct clustering of the microbial taxa was also observed among the different treatment groups through Bray-Curtis distance based on PCoA; the WJW group exhibited a closer resemblance to the Ctrl group when compared to the colitis group (Fig. 3B). In order to compare the overall structure and composition of the intestinal microbiota among different groups, the degree of bacterial taxonomic similarity was analyzed specifically at the phylum level. In the DSS-induced colitis group, there was a notable reduction of Firmicutes (10.2%) and a concomitant increase in Bacteroidetes (10%) compared to the Ctrl group. However, WJW administration effectively restored the levels of phyla to normal (Fig. 3C). Consistent with previous reports31, the Firmicutes/Bacteroidetes (F/B) ratio was significantly lower in the colitis mice compared to the normal group, and increased following WJW administration (Fig. 3C). At the family level, the DSS-induced colitis group exhibited a significant decrease in the relative abundance of Lactobacillaceae and Muribaculaceae, while the abundance of Peptostreptococcaceae, Bacteroidaceae, Tannerellaceae and Enterococcaceae significantly increased in comparison to the Ctrl group. WJW supplementation reversed the proportion of Lactobacillaceae (from 52% in Ctrl to 7% in Colitis, P < 0.001; from 7% in Colitis to 21% in WJW, P < 0.05), Muribaculaceae [from 11% (Ctrl) to 4% (Colitis), P < 0.05; from 4% (Colitis) to 10% (WJW), P < 0.05], and Bacteroidaceae [from 0.9% (Ctrl) to 26% (Colitis), P < 0.001; from 26% (Colitis) to 3% (WJW), P < 0.001] (Fig. 3D). While DSS induction had no effect on the abundance of Akkermansiaceae, Lachnospiraceae, and Prevotellaceae, WJW treatment significantly increased all three families. At the genus level, the administration of WJW effectively restored the abundance of Lactobacillus, Bacteroides and Allobaculum back to normal level. In addition, following WJW intervention, the proportion of Akkermansia, which exerts a protective effect on the gut barrier, as well as the common probiotic Bifidobacterium showed a notable increase compared to the colitis group (Fig. 3E)32,33. The abundance of Lactobacillus, the best characterized and widely commercialized probiotic, also significantly decreased in the colitis group (from 52% to 7%, P < 0.001) and increased after WJW treatment (from 7% to 21%, P < 0.05) (Fig. 3E)32,33. LEfSe analysis demonstrated distinct taxa in the colitis and WJW-treated groups. Furthermore, LDA with LEfSe confirmed that the Lachnospiracae and Lactobacilliaceae families were clearly altered after WJW treatment. Interestingly, Lactobacillus and Lactobacillus murinus showed higher LDA scores in the WJW group compared to that in the colitis group, suggesting that Lactobacillus could mediate the preventive effects of WJW on colitis (Fig. 3F).
Figure 3.
WJW reshaped the gut microbiota composition in DSS-induced colitis mice. Microbial composition of the mice was analyzed with 16S rRNA pyrosequencing technology. (A) Alpha diversity analysis of gut bacterial diversity (Shannon index) and richness [Observed operational taxonomic units (OTUs)] from different mouse groups (n = 7–8). (B) Bray–Curtis principal coordinate analysis (PCoA) analysis of gut microbiota based on the OTU level among three groups (n = 9–10). (C) Bacterial taxonomic profiling at the phylum level of intestinal bacteria and Firmicutes-to-Bacteroidetes ratio resulted from different groups (n = 8). (D, E) Relative abundance of dominant bacteria in families (D) and genus (E) in feces from Ctrl, colitis and WJW groups. (F) Taxonomic cladogram generated from linear discriminant analysis (LDA) effect size (LEfSe) analysis and differentially enriched gut microbiota in indicated group of mice fecal microbiome by LDA, LDA score >4. All analyses were based on a high dose of WJW (300 mg/kg). Data are presented as the mean ± SEM. Significant differences between the two groups were evaluated by a two-tailed Wilcoxon rank-sum test and the rest were determined using one-way ANOVA with Dunnett's t test for multiple-group comparisons. ∗P < 0.05 and ∗∗∗P < 0.001 vs. Ctrl group, #P < 0.05 and ##P < 0.01 vs. colitis group.
In order to ascertain the crucial role of gut microbiota in mediating the beneficial effects of WJW, we depleted the exiting gut flora in the colitis and WJW-treated groups using a cocktail of ABX, including vancomycin, metronidazole, neomycin sulfate, and ampicillin. Surprisingly, no significant distinctions were found between the ABX-Colitis and ABX-WJW groups regarding body weight, DAI, colonic weight/length ratio, spleen weight/body weight ratio, MPO activity, and inflammatory factors (Supporting Information Fig. S2A–S2C). In addition, the protective effects of WJW on intestinal barrier function and the expression of TJ proteins were abolished when the gut microbiota was depleted (Fig. S2D and S2E). Taken together, WJW could alleviate the progression of DSS-induced colitis by altering the composition of gut microbiota composition and increasing the abundance of Lactobacillus, and the ameliorative effects of WJW were dependent on the gut microbiota.
3.4. WJW enriched gut microbiota-derived tryptophan metabolites
Small-molecule microbial metabolites mediate host-microbiota interactions and modulate host physiology34. Therefore, we hypothesized that WJW improved the symptoms of DSS-mediated colitis by promoting synthesis of secondary microbial metabolites. To this end, we analyzed the fecal and serum metabolites of different groups using our previous developed precision-targeted metabolomics14.
PLS-DA model, distinct clusters were observed for the fecal and serum metabolites of the Ctrl group, colitis group, and WJW-treated group (Fig. 4A and B). These findings suggested that WJW protected against colitis progression by reshaping the metabolic profile of the gut. According to the heat map presented in Fig. 4C, DSS administration significantly affected the composition of fecal metabolites in comparison to the Ctrl group, and WJW administration partially restored the levels of 29 metabolites to the normal. In addition, the WJW group exhibited an increase in 10 metabolites and a decrease in 3 metabolites in feces compared to the colitis group (Supporting Information Table S4). Likewise, WJW intervention partially counteracted the metabolite changes induced by DSS in the serum, resulting in a significant upregulation of 6 metabolites and downregulation of 3 metabolites (Fig. 4D and Supporting Information Table S5). The elimination of the gut microbiota using ABX cocktail diminished the metabolic effects of WJW in the feces and serum (Supporting Information Fig. S3A–S3D), suggesting that the impact of WJW on small-molecule metabolism in colitis is dependent on the gut microbiota. The altered metabolic pathways were further analyzed using the MetaboAnalyst 5.0 (www.metaboanalyst.ca) program. Although the metabolites’ alteration included BAs metabolites (deoxycholic acid and cholic acid) and SCFAs (glutaric acid), the Trp metabolic pathway involving IA, IAA, Trp, Indole-3-propionic acid (IPA), and Serotonin (5-HT) was enriched in the feces and serum topology maps (Fig. 4E, Supporting Information Tables S4 and S5), indicating the potential role of Trp metabolites in the therapeutic mechanism of action of WJW. Therefore, we next evaluated the levels of Trp metabolites associated with the indole pathway, kynurenine pathway, and 5-HT pathway35 in the fecal and serum samples of different groups.
Figure 4.
WJW restored the metabolic disorder under colitis and enriched microbiota-derived tryptophan (Trp) metabolites. Discovery and characterization of the important differential metabolites with targeted metabolomics assay. (A, B) Partial least-squares discriminant analysis (PLS-DA) for discriminating the fecal (A) and serum (B) metabolome from indicated groups (n = 8). (C, D) Heatmaps overview of the differential metabolites in feces (C) and serum (D) that were altered by colitis compared with WJW-treated mice. Hashes represent metabolites whose abundance in healthy mice was changed by colitis and then modulated obviously by WJW. (E) Pathway enrichment analysis of the significantly altered metabolites by WJW treatment in the feces and serum. (F, G) Comparison of relative abundance of Trp metabolism-related small molecules in feces (F) and serum (G) by LC–MS (n = 8). (H) Spearman correlation between the colitis-related parameters and Trp metabolites in feces and serum. The red spots indicate a positive correlation, while blue color show a negative correlation. The intensity of the color is proportional to the strength of Spearman correlation. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001. (I) Scatterplot with Spearman's rank correlations between the relative abundances of Lactobacillus genera and indole-3-acetic acid (IAA) levels in feces and serum. All analyses were based on a high dose of WJW (300 mg/kg) (n = 8). Data are presented as the mean ± SEM. The differences of abundance distributions among metabolites between two groups were measured by the Mann–Whitney U test with Benjamini–Hochberg false discovery rate correction. ∗P < 0.05 and ∗∗P < 0.01 vs. Ctrl group, #P < 0.05 vs. colitis group. IA: 3-indoleacrylic acid; IPA: indole-3-propionic; IAA: indole-3-acetic acid; 5-HT: serotonin.
As depicted in Fig. 4F, the Trp level in feces did not notably increase in colitis mice compared to the normal group, while WJW treatment led to a considerable decrease in its levels. Specifically, IPA, IAA, and 5-HT levels were significantly reduced in the feces of the colitis mice, and WJW treatment increased the levels of IPA and IAA. Interestingly, the fecal IA levels remained unchanged in colitis group, but increased approximately threefold following WJW treatment compared to that in the normal group (Fig. 4F). The levels of Trp and N-acetyl-l-tryptophan in serum were respectively lower and higher in the colitis mice compared to both the normal and WJW-treated mice; however, the difference was not statistically significant. On the other hand, the levels of IAA, IA, and 5-HT in the serum of colitis mice were significantly decreased compared to the Ctrl group. However, WJW supplementation restored the levels of IAA and 5-HT back to normal (Fig. 4G). Overall, these results suggest that IAA and IA might potentially mediate the protective effects of WJW. Therefore, we evaluated the relationship between the levels of these Trp metabolites and common colitis phenotypes, such as weight loss, DAI, and inflammation indices. As shown in Fig. 4H, the serum and fecal IAA levels were significantly associated with colitis-related characteristics. However, the regulatory effects of WJW on IAA production were abolished in the colitis mice treated with the ABX cocktail (Fig. S3E and S3F), suggesting a crucial role of the gut microbiota in WJW-mediated Trp metabolism. Finally, Spearman's rank correlation analysis demonstrated a notable positive correlation between the levels of serum and fecal IAA and the abundance of Lactobacillus in the group treated with WJW (Fig. 4I). Overall, these findings suggest that WJW enriched IAA in the gut through the increased abundance of Lactobacillus, which could be the basis of its protective effect against colitis.
3.5. WJW improved the intestinal mucosal barrier function and integrity in the colitis model
To investigate the potential correlation between the anti-colitis effects of WJW and intestinal barrier function, we assessed mucosal barrier permeability using FITC-dextran as the tracer. Additionally, we analyzed the expression levels of various TJ proteins, including ZO-1, Occludin, Claudin-1, Claudin-2, and Claudin-4. As shown in Fig. 5A, the serum levels of FITC-dextran exhibited a significant increase in DSS-induced colitis mice compared to the Ctrl group. However, treatment with WJW resulted in a notable reduction in the serum levels of FITC-dextran. Furthermore, WJW also upregulated the mRNA levels of Tjp1, Ocln, and Muc2 and significantly downregulated that of the TJ leaky protein Cldn236 relative to the colitis group (Fig. 5B). As expected, ZO-1, Occludin, and Mucin-2 proteins were all significantly upregulated, whereas Claudin-2 was downregulated in the WJW-treated group when compared to the untreated colitis group, suggesting that WJW could restore the intestinal barrier integrity (Fig. 5C). Indeed, immunofluorescence staining revealed the presence of ZO-1 and Occludin proteins primarily along the epithelial cell membranes in the healthy intestines. However, in the inflamed tissues of the colitis group, their expression was notably reduced and observed only in sparse amounts. Remarkably, the administration of WJW significantly enhanced the expression of TJ proteins in both the spinous and granular layers of the mucosa (Fig. 5D). In addition, in the colitis mice, PAS staining revealed a significant loss of goblet cells, which are involved in mucin production and storage; the WJW treatment restored this loss (Fig. 5D)37. Taken together, WJW effectively enhanced the intestinal barrier function in colitis mice by stimulating TJ protein expression and gut mucin production.
Figure 5.
WJW relieved gut barrier disruption in DSS-induced colitis. Colitis was induced by administering 2.5% DSS dissolved in drinking water of mice for consecutive 10 days. WJW at dosage of 150 mg/kg (L) and 300 mg/kg (H) were orally administered daily in indicated group during colitis process. (A) Intestinal permeability from different groups (n = 3). (B) The mRNA levels of tight junction (TJ) and mucin related molecules involving Tjp1, Ocln, Cldn1, Cldn2, Cldn4, Muc1 and Muc2 (n = 3). (C) Representative immunoblots and the relative expression levels of zonula occludens-1 (ZO-1), Occludin, Claudin-2 and Mucin-2 proteins (n = 3). (D) Representative immunofluorescence images showing in situ expression of ZO-1, Occludin proteins and images of Periodic Acid-Schiff (PAS) stained colonic sections (n = 3, scale bars = 100 μm). Data are presented as the mean ± SEM. Statistical significance was determined using one-way ANOVA with Dunnett's t test for multiple-group comparisons. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 vs. Ctrl group, #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. colitis group, &P < 0.05, &&&P < 0.001 vs. WJW-H group.
3.6. WJW improved gut barrier function by activating the AhR signaling pathway in a gut microbiota-dependent manner
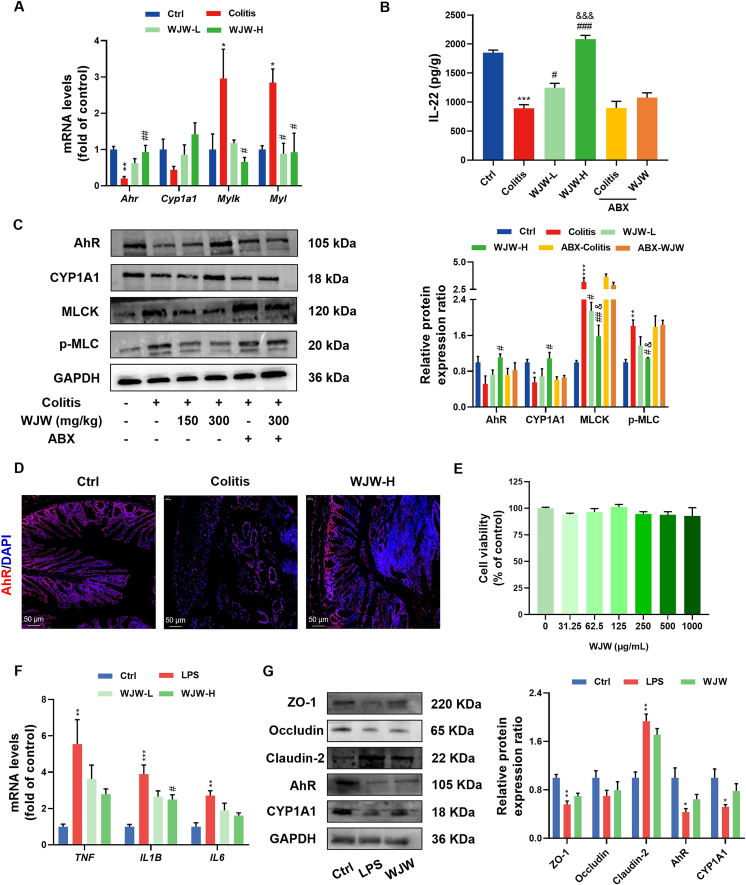
Since Trp metabolites formed in the gut during microbial fermentation activate the AhR pathway, we hypothesized that the potential protective effects of WJW on the integrity of intestinal barrier could be associated with the indirect activation of AhR signaling via the Trp-derived indole compounds38,39. DSS treatment led to an obvious downregulation in Ahr and Cyp1a1 mRNA and protein levels, whereas WJW enhanced AhR expression and activity when compared to the colitis group (Fig. 6A and C). Immunofluorescence staining for AhR showed consistent results (Fig. 6D). AhR activation has been shown to enhance epithelial barrier and ameliorate colitis symptoms in mice by inducing IL-22 production and suppressing MLCK–pMLC signaling39,40. Consistent with previous findings40, we found that DSS intervention stimulated the MLCK–pMLC signaling pathway and decreased IL-22 concentration in the colonic tissue. However, administration of WJW reversed the upregulation of MLCK and phosphorylated MLC and increased IL-22 levels in the colitis model (Fig. 6A–C). Interestingly, depletion of the intestinal microbiota abrogated the effects of WJW on the AhR/IL-22 and AhR/MLCK–pMLC pathways (Fig. 6B and C).
Figure 6.
Involvement of aryl hydrocarbon receptor (AhR) signaling pathway in the effect of WJW on intestinal integrity is associated with gut microbiota. (A–D) After antibiotic treatment for 10 days, mice were given 2.5% DSS drinking water and administrated with water or WJW (300 mg/kg) orally in indicated group. (A, C) The mRNA and protein levels of AhR signaling pathway involving Ahr, Cyp1a1, Mylk and Myl in indicated groups (n = 3). (B) IL-22 concentration from different treatment groups (n = 3). (D) Immunofluorescence staining for AhR (red) and cell nuclei (blue) in mouse colonic sections of three groups (n = 3, scale bars = 50 μm). (E–G) Caco-2 cells were incubated with the 1 μg/mL LPS or/and 250 (L) or 500 (H) mg/L WJW for 24 h. (E) Cell viability was determined using the Cell Counting Kit-8 assay after 24 h incubation with different concentrations of WJW in Caco-2 cells (n = 3). (F) The mRNA level of proinflammatory cytokines TNF, IL1B and IL6 from different groups (n = 3). (G) Representative immunoblots and the relative expression levels of intestinal barrier integrity and AhR signaling pathway proteins among three groups (n = 3). The analyses were based on a high dose of WJW (300 mg/kg or 500 mg/L). Data are presented as the mean ± SEM. Statistical significance was determined using one-way ANOVA with Dunnett's t test for multiple-group comparisons. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 vs. Ctrl group, #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. colitis or LPS group, &P < 0.05 and &&&P < 0.001 vs. ABX-WJW group.
To further characterize the crucial role of intestinal microbiota in intestinal barrier defense by WJW, we treated LPS-induced Caco-2 cells with 250 and 500 μg/mL WJW for 24 h and analyzed the colitis-related pathways (Fig. 6E). As shown in Fig. 6F, WJW significantly reversed the LPS-induced increase in the mRNA levels of Il1b in a dose-dependent manner. However, WJW treatment did not activate AhR signaling or upregulate TJ proteins in the Caco-2 cells (Fig. 6G), which confirmed that the protective effects of WJW on intestinal barrier dysfunction are dependent on the gut microbiota. Overall, these findings suggested that WJW exhibited the potential to alleviate murine colitis by inducing AhR activity and suppressing MLCK–pMLC signaling, thereby restoring the gut barrier function in a gut bacteria-dependent manner.
The involvement of AhR in the protective effects of WJW was directly assessed by treating the DSS-induced colitis and WJW-treated mice with the AhR antagonist CH-223191. As expected, CH-223191 significantly decreased AhR expression and its target genes Cyp1a1 and Il22 (Supporting Information Fig. S4E and S4F). Furthermore, blocking AhR signal transduction abolished WJW-induced improvements in colitis symptoms, colonic inflammation, and intestinal barrier permeability (Fig. S4A–S4D). Additionally, the upregulation of TJ proteins and Mucin-2 in the WJW group was also abated after AhR inhibition (Fig. S4F). Taken together, activation of AhR signaling is crucial for the protective effects of WJW against the gut epithelial barrier injury in colitis.
3.7. IAA improved intestinal functions and mitigated colitis by activating the AhR signaling pathway
Since IAA and IA were identified as crucial signaling molecules for the therapeutic action of WJW, we next confirmed their effects on intestinal barrier function and DSS-induced colitis. As shown in Fig. 7A and B, both IAA and IA rescued the body weight loss, DAI, colon shortening, and splenomegaly in the DSS-induced colitis model and demonstrated the same therapeutic effect as 5-ASA. Moreover, both metabolites reversed the elevation in MPO and inflammatory cytokines in colon tissues and improved barrier permeability (Fig. 7C–E). The improvement effects of IAA on the disrupted gut barrier were stronger than that of IA (Fig. 7A–F), suggesting that IAA might be the major metabolite responsible for WJW-induced gut barrier protection in colitis mice. Based on our findings so far, we hypothesized that IAA-mediated gut barrier protection was associated with the activation of AhR signaling. We examined this hypothesis using the LPS-induced Caco-2 cell monolayer model. IAA did not have significant impact on the viability of Caco-2 cells at concentrations ≤1 mmol/L (Fig. 8A), and 1 mmol/L was used for the subsequent experiments. Fig. 8B–D demonstrates that LPS stimulation significantly increased the mRNA levels of pro-inflammatory cytokines (TNF, IL1B and IL6) and disrupted the expression of intestinal barrier-related proteins (Occludin, ZO-1, and Claudin-2) in the Caco-2 cells. The changes were reversed by IAA treatment. However, AhR blockade using CH-223191 significantly hindered the regulatory effects of IAA on the levels of inflammatory cytokines and TJ proteins (Fig. 8B and C). Taken together, IAA could enhance the integrity of the intestinal barrier and mitigate colon inflammation partially through AhR signaling.
Figure 7.
The anti-colitis effects of IAA might be better than indole-3-acrylate (IA). Colitis was induced by administering 2.5% DSS dissolved in drinking water of mice for consecutive 10 days. IAA and IA at dosage of 20 mg/kg, 5-ASA (100 mg/kg) were orally administered daily in indicated group during colitis production. (A) Percentage body weight and DAI change during 10 consecutive days with DSS treatment (n = 6). (B) Macro observation of colon and spleen, colon weight to length ratio and spleen weight to body weight ratio in indicated group (n = 6). (C) MPO activity and inflammatory cytokine concentration (TNF-α, IL-1β and IL-10) in colon tissues (n = 3). (D) Intestinal permeability from different mouse groups (n = 3). (E) Representative immunoblots and the relative expression levels of ZO-1, Occludin and Mucin-2 proteins (n = 3). (F) Representative immunofluorescence images showing in situ expression of ZO-1 and Occludin proteins (n = 3). Data are presented as the mean ± SEM. Statistical significance was determined using one-way or two-way ANOVA with Dunnett's t test for multiple-group comparisons. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 vs. Ctrl group, #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. colitis group.
Figure 8.
IAA protected against epithelium barrier impairment through the AhR signaling pathway in vitro. Caco-2 cells were stimulated with 1 μg/mL LPS and 500 μmol/L IAA or 20 μmol/L AhR specific antagonist CH-223191 for the IAA or CH-223191 treatment group or in combination with 20 μmol/L CH-223191 for the IAA plus CH-223191 treatment group. (A) Cell viability was determined using the Cell Counting Kit-8 assay after 24 h incubation with different concentrations of IAA in Caco-2 cells (n = 3). (B) The mRNA level of proinflammatory cytokines TNF, IL1B and IL6 from different groups (n = 3). (C) Representative immunoblots and the relative expression levels of intestinal barrier integrity and AhR signaling pathway proteins among three groups (n = 3). (D) Representative immunofluorescence images of ZO-1, Occludin and AhR localization (red) in Caco-2 cells (n = 3, scale bars = 50 μm). Data are presented as the mean ± SEM. Statistical significance was determined using one-way ANOVA with Dunnett's t test for multiple-group comparisons. ∗P < 0.05 and ∗∗P < 0.01 vs. Ctrl group, #P < 0.05 and ##P < 0.01 vs. LPS group, &P < 0.05 vs. CH-223191-IAA group.
4. Discussion
Metabolic reprogramming due to gut dysbiosis, especially loss of microbial Trp metabolism, is a key contributor to intestinal barrier disruption and the pathological progression of IBD1,8. A better understanding of the metabolic pathways implicated in the development of IBD can help in the development of novel drugs16. TCM formulations offer numerous advantages, such as multiple molecular/pathway targets, cost-effectiveness, and good safety profile of the natural plant-based components in treating gastrointestinal tract diseases by regulating gut microbiota41. Several studies have indicated that WJW is an attractive candidate for the management of IBD owing to its beneficial effects on intestinal functions17,42. In this study, we provided novel evidence indicating that WJW could alleviate the symptoms of DSS-induced colitis and reduce colonic inflammation in a dose-dependent manner through restoration the gut microbiota and enhancing the abundance of the probiotic Lactobacillus. Moreover, the gut microbial Trp metabolite IAA contributed to the therapeutic effects of WJW by improving AhR-dependent intestinal barrier functions. Taken together, our results suggested that targeting the Lactobacillus–IAA–AhR axis shows great potential as a viable approach for the prevention and treatment of IBD and other intestinal inflammatory disorders.
The gut microbiome plays a critical role in maintenance of intestinal homeostasis43, and gut dysbiosis might be associated with the pathogenesis of gastrointestinal disorders. IBD patients show significant alterations in the gut microbiota compared to healthy individuals, such as lower microbial diversity, decreased F/B ratio, lower abundance of Lactobacillus, and a higher abundance of Enterobacteriaceae, Proteus, and Actinomycetes43, 44, 45. In this study, we found that WJW treatment counteracted the gut dysbiosis associated with DSS-induced colitis, resulting in an increase in both the richness and diversity of gut microbiota. Furthermore, depletion of the gut microbiota via antibiotic intervention thoroughly blunted the therapeutic efficacy of WJW, thereby underscoring the critical role of the resident microbes in mitigating intestinal inflammation. Bacteroidetes and Firmicutes are the dominant phyla among gut bacteria, and IBD patients exhibit a significantly lower F/B ratio compared to healthy controls31,46. WJW increased the F/B ratio in the colitis mice, although the effect was not significant. In fact, some recent studies have challenged the long-held assumption of phylum composition being a potential cause of disease and identified either a lack of, or even a positive association between phylum composition and diseases in human subjects47,48. Surprisingly, 16S rRNA gene sequencing revealed high LDA scores of Lactobacillus and L. murinus in the WJW-treated group compared to the colitis group, indicating that Lactobacillus could mediate the protective effects of WJW against colitis. Although we did not explore the ameliorative effects of Lactobacillus, previous studies have shown that it can alleviate intestinal inflammation in DSS-induced colitis models by improving the gut barrier function and microbial composition49,50. The probiotic L. murinus, which was also enriched following WJW treatment, has been reported to promote the expansion of Tregs. Mice colonized with L. murinus exhibited increased resistance to DSS-induced colitis51,52. These findings suggest that Lactobacillus, especially L. murinus, could be a potential target for WJW in the amelioration of colitis.
IBD patients also exhibit distinct gut metabolite profiles compared to healthy individuals. In particular, SCFAs, BAs and Trp metabolites have been implicated in the development of IBD8. Furthermore, the metabolic products secreted or regulated by the gut microbiota have therapeutic potential for IBD14,15. In the present study, WJW treatment restored the microbial catabolites in the colitis model, and this effect was completely abolished following depletion of the gut microbiota with ABX. The Trp catabolites IAA and IA were identified as crucial signaling molecules involved in the therapeutic action of WJW. However, only IAA levels in the serum and feces showed significant association with the pathological features of colitis, including body weight loss, DAI scores, and inflammatory cytokines. In addition, a notable positive correlation was found between the IAA levels and the abundance of the probiotic Lactobacillus. This was consistent with a previous study showing that Lactobacillus could metabolize Trp to IAA and other indole compounds53. The regulatory effects of WJW on IAA and other Trp metabolites were abrogated after ABX-mediated depletion of the gut microbiota, indicating that the gut microbiota might be an indispensable factor for the metabolic impact of WJW.
Gut barrier dysfunction is characterized by the loss of TJ proteins and increased intestinal permeability and is a common pathological feature of IBD16,54. WJW significantly increased the expression levels of intestinal barrier-related proteins, including ZO-1, Occludin, and Mucin-2, which improved the intestinal permeability in the DSS-treated mice. Furthermore, WJW also upregulated AhR and its downstream target gene IL-22, which is known to increase intestinal epithelial regeneration55. Therefore, WJW improved the intestinal barrier function partly by restoring the gut microbiota, which in turn promoted Trp metabolism and activated AhR signaling in the colon. These regulatory effects of WJW were abolished following antibiotic-mediated depletion of the intestinal microbiota. AhR plays a vital role in the maintenance of the intestinal barrier in both patients with IBD and animal models40. The activation of AhR in the intestinal tract is contingent upon the levels of Trp and its metabolites56. In fact, most Trp metabolites serve as ligands for AhR, binding to the receptor and improving gut barrier function57. Additionally, heighted expression and activation of MLCK have been identified in both human subjects with IBD and animal models of colitis58,59. Since AhR activation protects the intestinal epithelium from the damaging effects of TNF-α through suppression of the MLCK–pMLC signaling pathway39, we hypothesized that WJW also targets MLCK–pMLC activation40. As expected, DSS intervention activated the MLCK–pMLC pathway, while administration of WJW reversed the upregulation of MLCK and MLC phosphorylation in the colon. Moreover, depletion of the intestinal microbiota abolished the suppressive effects of WJW on the MLCK–pMLC signaling pathway. Consistent with this, while WJW decreased IL1B levels in the LPS-stimulated Caco-2 cells in a concentration-dependent manner, it did not restore intestinal barrier proteins and AhR activation in the in vitro model of colitis. Furthermore, AhR blockade in the colitis mice with CH-223191 neutralized the effects of WJW on the pathological and molecular indices of colitis. Taken together, our data suggest that WJW might ameliorate DSS-induced colitis by improving the gut barrier by activating AhR and partially suppressing the MLCK–pMLC pathway in a gut microbiota-dependent manner.
Indole derivatives produced by the microbial catabolism of Trp protect the intestinal epithelial barrier by upregulating specific genes60,61. For instance, AhR activation by IAA and other Trp metabolites induces colonic expression of IL-22, which promotes intestinal regeneration and barrier repair62. Intact gut barrier prevents the passage of pathogenic bacteria and toxins through the intestinal mucosa into other tissues and organs, resulting in lower immune stress and inflammation63. However, the physiological functions of IAA and IA in colitis and intestinal barrier function are not completely understood yet. We found that both metabolites demonstrated the same therapeutic effects as 5-ASA on the colitis symptoms and inflammation. However, IAA was more effective compared to IA in restoring the gut barrier function, indicating that IAA might be the dominant Trp catabolite mediating the protective effects of WJW. Indeed, IAA was the sole metabolite (in both serum and feces) that was significantly associated with all colitis-related characteristics in the WJW-treated group. Consistent with the in vivo findings, IAA also exhibited anti-inflammatory and intestinal barrier protective effects on the LPS-induced Caco-2 cell monolayer model via AhR activation. Taken together, these results suggest that IAA produced during microbial fermentation in the gut could stimulate AhR, which played a vital role in improving the intestinal barrier function in WJW-treated mice.
Our previous studies and other groups have demonstrated that the major components of WJW, including berberine, paeoniflorin and evodiamine, could improve the gut barrier function during the development of DSS-induced colitis in murine models14,64,65. These studies indicated that the gut microbiota, along with microbial metabolites, plays a crucial role in WJW components-dependent alleviation of gut barrier function. Additionally, Lactobacillus genus was found to be the predominant distinct gut microbiota responsible for distinguishing between colitis and treatment groups, aligning with the findings of our current study on WJW. However, the metabolomics analysis of WJW components in relation to colitis has been contentious. Some studies showed that berberine alleviated DSS-induced colitis by improving bile acid metabolic pathway in liver and gut or inhibiting the arachidonic acid metabolic pathway and its multiple markers in serum64,65. While our prior investigation suggested that the therapeutic role of berberine in colitis was mostly attributed to the activation AhR by gut-microbiota-associated Trp metabolites14. Although this mechanism for berberine appears to be parallel that observed in the current study on WJW, we are unable to definitively ascertain that the efficacy of WJW solely derives from berberine, as TCM is based on ‘Jun-Chen-Zuo-Shi’ (also known as ‘sovereign-minister-assistant-courier’) rule and featured as ‘multiple ingredients and multiple targets’. Paeoniflorin, another major component from WJW, was reported to induce significant shifts in metabolic pathways associated with Trp, with the inhibition of indole-3-lactate being a primary target of paeoniflorin for colitis14. Yet, our previous research indicated that paeoniflorin administration significantly improved BAs dysmetabolism in colitis mice model, leading to increased levels of deoxycholic acid and lithocholic acid, both of which are known to promote intestinal homeostasis66. Evodiamine was found to significantly enhance the abundance of L. acidophilus and increase the production of SCFAs in colitis rat, especially acetate, which have the potential to improve the barrier function of the host's intestinal epithelium, thereby protecting the host from lethal infection21. Generally, these major components in WJW exhibited similar improvement effects on the DSS-induced intestinal bacteria imbalance, yet with multifarious regulation effects on microbial metabolic pathways as well as mechanisms during their therapeutic effects. Give the still incomplete knowledge on the details of microbial metabolism, we are unable to conclude that the anti-colitis effects of WJW come mostly from these three components. Further research on the compatibility of components in WJW is needed to explore the active constituents and their interactions with intestinal microbes and microbial metabolites.
To summarize, WJW reinstated the intestinal microbiota in the colitis model, increased the abundance of Lactobacillus, and restored microbial Trp metabolism. The subsequent elevation of IAA levels stimulated AhR signaling, leading to a substantial improvement in gut-barrier integrity and suppression of colonic inflammation.
5. Conclusions
WJW alleviated colonic inflammation and gut barrier dysfunction in the DSS-induced colitis model by restoring the gut microbiota. The therapeutic efficacy of WJW was primarily attributed to the activation of AhR by Trp metabolites, and the Lactobacillus–IAA–AhR pathway could be the major target of WJW. Our study provides novel insights into underlying mechanisms of IBD and highlights the therapeutic potential of WJW in individuals with intestinal dysfunction.
Author contributions
Wanghui Jing: Writing – review & editing, Writing – original draft, Supervision, Project administration. Sijing Dong: Investigation, Formal analysis, Data curation. Yinyue Xu: Investigation, Formal analysis, Data curation. Jingjing Liu: Methodology, Formal analysis, Data curation. Jiawei Ren: Methodology, Investigation. Xue Liu: Investigation. Min Zhu: Investigation. Menggai Zhang: Investigation. Hehe Shi: Investigation. Na Li: Investigation. Peng Xia: Investigation. Haitao Lu: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization. Sicen Wang: Supervision, Project administration, Conceptualization.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by the National Natural Scientific Foundation of China (82174059), Outstanding young and middle-aged science and technology talents of Shaanxi Administration of Traditional Chinese Medicine (2023-ZQNY-004, China), the Natural Science Foundation of Shaanxi Province (2022SF-123, China), Innovative talent team introduction project in Xinzhou City (20220701, China), the Project of Shaanxi Administration of Traditional Chinese Medicine (No. 2022-SLRH-YQ-001, China), the SKL-GRF Grant from State Key Laboratory of Environmental and Biological Analysis at Hong Kong Baptist University (SKLP_2425_P02, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2024.11.009.
Contributor Information
Wanghui Jing, Email: jingwanghui1987@163.com.
Haitao Lu, Email: Haitaolyu@hkbu.edu.hk.
Sicen Wang, Email: wangsc@mail.xjtu.edu.cn.
Appendix A. Supporting information
The following is the Supporting Information to this article.
References
- 1.Barbara G., Barbaro M.R., Fuschi D., Palombo M., Falangone F., Cremon C., et al. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.718356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh S., Whitley C.S., Haribabu B., Jala V.R. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol Hepatol. 2021;11:1463–1482. doi: 10.1016/j.jcmgh.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee M., Chang E.B. Inflammatory bowel diseases (IBD) and the microbiome-searching the crime scene for clues. Gastroenterology. 2021;160:524–537. doi: 10.1053/j.gastro.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu Y.F., Lyu J., Wang S.S. The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y.Y., Zhu M., Wu J., Luo L.B., Dong S.J., Zhang M.G., et al. A mannitol-modified emodin nano-drug restores the intestinal barrier function and alleviates inflammation in a mouse model of DSS-induced ulcerative colitis. Chin Med. 2023;18:98. doi: 10.1186/s13020-023-00801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 7.Mehandru S., Colombel J.F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat Rev Gastroenterol Hepatol. 2021;18:83–84. doi: 10.1038/s41575-020-00399-w. [DOI] [PubMed] [Google Scholar]
- 8.Lavelle A., Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 9.Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Rauf A., Khalil A.A., Rahman U.U., Khalid A., Naz S., Shariati M.A., et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): an updated review. Crit Rev Food Sci Nutr. 2022;62:6034–6054. doi: 10.1080/10408398.2021.1895064. [DOI] [PubMed] [Google Scholar]
- 11.Tan J.K., Macia L., Mackay C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J Allergy Clin Immunol. 2023;151:361–370. doi: 10.1016/j.jaci.2022.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Collins S.L., Stine J.G., Bisanz J.E., Okafor C.D., Patterson A.D. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. 2023;21:236–247. doi: 10.1038/s41579-022-00805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaap F.G., Trauner M., Jansen P.L. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11:55–67. doi: 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
- 14.Jing W.H., Dong S.J., Luo X.L., Liu J.J., Wei B., Du W., et al. Berberine improves colitis by triggering AhR activation by microbial tryptophan catabolites. Pharmacol Res. 2021;164 doi: 10.1016/j.phrs.2020.105358. [DOI] [PubMed] [Google Scholar]
- 15.Dong S.J., Zhu M., Wang K., Zhao X.Y., Hu L.L., Jing W.H., et al. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol Res. 2021;171 doi: 10.1016/j.phrs.2021.105767. [DOI] [PubMed] [Google Scholar]
- 16.Gou H.Y., Su H., Liu D.H., Wong C.C., Shang H.Y., Fang Y., et al. Traditional medicine Pien Tze Huang suppresses colorectal tumorigenesis through restoring gut microbiota and metabolites. Gastroenterology. 2023;165:1404–1419. doi: 10.1053/j.gastro.2023.08.052. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y., Xiao S.M., Gong Z.P., Zhu X.X., Yang Q., Li Y.J., et al. Wuji Wan formula ameliorates diarrhea and disordered colonic motility in post-inflammation irritable bowel syndrome rats by modulating the gut microbiota. Front Microbiol. 2017;8:2307. doi: 10.3389/fmicb.2017.02307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan J., Wang Y., An R., Wang S., Li S.J., Jia J.Y., et al. Simultaneous determination of six alkaloids and one monoterpene in rat plasma by liquid chromatography-tandem mass spectrometry and pharmacokinetic study after oral administration of a Chinese medicine Wuji Pill. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;895–896:154–161. doi: 10.1016/j.jchromb.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 19.Tian T.T., Jin Y.R., Ma Y.H., Xie W.W., Xu H.J., Du Y.F. Simultaneous quantification of 11 constituents in Wuji pill using ultra performance liquid chromatography coupled with a triple quadrupole electrospray tandem mass spectrometry. J Chromatogr Sci. 2016;54:237–245. doi: 10.1093/chromsci/bmv140. [DOI] [PubMed] [Google Scholar]
- 20.Fan Q.L., Guan X.J., Hou Y.L., Liu Y.L., Wei W., Cai X.Y., et al. Paeoniflorin modulates gut microbial production of indole-3-lactate and epithelial autophagy to alleviate colitis in mice. Phytomedicine. 2020;79 doi: 10.1016/j.phymed.2020.153345. [DOI] [PubMed] [Google Scholar]
- 21.Wang M.X., Lin L., Chen Y.D., Zhong Y.P., Lin Y.X., Li P., et al. Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol Res. 2020;159 doi: 10.1016/j.phrs.2020.104978. [DOI] [PubMed] [Google Scholar]
- 22.Fei F., Yang H.L., Peng Y., Wang P., Wang S.Y., Zhao Y.Q., et al. Sensitive analysis and pharmacokinetic study of the isomers paeoniflorin and albiflorin after oral administration of Total Glucosides of White Paeony Capsule in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1022:30–37. doi: 10.1016/j.jchromb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Ma J.Y., Feng R., Tan X.S., Ma C., Shou J.W., Fu J., et al. Excretion of berberine and its metabolites in oral administration in rats. J Pharm Sci. 2013;102:4181–4192. doi: 10.1002/jps.23718. [DOI] [PubMed] [Google Scholar]
- 24.Oh S.Y., Cho K.A., Kang J.L., Kim K.H., Woo S.Y. Comparison of experimental mouse models of inflammatory bowel disease. Int J Mol Med. 2014;33:333–340. doi: 10.3892/ijmm.2013.1569. [DOI] [PubMed] [Google Scholar]
- 25.Kim J.J., Shajib M.S., Manocha M.M., Khan W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp. 2012;60 doi: 10.3791/3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada M., Ohkusa T., Okayasu I. Occurrence of dysplasia and adenocarcinoma after experimental chronic ulcerative colitis in hamsters induced by dextran sulphate sodium. Gut. 1992;33:1521–1527. doi: 10.1136/gut.33.11.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira D.L., Pugine S.M., Ferreira M.S., Lins P.G., Costa E.J., de Melo M.P. Influence of indole acetic acid on antioxidant levels and enzyme activities of glucose metabolism in rat liver. Cell Biochem Funct. 2007;25:195–201. doi: 10.1002/cbf.1307. [DOI] [PubMed] [Google Scholar]
- 28.Tian T., Xu X., Li X., Zhang W.H., Lu H.T. Precision-characterization and quantitative determination of main compounds in Si-Ni-San with UHPLC–MS/MS based targeted-profiling method. J Pharm Biomed Anal. 2021;194 doi: 10.1016/j.jpba.2020.113816. [DOI] [PubMed] [Google Scholar]
- 29.Li D.T., Feng Y., Tian M.L., Ji J.F., Hu X.S., Chen F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome. 2021;9:83. doi: 10.1186/s40168-021-01028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J.J., Luo X.L., Guo R., Jing W.H., Lu H.T. Cell metabolomics reveals berberine-inhibited pancreatic cancer cell viability and metastasis by regulating citrate metabolism. J Proteome Res. 2020;19:3825–3836. doi: 10.1021/acs.jproteome.0c00394. [DOI] [PubMed] [Google Scholar]
- 31.Kabeerdoss J., Jayakanthan P., Pugazhendhi S., Ramakrishna B.S. Alterations of mucosal microbiota in the colon of patients with inflammatory bowel disease revealed by real time polymerase chain reaction amplification of 16S ribosomal ribonucleic acid. Indian J Med Res. 2015;142:23–32. doi: 10.4103/0971-5916.162091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caenepeel C., Sadat Seyed Tabib N., Vieira-Silva S., Vermeire S. Review article: how the intestinal microbiota may reflect disease activity and influence therapeutic outcome in inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52:1453–1468. doi: 10.1111/apt.16096. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y., Sugihara K., Gillilland M.G., 3rd, Jon S., Kamada N., Moon J.J. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat Mater. 2020;19:118–126. doi: 10.1038/s41563-019-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roager H.M., Licht T.R. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9:3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krautkramer K.A., Fan J., Backhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2021;19:77–94. doi: 10.1038/s41579-020-0438-4. [DOI] [PubMed] [Google Scholar]
- 36.Qu C., Yuan Z.W., Yu X.T., Huang Y.F., Yang G.H., Chen J.N., et al. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharmacol Res. 2017;121:70–82. doi: 10.1016/j.phrs.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Cornick S., Kumar M., Moreau F., Gaisano H., Chadee K. VAMP8-mediated MUC2 mucin exocytosis from colonic goblet cells maintains innate intestinal homeostasis. Nat Commun. 2019;10:4306. doi: 10.1038/s41467-019-11811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stockinger B., Shah K., Wincent E. AHR in the intestinal microenvironment: safeguarding barrier function. Nat Rev Gastroenterol Hepatol. 2021;18:559–570. doi: 10.1038/s41575-021-00430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu M., Wang Q.M., Ma Y.H., Li L.Z., Yu K., Zhang Z.C., et al. Aryl hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight junction integrity. Int J Biol Sci. 2018;14:69–77. doi: 10.7150/ijbs.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han B., Sheng B.F., Zhang Z.C., Pu A.M., Yin J.H., Wang Q.M., et al. Aryl hydrocarbon receptor activation in intestinal obstruction ameliorates intestinal barrier dysfunction via suppression of MLCK–MLC phosphorylation pathway. Shock. 2016;46:319–328. doi: 10.1097/SHK.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y.K., Pan Y., Hou M.R., Luo R.S.Q., He J.W., Lin F., et al. Danggui Shaoyao San ameliorates the lipid metabolism via the PPAR signaling pathway in a Danio rerio (zebrafish) model of hyperlipidemia. Biomed Pharmacother. 2023;168 doi: 10.1016/j.biopha.2023.115736. [DOI] [PubMed] [Google Scholar]
- 42.Gong Z.P., Yang Q., Wang Y.J., Weng X.G., Li Y.J., Dong Y., et al. Pharmacokinetic differences of Wuji pill components in normal and chronic visceral hypersensitivity irritable bowel syndrome rats attributable to changes in tight junction and transporters. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.948678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni J., Wu G.D., Albenberg L., Tomov V.T. Gut microbiota and IBD: causation or correlation?. Nat Rev Gastroenterol Hepatol. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H.M., Wang K., Hao M.D., Liu Y., Liang X.Q., Yuan D.J., et al. The role of intestinal microecology in inflammatory bowel disease and colorectal cancer: a review. Medicine (Baltim) 2023;102 doi: 10.1097/MD.0000000000036590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin J.J., Li R.Q., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sender R., Fuchs S., Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Cani P.D., Van Hul M., Lefort C., Depommier C., Rastelli M., Everard A. Microbial regulation of organismal energy homeostasis. Nat Metab. 2019;1:34–46. doi: 10.1038/s42255-018-0017-4. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y., Lee H.Y. Revisiting the bacterial phylum composition in metabolic diseases focused on host energy metabolism. Diabetes Metab J. 2020;44:658–667. doi: 10.4093/dmj.2019.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashraf R., Shah N.P. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr. 2014;54:938–956. doi: 10.1080/10408398.2011.619671. [DOI] [PubMed] [Google Scholar]
- 50.Curro D., Ianiro G., Pecere S., Bibbo S., Cammarota G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br J Pharmacol. 2017;174:1426–1449. doi: 10.1111/bph.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan L., Ma M.F., Wang Y.M., Dai W., Fu T.Y., Wang L.H., et al. Polyguluronate alleviates ulcerative colitis by targeting the gut commensal Lactobacillus murinus and its anti-inflammatory metabolites. Int J Biol Macromol. 2024;257 doi: 10.1016/j.ijbiomac.2023.128592. [DOI] [PubMed] [Google Scholar]
- 52.Tang C., Kamiya T., Liu Y., Kadoki M., Kakuta S., Oshima K., et al. Inhibition of Dectin-1 signaling ameliorates colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe. 2015;18:183–197. doi: 10.1016/j.chom.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Zelante T., Iannitti R.G., Cunha C., De Luca A., Giovannini G., Pieraccini G., et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Villablanca E.J., Selin K., Hedin C.R.H. Mechanisms of mucosal healing: treating inflammatory bowel disease without immunosuppression?. Nat Rev Gastroenterol Hepatol. 2022;19:493–507. doi: 10.1038/s41575-022-00604-y. [DOI] [PubMed] [Google Scholar]
- 55.Lindemans C.A., Calafiore M., Mertelsmann A.M., O'Connor M.H., Dudakov J.A., Jenq R.R., et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei Y.L., Chen Y.Q., Gong H., Li N., Wu K.Q., Hu W., et al. Fecal microbiota transplantation ameliorates experimentally induced colitis in mice by upregulating AhR. Front Microbiol. 2018;9:1921. doi: 10.3389/fmicb.2018.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jennis M., Cavanaugh C.R., Leo G.C., Mabus J.R., Lenhard J., Hornby P.J. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil. 2018;30 doi: 10.1111/nmo.13178. [DOI] [PubMed] [Google Scholar]
- 58.Blair S.A., Kane S.V., Clayburgh D.R., Turner J.R. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 59.Su L.P., Nalle S.C., Shen L., Turner E.S., Singh G., Breskin L.A., et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scott S.A., Fu J.J., Chang P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2020;117:19376–19387. doi: 10.1073/pnas.2000047117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J.J., Zhang L., Wu T., Li Y.F., Zhou X.J., Ruan Z. Indole-3-propionic acid improved the intestinal barrier by enhancing epithelial barrier and mucus barrier. J Agric Food Chem. 2021;69:1487–1495. doi: 10.1021/acs.jafc.0c05205. [DOI] [PubMed] [Google Scholar]
- 62.Lamas B., Richard M.L., Leducq V., Pham H.P., Michel M.L., Da Costa G., et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu L.C. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci. 2018;25:79. doi: 10.1186/s12929-018-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun X.J., Zhang Y., Cheng G., Zhu T.X., Zhang Z.G., Xiong L., et al. Berberine improves DSS-induced colitis in mice by modulating the fecal-bacteria-related bile acid metabolism. Biomed Pharmacother. 2023;167 doi: 10.1016/j.biopha.2023.115430. [DOI] [PubMed] [Google Scholar]
- 65.Yang T., Qin N.P., Liu F.H., Zhao Y.H., Liu W.N., Fan D.M. Berberine regulates intestinal microbiome and metabolism homeostasis to treat ulcerative colitis. Life Sci. 2024;338 doi: 10.1016/j.lfs.2023.122385. [DOI] [PubMed] [Google Scholar]
- 66.Wang X., Zhu M., Dong S.J., Xu Y.Y., Jing W.H., Wang S.C. The regulatory effect of paeoniflorin on gut microbiota and bile acid metabolism in colitis mice. Acta Pharm Sin. 2021;56:1811–1819. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.