Abstract
Colonic mucosal healing is the ultimate goal of ulcerative colitis (UC) treatment, but it remains difficult to realize. Given the higher incidence of UC in males and the beneficial effect of estrogen on UC, we conducted this study to examine the therapeutic potential of estrogen receptor β (ERβ), the primary ER subtype in colon, on mucosal healing in UC. Our study is the first to report that ERβ activation degree was positively correlated with mucosal healing in patients with UC. Furthermore, ERβ activation enhanced mucosal healing in mice with dextran sulfate sodium-induced and biopsy-induced colonic injuries. Mechanistically, ERβ activation promoted autophagy of colonic epithelial cells by inhibiting branched-chain amino acid transport, leading to focal adhesion kinase (FAK) activation. Activated FAK promoted focal adhesion turnover and colonic epithelial cell migration, ultimately facilitating mucosal healing. ERβ−/− colitis mice exhibited impaired mucosal healing compared to wild-type littermates, highlighting the crucial effect of ERβ. Importantly, combination with ERβ-agonist diarylpropionitrile enhanced the amelioration of 5-aminosalicylic acid, a standard UC treatment agent, against mouse colitis. These findings attest to the crucial role of ERβ activation in colonic mucosal healing and may further inform the development of novel strategies for UC treatment.
Key words: Ulcerative colitis, Mucosal healing, Estrogen receptor β, Large neutral amino acid transporter 1, Branched-chain amino acids transport, Autophagy, Focal adhesion kinase, Cell migration
Graphical abstract
ERβ activation promoted autophagy of colonic epithelial cells by inhibiting branched-chain amino acid transport, leading to FAK activation and epithelial cell migration to facilitate mucosal healing in colon.
1. Introduction
Ulcerative colitis (UC) is a disease that is characterized by colonic mucosal ulceration and chronic inflammation1. The colonic mucosal barrier, primarily consisting of tightly arranged epithelial cells, serves as the final defense in protecting the body from harmful intestinal substances. When the colonic mucosa sustains damage, intestinal antigens penetrate into the lamina propria, triggering the activation of immune cells and precipitating intestinal inflammation2.
Immunosuppressive and anti-inflammatory therapies have historically served as the cornerstone of UC treatment, but they elicit a poor response in a considerable portion of patients, possibly because they do not directly promote mucosal healing. This limitation allows for the continued invasion of intestinal antigens into the lamina propria, leading to persistent intestinal inflammation3,4. Furthermore, patients with UC who exhibit mucosal healing display notably lower rates of complications, hospitalization risks, and surgical interventions compared to those without mucosal healing, indicating that mucosal healing is a critical therapeutic endpoint in clinical treatment5. Therefore, developing and identifying drugs that can directly promote mucosal repair are crucial for improving therapeutic outcome of UC and patient's quality of life.
UC incidence exhibits a noteworthy gender disparity, with a male-to-female ratio of 2.4:1 among patients in mainland China6. An epidemiological survey encompassing 11 countries in Asia indicated that the incidence of UC is 42% higher in males than in females7. The clinical observations have shown that UC symptoms often worsen during menopause or menstruation due to decreased estrogen levels, whereas symptoms tend to improve during pregnancy or estrogen replacement therapy when estrogen levels rise8,9. Estrogen exerts its biological functions through binding to three estrogen receptors subtypes: G protein-coupled estrogen receptors, estrogen receptor α and estrogen receptor β (ERβ). In colonic tissues, especially within colonic epithelial cells, ERβ is the primary subtype10,11. Moreover, ERβ activation has been reported to ameliorate colitis in model animals, but its role in mucosal healing is unclear12. As the promotion of mucosal healing is the ultimate goal of UC treatment, it is crucial to more deeply investigate the potential and mechanisms of ERβ activation in colonic wound healing.
Findings of the present study are the first to confirm a positive correlation between ERβ activation degree in colonic epithelial cells and mucosal healing in patients with UC. The activation of ERβ can enhance autophagy by suppressing branched-chain amino acid transport, thereby accelerating colonic epithelial cell migration and ultimately promoting mucosal healing. Additionally, combining the ERβ-agonist with 5-aminosalicylic acid (5-ASA), the first-line UC therapeutic agent, generates a synergistic anti-colitis effect, which may become a novel and improved therapeutic strategy for UC.
2. Materials and methods
2.1. Human samples
Human colonic biopsy tissue sections were collected from 6 UC patients and 6 healthy controls at Jiangsu Province Hospital of Traditional Chinese Medicine (Nanjing, China). This human study adhered strictly to the ethical principles outlined in the Declaration of Helsinki and received official approval from the Ethics Committee of Jiangsu Provincial Hospital of Traditional Chinese Medicine (Approval Number: 2022NL-135-02). Prior to the participation, all patients and healthy controls were thoroughly informed about the content and objectives of the study to ensure the compliance of the research and the protection of patients’ rights.
2.2. Immunofluorescence staining of colon tissue sections
Colon tissue sections underwent dewaxing using graded ethanol, followed by antigen retrieval in citrate buffer at 95 °C. After cooling, the sections were blocked with 3% bovine serum albumin solution for 1 h at room temperature to minimize nonspecific binding. Subsequently, the sections were delicately coated with primary antibodies and incubated at 37 °C for 2 h in humidified chamber to allow for specific antigen-antibody interactions. Thereafter, fluorescent secondary antibodies were added dropwise, and the sections were incubated in a dark environment at 37 °C for additional 2 h to amplify the fluorescence signal. Finally, DAPI (Beyotime Biotechnology, Shanghai, China) solution was added dropwise, and the sections were incubated in a dark environment at 37 °C for 15 min to stain the nuclei. Images were captured using LMS800 laser confocal fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany), and the average fluorescence intensity of each image was analyzed using ImageJ software (version: 1.53q; NIH, Bethesda, USA).
2.3. Animals
Male C57BL/6 mice aged 8 weeks were purchased from Yangzhou University (Yangzhou, China). Additionally, ERβ+/− mice aged 8 weeks were purchased from Shanghai Model Organisms Center (Shanghai, China). To generate ERβ−/− mice, male and female ERβ+/− mice were crossed, and tail tissue samples were collected from their offspring for genotyping purposes (Supporting Information Fig. S1A and S1B). Littermate wild-type mice were retained as comparative controls. Moreover, flow cytometry was used to detect the frequencies of CD4+ T cells, CD8+ T cells, B cells, macrophages, neutrophils and monocytes in the spleens and mesenteric lymph nodes of ERβ−/− mice and their littermate wild-type mice. The results show that the knockout of ERβ did not cause significant abnormalities in the immune system of mice (Fig. S1C–S1E).
The mice were raised in a stable and comfortable environment at a constant temperature of 24 °C, and provided with free access to water and standard feed. This animal study underwent rigorous ethical scrutiny and approval by the Animal Ethics Committee of China Pharmaceutical University (Approval Number: 2022-08-013).
2.4. Induction of colitis in mice and drug administration
To assess the effect of ERβ activation on mucosal healing in mice with colitis, the mice were randomly assigned to 8 groups: normal group, dextran sulfate sodium (DSS; MP Biomedicals, Aurora, USA)-induced colitis group, diarylpropionitrile (DPN; 1, 2.5, 5 mg/kg; Sigma–Aldrich, St. Louis, MO, USA) groups and liquiritigenin (LIQ; 10, 20, and 40 mg/kg; Yuanye Biotechnology, Shanghai, China) groups, with 15 mice in each group. The mice consumed 3.5% DSS solution freely for 5 days to induce colitis. Starting from the 6th day, the mice were administered DPN (intraperitoneal injection) and LIQ (oral administration) once daily for 5 consecutive days. On the 10 th day, all mice were euthanized for subsequent analysis. DPN was dissolved in DMSO and then diluted with isotonic saline (final DMSO concentration of 1%), while LIQ was fully suspended in 0.5% CMC-Na.
To investigate the effect of ERβ knockout on mucosal healing of colitis mice, littermate wild type mice and ERβ−/− mice were randomly assigned to 5 groups: normal group, DSS-induced colitis group, DPN (2.5 mg/kg) group and ERβ−/− group, ERβ−/− + DPN group. DSS-induced colitis and the administration of DPN in mice were carried out in accordance with established protocols. On the 10th day, all mice were euthanized for further analysis.
To assess the therapeutic synergies of combining the ERβ-specific agonist DPN with the widely used anti-inflammatory agent 5-ASA (Ethypharm, Shanghai, China) against colitis, mice were randomly assigned to 5 groups: normal group, DSS-induced colitis group, DPN (2.5 mg/kg) groups, 5-ASA (100 mg/kg) group and DPN + 5-ASA group. 5-ASA was fully suspended in 0.5% CMC-Na. DSS-induced colitis and the administration of DPN in mice were carried out in accordance with established protocols. On the 10th day, all mice were euthanized for further analysis.
2.5. FITC-dextran intestinal permeability assay
On the 10th day of the DSS-induced colitis model, mice were orally administered with 44 mg/100 g of fluorescein isothiocyanate (FITC)-dextran (dissolved in water; Puch Biotechnology, Chengdu, China). After 4 h, the mice were euthanized, and blood samples were collected for serum preparation. The concentration of FITC-dextran in the serum was accurately measured at the wavelength of 485/530 nm13.
2.6. Colonic endoscope
A high-resolution, miniaturized colonoscopic system equipped with biopsy forceps (Vilber Lourmat, Paris, France) was used to capture images of colonic mucosa on Days 6, 8 and 10 in mice with DSS-induced colitis. The percentage of wound healing was quantitatively analyzed as previously reported14.
For biopsy experiment, mucosal injuries were created in descending colon of each mouse using the biopsy forceps. Images of the injured mucosa in mice were captured at 24 and 72 h post-injury, and the percentage of wound healing was quantitatively analyzed as previously reported14.
2.7. Extraction of primary mouse epithelial cells
The colonic tissues of mice were minced into 1 mm3 fragments and thoroughly washed in phosphate buffered saline containing 1 × penicillin–streptomycin–amphotericin (Shenggong Biotechnology, Shanghai, China). Subsequently, the tissue fragments were placed into mixed digestion solution containing 0.1 mg/mL neutral protease (Shenggong Biotechnology) and 0.1 mg/mL collagenase XI (Shenggong Biotechnology), maintained at 37 °C for 45 min. After digestion, the mixture was centrifuged and the cell pellet was resuspended. The purity of colon epithelial cells was determined using flow cytometry. Only samples with exceeding 90% purity were deemed suitable for subsequent experiments (Fig. S1F).
2.8. Cell culture
Normal human colonic epithelial cells (NCM460 cells) and human colorectal cancer epithelial cells (HT-29 cells) were purchased from the Chinese Academy of Medical Sciences (Beijing, China) in 2022 and underwent short tandem repeats sequencing for authentication. These cells were cultured in RPMI 1640 medium (Gibco, Grand Island, USA) supplemented with 10% fetal bovine serum (Gibco), and maintained at 37 °C with 5% CO2. Once the cell density reached 1 × 106 cells/mL, a 0.1% trypsin solution (Gibco) was utilized for digestion and cell passage. Typically, the passaging ratio is 1:2 to 1:3, and passage was conducted every 2–3 days to ensure that the cells remain in the logarithmic growth phase and avoid excessive proliferation. Throughout the experimental period, no mycoplasma infection was warranted.
2.9. Focal adhesion turnover assay
NCM460 cells transfected paxillin-pmCherry plasmid (Synbio Technologies, Suzhou, China) were seeded onto confocal dish coated with 10 μg/mL fibronectin (Yeasen, Shanghai, China). Subsequently, LMS800 confocal fluorescence microscopy was used to capture cell images every 4 min for a duration of 1 h. For analysis, only focal adhesions that could be fully traced from their appearance to disappearance were measured, with 1–2 focal adhesions were measured per cell. Afterwards, the fluorescence intensity curves of focal adhesion over time were plotted using GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA), and the assembly and disassembly time of focal adhesions was calculated15,16.
2.10. Autophagy flux assay
NCM460 cells transfected pmCherry-EGFP-Light chain 3 (LC3) plasmid (Synbio Technologies) were seeded onto confocal dish. They were fixed with 4% formaldehyde (Sigma–Aldrich) for 15 min, and then the cell nuclei were stained using DAPI reagent. Finally, LMS800 confocal fluorescence microscopy was employed to capture images.
2.11. Transmission electron microscopy
NCM460 cells were collected and fixed with 2.5% glutaraldehyde solution (Powerful Biology, Wuhan, China) and 1% osmic acid (Powerful Biology), each for 1 h. The samples underwent dehydration and were consecutively transferred to infiltrating agents with acetone-epoxy resin ratios of 2:1 and 1:1. Fully infiltrated samples were embedded in epoxy resin and securely placed in embedding molds. The samples were then polymerized in a 60 °C incubator for 48 h. Finally, ultra-thin sections were meticulously prepared using an ultramicrotome (Leica, Wetzlar, Germany) and observed under a transmission electron microscope (FEI, Hillsboro, USA).
2.12. Cut & Tag
Cells were collected, washed with 500 μL of washing buffer and centrifuged to remove supernatants. Con A-coated magnetic beads (Vazyme, Nanjing, China) were prepared in accordance with the manufacturer's guidelines, and 10 μL of activated magnetic beads were added to each sample for incubation at room temperature for 10 min. Subsequently, the cells were co-incubated with ERβ antibody, secondary antibody (goat anti-rabbit IgG H&L; Vazyme), and hyperactive PG-TN5/PA-TN5 transposase (Vazyme), in accordance with the kit's instructions. The cellular fragments were then generated, and DNA fragments were extracted from the samples for qPCR assay.
2.13. Transcriptomics assay
Total RNA was extracted from both DPN-treated NCM460 cell samples and untreated control samples utilizing Trizol reagent. To accurately determine the concentration and purity of total RNA in each sample, the Nano 300 spectrophotometer (Allsheng Instruments, Hangzhou, China) was employed. The total RNA samples were submitted to Shanghai Bioprofile Technology for professional processing. It is noteworthy that sequencing data typically contain low-quality reads, which could potentially compromise the accuracy of downstream analysis. The Cutadapt software (version: 2.7; Max Planck Institute, Heidelberg, Germany) was used to filter the sequencing data to obtain high quality sequence for further analysis. To identify the significance of differentially expressed genes between the two groups, we adopted criteria of |log2FoldChange| ≥ 1 and P-value less than 0.05. Furthermore, a comprehensive functional enrichment analysis was conducted to ensure the accuracy and reliability of the results.
2.14. Metabolomics assay
The polar metabolites in DPN-treated NCM460 cell samples and untreated control samples were extracted through repeated freeze-thaw cycles with addition of pre-cooled MeOH–H2O (8:2). The samples were vortexed for 1 min, followed by centrifugation to collect the supernatant. The supernatant extract was then transferred to Shanghai Bioprofile Technology for professional processing. To identify the significance of differentially metabolites between the two groups, we adopted criteria of |log2FoldChange| ≥ 1 and P-value less than 0.05. Kyoto encyclopedia of genes and genomes (KEGG) was used to identify enriched metabolic pathways.
2.15. Statistical analysis
The data are expressed in the format of mean ± standard error of the mean (SEM) to show clarity and reproducibility. To assess the differences among groups, we employed one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. A P-value below 0.05 was considered statistically significant. Correlation between various factors was analyzed by Pearson correlation analysis.
Other methods and materials are provided in the online supporting information.
3. Results
3.1. Correlation between ERβ activation in colonic epithelial cells and mucosal healing in patients with UC and colitis mice
To evaluate ERβ activation changes within the colon epithelial cells of patients with UC, we employed immunofluorescence staining for epithelial cell adhesion molecule (Ep-CAM; an epithelial cell marker) and ERβ in colon sections from patients with UC and healthy controls. Colocalization staining showed a notably lower ERβ expression within the colonic epithelium of patients with UC compared to healthy controls, with the lowest levels observed in the lesion areas (Fig. 1A–C). To confirm this finding, we analyzed the messenger RNA (mRNA) expression of ESR2 (the gene encoding ERβ expression) and its target gene Caveolin-1 (CAV1) in the Gene Expression Omnibus (GEO: GSE206285 and GSE59071) database, and found a significant expression downregulation of both ESR2 and CAV1 in the colonic biopsy mucosal tissues of patients with UC compared to those in healthy controls (Fig. 1D, Supporting Information Fig. S2A). A further correlation analysis demonstrated a positive correlation between the expression of ESR2 and CAV1 with the expression of mucosal healing-related factors (trefoil factor 3 [TFF3], epidermal growth factor [EGF], and occludin) (Fig. 1E–G, Fig. S2B–S2G).
Figure 1.
Correlation between ERβ expression in colonic epithelial cells and mucosal healing in UC patients and colitis mice. (A–C) Expression levels of ERβ and epithelial cell marker EP-CAM in colon tissue sections of health controls and UC patients were detected by co-localization immunofluorescence staining (n = 6; ##P < 0.01 versus healthy controls group; plotting scale: 100 μm). (D–G) Correlation between mucosal healing-related factors levels and ESR2 expression in colonic biopsy tissues of healthy controls and UC patients was analyzed by Pearson correlation analysis (GEO profile GES206285). (H–J) Expression levels of ERβ and epithelial cell marker EP-CAM in colon tissue sections of DSS-induced colitis mice and normal mice were detected by co-localization immunofluorescence staining (n = 12; ##P < 0.01 versus normal mice; plotting scale: 100 μm). (K) Quantification of Esr2 expression in the primary colonic epithelial cells originating from normal mice and DSS-induced colitis mice was conducted using RT-qPCR (n = 12; ##P < 0.01 versus normal mice). (L–N) Correlation between serum level of FITC-dextran, ulcer score, percentage of endoscopic wound healing and Esr2 expression in colitis mice and normal mice was analyzed by Pearson correlation analysis. (O–Q) Correlation between mucosal healing-related factor levels and Esr2 expression in colitis mice and normal mice was analyzed by Pearson correlation analysis. The data are expressed as means ± SEM.
The expression of Esr2 and Cav1 in the colonic epithelial cells of mice with DSS-induced colitis notably decreased, particularly in lesion regions, which was consistent with the findings in patients with UC (Fig. 1H–K, Fig. S2H). Subsequently, we evaluated several indicators reflecting colonic mucosal barrier injury and wound healing in colitis mice, including FITC-dextran permeability, ulcer scores derived from hematoxylin and eosin staining (H&E staining), and endoscopic wound healing (Fig. S2I–S2K). Correlation analysis revealed that Esr2 and Cav1 expression levels negatively correlated with FITC-dextran permeability and ulcer score but positively correlated with the percentage of mucosal healing (Fig. 1L–N, Fig. S2L–S2N). The expression levels of mucosal healing factors in colonic tissue of colitis mice were reduced and positively correlated with Esr2 and Cav1 expression, which was consistent with findings in humans (Fig. 1O–Q, Fig. S2O–S2T).
Our findings demonstrate that there is a positive correlation between ERβ activation and mucosal healing in both UC patients and colitis mice, suggesting that ERβ activation is a promising approach for facilitating mucosal healing in UC treatment.
3.2. ERβ activation promoted colonic mucosal healing in vivo
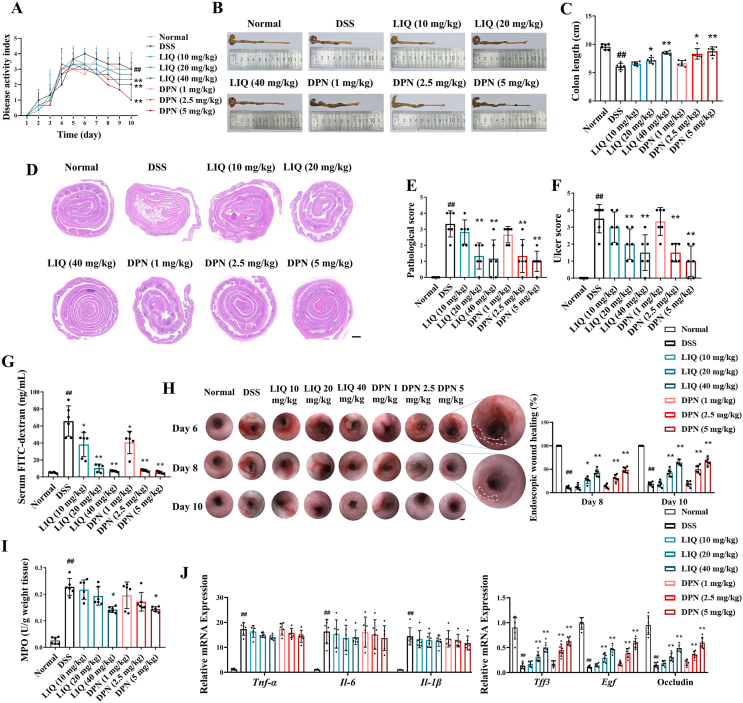
To ascertain the impact of ERβ activation on mucosal healing under the status of UC, we employed a specialized colitis model simulating mucosal injury and subsequent repair by adding DSS to drinking water and subsequently switching back to regular water. ERβ-agonists DPN (1, 2.5, and 5 mg/kg) and LIQ (10, 20, and 40 mg/kg) were administered during the repair phase. The results show that DPN and LIQ administration effectively reduced colon shortening, disease activity index (DAI) scores, and pathological scores in colitis mice (Fig. 2A–E).
Figure 2.
ERβ activation promoted colonic mucosal healing in mice with DSS-induced colitis. (A) The mice were provided with water containing 3.5% DSS for 5 days, and normal water for another 5 days. DPN (1, 2.5, 5 mg/kg, intraperitoneal injection) and LIQ (10, 20, 40 mg/kg, oral gavage) were given from Day 6 to Day 10 after induction of colitis, and the DAI score was evaluated. (B, C) The length of colons. (D–F) Representative images of Swiss roll colon section stained with H&E, alongside pathological scores and ulcer scores (plotting scale: 200 μm). (G) Assessment of FITC-dextran permeability across the colonic mucosal barrier. (H) Representative images of colonic endoscope displaying ulcer regions and quantifying the percentage of wound healing (plotting scale: 200 μm). (I) MPO activity of colon tissues. (J) Expression of mucosal healing-related factors and pro-inflammatory factors in colon tissues was conducted using RT-qPCR. The data are expressed as mean ± SEM (n = 6). Significant differences are designated as follows: ##P < 0.01 versus the Normal group; ∗P < 0.05 and ∗∗P < 0.01 versus the DSS group.
To characterize the effect of ERβ activation on mucosal healing, additional experiments were conducted. We observed that DPN and LIQ significantly downregulated the mucosal damage indicators, including ulcer scores and FITC-dextran intestinal permeability (Fig. 2F and G). Moreover, treatments with DPN and LIQ accelerated ulcer wound healing in colitis mice according to colonic endoscope images (Fig. 2H). To further investigate the effect of DPN and LIQ on mucosal healing, we labeled the epithelial cells in colon sections using EP-CAM. It was shown that LIQ (20 mg/kg) and DPN (2.5 mg/kg) could significantly promote epithelial restoration in ulcerated areas, and LIQ (40 mg/kg) and DPN (5 mg/kg) not only facilitated epithelial restoration but also enhanced crypt regeneration in ulcerated areas (Supporting Information Fig. S3). Interestingly, DPN and LIQ significantly upregulated mucosal healing factor expression while exerting only a mild inhibitory effect on myeloperoxidase (MPO) activity (a marker of neutrophil infiltration) and expression of pro-inflammatory cytokines (interleukin [Il]-6, Il-1β, and tumor necrosis factor [Tnf]-α) within the colon tissues of colitis mice (Fig. 2I and J). The findings suggest that ERβ activation can directly facilitate mucosal healing in colitis mice independent of its anti-inflammatory effect.
To further confirm the direct effect of ERβ activation on mucosal healing, we used a colonic biopsy-injured mouse model. Biopsy wounds were created on the descending colon mucosa of mice using biopsy scissors, 24 h after which DPN and LIQ were administered for three consecutive days. Images of the injured mucosa were captured on Days 1 and 3 post-injury (Fig. 3A and B). Image analysis revealed that DPN and LIQ significantly facilitated the healing of the mucosal biopsy wounds in mice (Fig. 3C and D). Ep-CAM staining further demonstrated that LIQ and DPN substantially shortened the length of biopsy wound cross-sections (Fig. 3E and F).
Figure 3.
ERβ activation promoted colonic mucosal healing in mice with biopsy-induced colonic injury. (A, B) Employing endoscope and biopsy scissors, biopsy lesions were generated within the dorsal aspect of the mucosa in the descending colon of anesthetized mice on Day 0. DPN (2.5 mg/kg, intraperitoneal injection) and LIQ (20 mg/kg, oral gavage) were given after 24 h of biopsy lesion generating. (C, D) Representative images of colonic endoscope displaying biopsy lesions on Days 1 and 3, and quantification of the wound healing percentage (plotting scale: 200 mm). (E, F) Representative images of immunofluorescence staining biopsy lesions on Day 3 and quantification of the length (plotting scale: 100 μm). The data are expressed as mean ± SEM (n = 6). Significant differences are designated as follows: ∗∗P < 0.01 versus the Control group.
In conclusion, ERβ activation can effectively and directly enhance colonic mucosal healing in vivo.
3.3. ERβ activation promoted colonic epithelial cell migration and wound healing through accelerating focal adhesion turnover
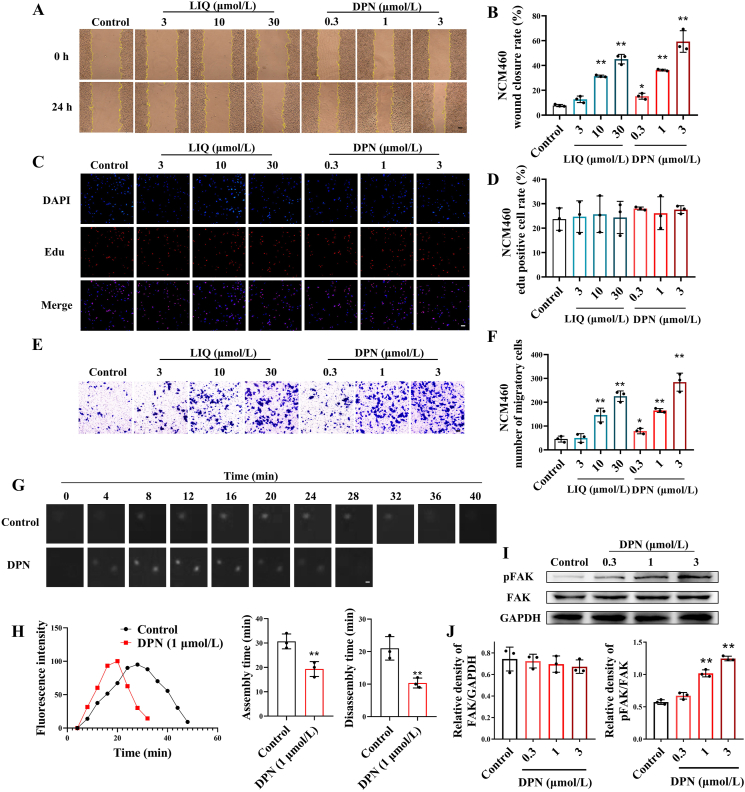
We investigated the in vitro effect of ERβ activation on colonic epithelial cell wound healing. Scratch assays revealed that DPN and LIQ could significantly promote wound healing in both NCM460 and HT-29 cells (Fig. 4A and B, Supporting Information Fig. S4A–S4C). We next assessed the effect of ERβ activation on colonic epithelial cell migration and proliferation, which are two pivotal factors contributing to wound healing17. The results show that both DPN and LIQ promoted colonic epithelial cell wound healing by enhancing cell migration, but not proliferation (Fig. 4C–F, Fig. S4D and S4E).
Figure 4.
ERβ activation promoted focal adhesion turnover, cell migration and wound healing of colonic epithelial cells. NCM460 cells were treated with DPN (0.3, 1, and 3 μmol/L) and LIQ (3, 10, and 30 μmol/L) for 24 h. (A, B) Wound healing rate of NCM460 cells (plotting scale: 50 μm). (C, D) Proliferation rate of NCM460 cells (plotting scale: 50 μm). (E, F) Migration of NCM460 cells (plotting scale: 50 μm). (G) Confocal microscopy time-lapse sequences of NCM460 cells expressing paxillin-mCherry (white) to monitor focal adhesion dynamics (plotting scale: 1 μm). (H) Example plots of paxillin-mCherry fluorescence intensity (y axis) over time (x axis), and statistics of focal adhesion assembly and depolymerization time. (I, J) Expression levels of FAK and p-FAK in NCM460 cells were detected by Western blotting. The data are expressed as mean ± SEM (n = 3). Significant differences are designated as follows: ∗P < 0.05 and ∗∗P < 0.01 versus the Control group.
The process of cell migration comprises 4 steps: pseudopod extension, focal adhesion assembly for stable anchoring, cytosolic contraction to generate migratory force, and focal adhesion disassembly to facilitate cell movement forward18. Focal adhesions turnover, a dynamic process involving assembly and disassembly, is the rate-limiting step in cell migration, which is regulated by focal adhesion kinase (FAK)19,20. To investigate the impact of ERβ activation on focal adhesion turnover, we transfected NCM460 cells with paxillin-pmCherry plasmid to visualize the focal adhesion component protein paxillin. Utilizing the time-lapse functionality of fluorescent confocal microscope, we captured images of the cells every 4 min for 1 h, observing focal adhesion assembly and disassembly. Analysis of the fluorescence intensity curve over time revealed that DPN significantly shortened focal adhesion assembly and disassembly processes (Fig. 4G and H). Moreover, DPN profoundly facilitated the activation of FAK (Fig. 4I and J). Combination with either FAK-specific inhibitor PF-573228 or siFAK weakened the acceleration of focal adhesion turnover, migration, and wound healing by DPN in NCM460 cells (Fig. 5A–F, Supporting Information Fig. S5A–S5F).
Figure 5.
ERβ activation promoted colonic epithelial cell migration and wound healing by accelerating focal adhesion turnover. NCM460 cells were treated with DPN (1 μmol/L) for 24 h in the presence or absence of siFAK. (A) Confocal microscopy time-lapse sequences of NCM460 cells expressing paxillin-mCherry (white) to monitor focal adhesion dynamics (plotting scale: 1 μm). (B–D) Example plots of paxillin-mCherry fluorescence intensity (y axis) over time (x axis), and statistics of focal adhesion assembly and depolymerization time. (E) Migration of NCM460 cells (plotting scale: 50 μm). (F) Wound healing rate of NCM460 cells (plotting scale: 50 μm). The data are expressed as mean ± SEM (n = 3). Significant differences are designated as follows: ∗∗P < 0.01 versus the Control group; nsP > 0.05 not significant versus the siFAK group.
In summary, the activation of ERβ accelerates focal adhesion turnover through activating FAK, ultimately promoting colon epithelial cell migration and wound healing in vitro.
3.4. ERβ activation accelerated focal adhesion turnover in colonic epithelial cells by enhancing autophagy
To further investigate how ERβ activation expedites focal adhesion turnover, we used a transcriptomic assay to compare the transcriptional profiles of NCM460 cells treated with DPN to those of untreated controls. Analysis of RNA-sequencing data revealed that autophagy-related genes were significantly upregulated in NCM460 cells treated with DPN. Notably, autophagy signaling emerged as the most significantly enriched pathway among the differentially expressed genes in both the Gene Ontology classification and KEGG analysis (Fig. 6A–C).
Figure 6.
ERβ activation enhanced autophagy in colonic epithelial cells and activated FAK by promoting autophagosome formation. NCM460 cells were treated with DPN (1 μmol/L) for 24 h in the presence or absence of 3-MA and CQ. (A) Volcano plot depiction of the differentially expressed genes between NCM460 cells treated with DPN and untreated NCM460 cells. (B) Scatter plots of enrichment results for the KEGG pathways and GO function enrichment for the NCM460 cells treated with DPN compared with the untreated NCM460 cells. The x-axis represents the rich factor, which calculates the proportion of differentially expressed genes to the total gene count within each KEGG or GO pathway. The color of each dot signifies the FDR value, while the dot's size corresponds to the number of genes associated with each pathway. (C) Relative changes of some representative genes in the screened autophagy signaling pathway. (D) Expression levels of LC3, ATG5, Beclin1 and SQSTM1 in NCM460 cells were detected by Western blotting. (E) Autophagosomes and autolysosomes in NCM460 cells were determined by transmission electron microscopy analysis (plotting scale: 1 μm). (F) NCM460 cells were stably transferred with pmCherry-EGFP-LC3 plasmid, and the autophagosomes (yellow fluorescence) and autolysosomes (red fluorescence) spots were observed by confocal microscopy (plotting scale: 5 μm). (G, H) Expression levels of FAK and p-FAK in NCM460 cells were detected by Western blotting. (I) The total protein of NCM460 cells was isolated and immunoprecipitated with antibody against FIP200, and the expression levels of FAK and FIP200 were analyzed by Western blotting. (J) Representative immunostaining images of NCM460 cells for FAK (red), FIP200 (green). The R represents Pearson's correlation CR coefficient value to show the signal colocalization status (plotting scale: 5 μm). The data are expressed as means ± SEM (n = 3). Significant differences are designated as follows: ∗P < 0.05 and ∗∗P < 0.01 versus the Control group; nsP > 0.05 not significant versus the 3-MA group; #P < 0.05 versus the CQ group.
The autophagy-related proteins Beclin1 and autophagy-related gene 5 (ATG5) were upregulated in NCM460 cells treated with DPN, which corroborated our transcriptomic findings. DPN also facilitated the conversion of LC3Ⅰ to LC3Ⅱ and accelerated sequestosome 1 (SQSTM1) degradation (Fig. 6D). Using transmission electron microscopy, we observed an increased autophagosomes and autolysosomes in DPN-treated NCM460 cells compared to controls (Fig. 6E). Transfection of NCM460 cells with the pmCherry-EGFP-LC3 plasmid caused LC3 to spontaneously express red (pmCherry) fluorescence and green (EGFP) fluorescence. Yellow puncta appeared when LC3 assembled into autophagosomes due to the overlay of the two types of fluorescence. As autophagosomes merged with acidic lysosomes and generated autolysosomes, the green fluorescence (sensitive to environmental pH) was quenched, resulting in the appearance of red fluorescent puncta. Confocal microscopy images revealed an increase in autophagosome and autolysosome formation in DPN-treated NCM460 cells (Fig. 6F).
Next, we investigated whether ERβ activation stimulates FAK through activating autophagy by treating NCM460 cells with the autophagy inhibitor 3-methyladenine (3-MA) and the autophagic flux (the fusion of autophagosomes with lysosomes to form autolysosomes) inhibitor chloroquine (CQ). 3-MA suppressed the stimulatory effect of DPN on FAK activation, while CQ had no significant impact, suggesting ERβ stimulates FAK mainly through autophagosome formation (Fig. 6G and H).
FAK-family interacting protein of 200 kDa (FIP200), an autophagy-related protein, binds to FAK and suppresses its activation under physiological conditions21,22. We next investigated the interaction between FAK and FIP200 during ERβ-induced autophagy. Coimmunoprecipitation assay revealed that DPN treatment significantly inhibited the interaction between FAK and FIP200 in NCM460 cells (Fig. 6I). Furthermore, the colocalization staining images clearly showed that the focal adhesions marked by FAK (red fluorescent puncta) and autophagosomes marked by FIP200 (green fluorescent puncta) were not colocalized in DPN-treated NCM460 cells (Fig. 6J). The inhibitory effect of DPN on the interaction between FIP200 and FAK, which typically facilitates focal adhesion turnover and wound healing, was notably attenuated after combination with siATG5 (Fig. 7A–F, Supporting Information Fig. S6A–S6C). 3-MA treatment demonstrated a similar effect to that of siATG5, while CQ exerted no notable influence on the effect of DPN (Supporting Information Figs. S7A–S7G, S8A–S8G).
Figure 7.
ERβ activation accelerated focal adhesion turnover in colonic epithelial cells by enhancing autophagy. NCM460 cells were treated with DPN (1 μmol/L) for 24 h in the presence or absence of siATG5. (A, B) The total protein was isolated and immunoprecipitated with antibody against FIP200, and the expression levels of FAK and FIP200 were analyzed by Western blotting. (C) Representative immunostaining images of NCM460 cells for FAK (red), FIP200 (green). The R represents Pearson's correlation CR coefficient value to show the signal colocalization status (plotting scale: 5 μm). (D) Expression levels of FAK and p-FAK in NCM460 cells were detected by Western blotting. (E) Confocal microscopy time-lapse sequences of NCM460 cells expressing paxillin-mCherry (white) to monitor focal adhesion dynamics (plotting scale: 1 μm). (F) Example plots of paxillin-mCherry fluorescence intensity (y axis) over time (x axis), and statistics of focal adhesion assembly and depolymerization time. The data are expressed as means ± SEM (n = 3). Significant differences are designated as follows: ∗P < 0.05 and ∗∗P < 0.01 versus the Control group; nsP > 0.05 not significant versus the siATG5 group.
These findings indicate that ERβ activation suppresses the interaction between FAK and FIP200 by triggering autophagy. This suppression leads to the activation of FAK, which in turn accelerates focal adhesion turnover and ultimately facilitates colonic epithelial cell migration and wound healing.
3.5. ERβ activation promoted autophagy in colonic epithelial cells by suppressing branched-chain amino acid transport
Autophagy, linked to cellular metabolism, is activated when cells lack nutrients like amino acids or fatty acids23. To explore the mechanism behind ERβ activation-induced autophagy, we employed LC–MS/MS to analyze metabolites in DPN-treated NCM460 cells and the untreated controls. Amino acids and short peptides were the most altered metabolite types after DPN treatment (Fig. 8A, Supporting Information Fig. S9). KEGG analysis further demonstrate that metabolites related to amino acid metabolism and transmembrane transport were significantly enriched (Fig. 8B). The most notable changes were observed in branched-chain amino acids (valine, leucine, isoleucine), with a significant decrease within DPN-treated NCM460 cells (Fig. 8C and D).
Figure 8.
ERβ activation suppressed branched-chain amino acid transport in colonic epithelial cells by downregulating the expression of LAT1. NCM460 cells were treated with DPN (1 μmol/L) for 24 h in the presence or absence of LAT1-Plasmid. (A) Pie chart based on the main metabolic differential types between NCM460 cells treated with DPN and untreated NCM460 cells. (B) Bar chart of enrichment results for the KEGG pathways of the NCM460 cells treated with DPN compared with the untreated NCM460 cells. The x axis shows the negative log transformation of P-values. The y axis shows the pathway. (C) Relative changes of some representative metabolites in the screened amino acids transport pathway. (D) The levels of leucine, isoleucine and valine in NCM460 cells were detected by ELISA kits. (E) Expression level of LAT1 in NCM460 cells was detected by Western blotting. (F) The DNA sequence of LAT1, marked with five silencers. (G) Schematic diagram of the Cut & Tag experimental, sourced from Vazyme Biotech Co., Ltd. Expression of LAT1 silencers binding with ERβ was detected by Cut & Tag-qPCR. (H) Expression levels of p-mTORC1 and mTORC1 in NCM460 cells were detected by Western blotting. The data are expressed as mean ± SEM (n = 3). Significant differences are designated as follows: ∗P < 0.05 and ∗∗P < 0.01 versus the Control group.
Since branched-chain amino acids are essential amino acids that must be obtained externally, we paid attention to the branched-chain amino acid transporter, large neutral amino acid transporter 1 (LAT1)24. DPN treatment in NCM460 cells led to a decrease in LAT1 expression (Fig. 8E). Upon activation by ligands, ERβ can enter the nucleus and regulate the transcription of downstream target genes through directly binding to the estrogen transcription element sequence located in the promoters, enhancers and silencers of the target genes or by interacting with other transcription factors25. ERβ activation potentially suppresses the expression of the LAT1 by binding to its silencer region. We identified 5 silencer sequences in the LAT1 gene form Genebank, and Cut & Tag assay revealed a marked enhancement in the binding of DPN-activated ERβ to silencer2 within the LAT1 gene sequence (Fig. 8F and G). These results suggest that activated ERβ binds to silencer2 on the LAT1 gene sequence, thereby suppressing the expression of LAT1 in colon epithelial cells. mTOR, a key autophagy regulator, could be activated by elevated amino acid levels26. Our result show that ERβ activation suppressed mTOR activation by inhibiting amino acid transport, thereby activating autophagy (Fig. 8H).
To validate the conclusion, we co-administered the LAT1-Plasmid with DPN in NCM460 cells. The results show that the LAT1-Plasmid reversed DPN's inhibition on amino acid transport and autophagy activation (Fig. 9A–D). LAT1-Plasmid also negated the inhibitory effect of DPN on FIP200-FAK binding and FAK activation, thereby suppressing downstream effects like focal adhesion turnover, cell migration, and wound healing. (Supporting Information Fig. S10A–S10G).
Figure 9.
ERβ activation promoted autophagy in colonic epithelial cells by suppressing branched-chain amino acid transport. NCM460 cells were treated with DPN (1 μmol/L) for 24 h in the presence or absence of LAT1-Plasmid. (A) The levels of leucine, isoleucine and valine in NCM460 cells were detected by ELISA kits. (B) Expression levels of LC3, ATG5, Beclin1 and SQSTM1 in NCM460 cells were detected by Western blotting. (C) Autophagosomes and autolysosomes in NCM460 cells were determined by transmission electron microscopy analysis (plotting scale: 1 μm). (D) NCM460 cells were stably transferred with pmCherry-EGFP-LC3 plasmid, and the autophagosomes (yellow fluorescence) and autolysosomes (red fluorescence) spots were observed by confocal microscopy (plotting scale: 5 μm). The data are expressed as mean ± SEM (n = 3). Significant differences are designated as follows: ∗P < 0.05 and ∗∗P < 0.01 versus the Control group; nsP > 0.05 not significant versus the LAT1-Plasmid group.
Finally, we conducted a joint analysis of metabolomic and transcriptomic data. The comprehensive KEGG signaling pathway enrichment analysis revealed that autophagy, branched-chain amino acid transport and the mTOR signaling pathway topped the list, ranking in the top three positions based on their highest average scores (Supporting Information Fig. S11). This finding provides further reinforcement to our previous conclusions.
The findings of this section indicate that activated ERβ binds to silencer2 within the LAT1 gene sequence, suppressing LAT1 expression, branched-chain amino acid transport and mTOR activation, ultimately leading to the activation of autophagy in colonic epithelial cells.
3.6. Insufficient ERβ activation led to increased branched-chain amino acid transport and inhibition of autophagy in colonic epithelial cells, ultimately resulting in deceleration of wound healing
To validate our previous findings, we simulated the effect of insufficient ERβ activation on wound healing by utilizing siRNA to knockdown ERβ expression in NCM460 cells, while concurrently treating with the ERβ-agonist DPN. Insufficient ERβ activation led to increased LAT1 expression, branched-chain amino acid transport, and mTOR activation in NCM460 cells compared to cells treated with DPN alone (Supporting Information Fig. S12A–S12D). Subsequently, mTOR activation due to insufficient ERβ activation led to impaired autophagy in NCM460 cells (Fig. S12E–S12G). Colocalization immunofluorescence staining and coimmunoprecipitation assays revealed that the compromised autophagy led to an elevated colocalization between FIP200 and FAK, thereby preventing the activation of FAK in NCM460 cells (Supporting Information Fig. S13A–S13C). Ultimately, the impaired activation of FAK resulted in slower focal adhesion turnover, delayed colon epithelial cell migration and wound healing (Fig. S13D–S13G). The pharmacological inhibitor of ERβ, PHTPP, exhibited similar effects with siESR2 in NCM460 cells (Supporting Information Fig. S14A–S14I).
Our findings further confirmed that ERβ activation can effectively promote wound healing in colonic epithelial cells in vitro by suppressing branched-chain amino acid transport and activating autophagy.
3.7. Colonic mucosal healing was impaired in ERβ−/− colitis mice
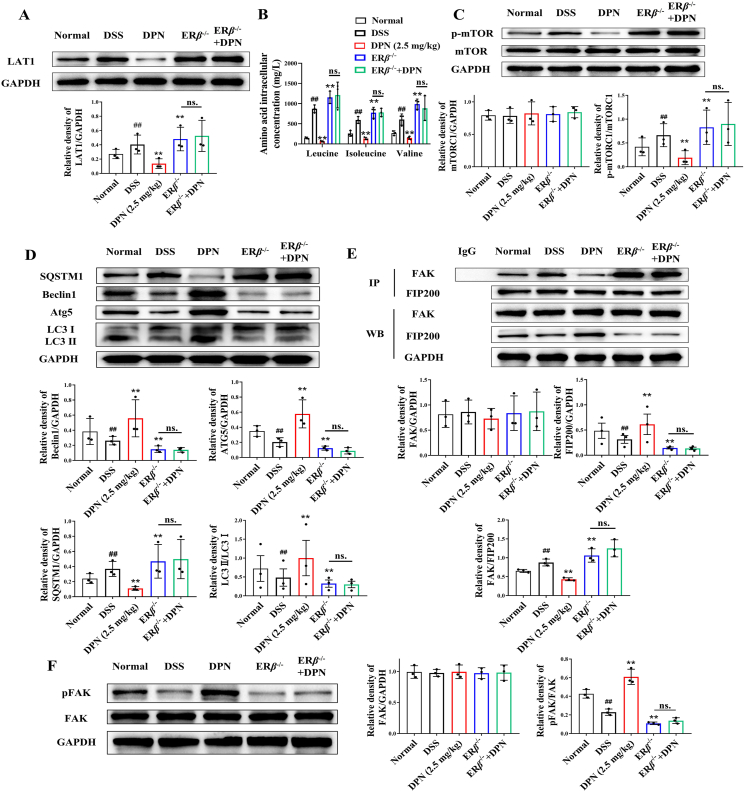
Comprehensive experiments on ERβ−/− mice were conducted to assess the effect of ERβ knockout on mucosal healing in colitis mice and to determine whether this knockout affects the wound healing-enhancing effect of the ERβ-agonist DPN. We found that ERβ−/− colitis mice exhibited higher DAI scores, higher pathological scores and shorter colon lengths compared to their wild-type colitis littermates (Fig. 10A–E). Notably, ERβ knockout completely abolished the ameliorative effect of DPN on mouse colitis.
Figure 10.
Colonic mucosal healing was impaired in ERβ−/− colitis mice. The wild type mice and ERβ−/− mice were provided with water containing 3.5% DSS for 5 days, and normal water for another 5 days. DPN (2.5 mg/kg, intraperitoneal injection) was given from Day 6 to Day 10 after induction of colitis. (A) The DAI score. (B, C) The length of colons. (D–F) Representative images of Swiss roll colon section stained with H&E, alongside pathological scores and ulcer scores (plotting scale: 200 μm). (G) Assessment of FITC-dextran permeability across the colonic mucosal barrier. (H, I) Representative images of colonic endoscope displaying ulcer regions and quantifying the percentage of wound healing (plotting scale: 200 μm). (J, K) Expression of mucosal healing-related factors and pro-inflammatory factors in colon tissues was conducted using RT-qPCR. The data are expressed as mean ± SEM (n = 6). Significant differences are designated as follows: ##P < 0.01 versus the Normal group; ∗∗P < 0.01 versus the DSS group; nsP > 0.05 not significant versus the ERβ−/− group.
ERβ−/− colitis mice exhibited more severe colonic mucosal barrier damage with increased FITC-dextran intestinal permeability and ulcer scores compared to wild-type mice (Fig. 10F and G). Colonic endoscope images and colonic Ep-CAM immunofluorescence-stained sections showed a remarkable delay in mucosal healing in ERβ−/− colitis mice compared to their wild-type colitis littermates, which was accompanied by a markedly downregulated expression of the factors related to mucosal healing (Fig. 10H–K, Supporting Information Fig. S15). Moreover, the absence of ERβ completely negated the beneficial effect of DPN on colonic mucosal barrier integrity and the promotion of mucosal healing in colitis mice.
To confirm our in vitro findings that ERβ facilitated colonic epithelial cell wound healing, we isolated primary epithelial cells from the colitis mice for further investigation. Primary colonic epithelial cells from ERβ−/− colitis mice exhibited an elevated expression of LAT1 compared to wild-type mice, leading to increased branched-chain amino acid transport and subsequent mTOR activation (Fig. 11A–C). mTOR activation suppressed autophagy, potentiating the binding between FAK and FIP200 and thereby inhibiting the activation of FAK (Fig. 11D–F). DPN could activate autophagy and FAK, which was reversed by ERβ knockout.
Figure 11.
Insufficient ERβ activation facilitated branched-chain amino acid transport, suppressed autophagy, and ultimately inhibited FAK activation in colonic epithelial cells. (A) Expression level of LAT1 in primary mouse colonic epithelial cells was detected by Western blotting. (B) The levels of leucine, isoleucine and valine in primary mouse colonic epithelial cells were detected by ELISA kits. (C) Expression levels of p-mTORC1 and mTORC1 in primary mouse colonic epithelial cells were detected by Western blotting. (D) Expression levels of LC3, ATG5, Beclin1 and SQSTM1 in primary mouse colonic epithelial cells were detected by Western blotting. (E) The total protein of primary mouse colonic epithelial cells was isolated and immunoprecipitated with antibody against FIP200, and the expression levels of FAK and FIP200 were analyzed by Western blotting. (F) Expression levels of p-FAK and FAK in primary mouse colonic epithelial cells were detected by Western blotting. The data are expressed as mean ± SEM (n = 3). Significant differences are designated as follows: ##P < 0.01 versus the Normal group; ∗∗P < 0.01 versus the DSS group; nsP > 0.05 not significant versus the ERβ−/− group.
Overall, ERβ−/− colitis mice exhibited impaired mucosal healing capability compared to their littermate wild-type controls, which led to a more severe colitis. Moreover, our experiments clarified the mechanisms underlying ERβ-mediated wound healing in colitis mice.
3.8. ERβ agonist significantly enhanced the amelioration of 5-ASA on colitis in mice
The currently available treatment strategies for UC primarily rely on anti-inflammatory drugs such as 5-ASA, which do not directly promote mucosal wounds healing, permitting intestinal antigens to invade and activate immune cells, and causing persistent inflammation. Encouraged by the findings that ERβ activation can directly enhance mucosal healing, we examined the potential benefit of combining ERβ-agonist DPN with 5-ASA for the treatment of colitis. It was shown that combining DPN with 5-ASA significantly reduced DAI scores, suppressed colon shortening, and downgraded pathological scores in colitis mice, surpassing the effects of either agent used alone (Fig. 12A–E). Furthermore, the combined use of DPN with 5-ASA further enhanced the acceleration of DPN on mucosal healing in colitis mice, whereas 5-ASA alone had no significant impact on mucosal healing (Fig. 12F–I, Supporting Information Fig. S16). Finally, DPN upregulated the expression of mucosal healing-related factors without a notably influence on pro-inflammatory cytokines, while 5-ASA downregulated the expression of pro-inflammatory cytokines without notable influence on the expression of mucosal healing-related factors in the colon tissues of colitis mice. The combination of DPN with 5-ASA potentiated each other's effects, evidenced by significant upregulation in the expression of mucosal healing–related factors and downregulation in the expression of pro-inflammatory cytokines (Fig. 12J and K).
Figure 12.
ERβ agonist enhanced the amelioration of 5-ASA on colitis in mice. The mice were provided with water containing 3.5% DSS for 5 days, and normal water for another 5 days. DPN (2.5 mg/kg, intraperitoneal injection) and 5-ASA (100 mg/kg, oral gavage) were given from Day 6 to Day 10 after induction of colitis. (A) The DAI score. (B, C) The length of colons. (D–F) Representative images of Swiss roll colon section stained with H&E, alongside pathological scores and ulcer scores (plotting scale: 200 μm). (G) Assessment of FITC-dextran permeability across the colonic mucosal barrier. (H, I) Representative images of colonic endoscope displaying ulcer regions and quantifying the percentage of wound healing (plotting scale: 200 μm). (J, K) Expression of mucosal healing-related factors and pro-inflammatory factors in colon tissues was conducted using RT-qPCR. The data are expressed as mean ± SEM (n = 6). Significant differences are designated as follows: ##P < 0.01 versus the Normal group; ∗P < 0.01 and ∗∗P < 0.01 versus the DSS group.
These findings indicate that the combined use of 5-ASA and ERβ-agonists exerts anti-inflammatory effect together with mucosal healing effect, which may be a more efficient therapeutic strategy for UC.
4. Discussion
Mucosal healing, defined as the restoration of epithelial integrity, is a critical endpoint in UC management. It alleviates UC symptoms and reduces the risk of disease relapse and complications, and is therefore critical to the treatment of UC27. Unfortunately, UC therapies tend to be centered on reducing inflammation, and there is a notable absence of drugs that directly promote mucosal healing. This highlights an urgent need for innovative therapeutic approaches that can address this deficiency.
The unexplained gender-related susceptibility of UC, with more males being affected, remains unexplained28. Exogenous estrogen supplementation has proven effective in relieving symptoms in patients with UC29. Targeting ERβ, the most enriched ER subtype in colon, may represent a novel therapeutic strategy for promoting mucosal healing in patients with UC. This study is the first to report a positive correlation between ERβ activation in colonic epithelial cells and mucosal healing in patients with UC and colitis mice. This suggests that activating ERβ may enhance mucosal healing in patients with UC. Our studies further demonstrated that the ERβ-agonists, DPN (injected intraperitoneally) and LIQ (administered orally), could significantly promote mucosal healing in vivo while exerting no significant impact on inflammation. Notably, LIQ has a very low oral bioavailability of approximately 1.3%. Consequently, the majority of LIQ is enriched in the intestine and can hardly be absorbed into the blood, which allows them to reach the damaged part of the colon epithelium for action30,31. This evidence indicates that the activation of ERβ directly facilitates mucosal healing independent of the suppression of inflammation.
Mucosal healing involves three stages. Firstly, colon epithelial cells migrate from neighboring crypts to cover the wound bed. Secondly, the migrated cells proliferate to accelerate wound repair. Finally, crypts are regenerated at the injury site17. Our mechanistic studies revealed that ERβ activation promoted mucosal healing through enhancing colonic epithelial cell migration. As the initial stage of mucosal healing, cell migration involves pseudopod extension, focal adhesion assembly for stable anchoring, cytoplasmic contraction for generating migratory force, and focal adhesion disassembly for enabling forward cell movement18. Accelerating focal adhesion turnover has the potential to enhance colonic epithelial cell migration. We found that ERβ activation accelerated focal adhesion turnover in colonic epithelial cells by activating FAK, an observation which provides insights into developing therapeutic strategies for mucosal healing.
Transcriptomics assay suggested that autophagy might serve as an intermediate mechanism for the activation of FAK following ERβ activation. Among the numerous autophagy-related proteins, FIP200 interacts with FAK and restrains its activation under physiological conditions. During the initial phase of autophagy, FIP200 needs to interact with various other autophagy-related proteins in order to form autophagosomes32. We speculated that this interaction between FIP200 and other autophagy-related proteins can disrupt FIP200–FAK interaction due to steric hindrance, leading to FAK activation. We found that ERβ activation triggered autophagy, suppressing the interaction between FIP200 and FAK, subsequently enhancing focal adhesion turnover and cell migration. This discovery may be used to promote mucosal healing and can potentially be applied in the treatment of tumor metastasis. Although autophagy is activated in tumor tissues and has been to promote focal adhesion turnover and tumor metastasis, the exact mechanisms underlying this process had not been previously clarified33,34. Our study provides insights into this mechanism, laying the foundation for the development of novel therapeutic strategies in treating tumor metastasis and other mucosal defect-related diseases.
Autophagy is primarily regulated by metabolism and nutrients35. The metabolomics assay in the current study initially revealed that ERβ activation significantly suppresses branched-chain amino acid transport. Further mechanistic studies demonstrated that ERβ activation suppresses branched-chain amino acid transport in colonic epithelial cells by inhibiting LAT1 expression. This suppression enhances autophagy by inhibiting mTOR activation. Amino acids play a complex array of roles in colitis, and kynurenine can modulate aberrant colonic immune responses, while sulfhydryl-containing amino acids exacerbate colitis through the production of sulfides36,37. Branched-chain amino acid supplements can increase intestinal permeability, which may be particularly harmful for patients with UC38,39.
The above-mentioned findings established the pivotal role of ERβ activation in mucosal healing. Further validation studies demonstrated that insufficient ERβ activation in colonic epithelial cell of ERβ−/− colitis mice resulted in impaired wound healing. Targeting ERβ thus holds promise for promoting mucosal healing, which not only opens a new avenue in UC treatment but also holds the potential to complement traditional anti-inflammatory drugs such as 5-ASA. Our results also demonstrated that the combined administration of the ERβ-agonist and 5-ASA in colitis mice significantly enhanced mucosal healing while simultaneously exerting anti-inflammatory effects. Consequently, this combination therapy potentiated the therapeutic benefits of both 5-ASA and DPN individually in colitis. This key finding may be further explored to improve UC treatment strategies.
5. Conclusions
The findings of this study represent the first demonstration of a positive correlation between the activation of ERβ in colonic epithelial cells and mucosal healing in patients with UC and colitis mice. Activated ERβ binds to silencer2 of LAT1 gene and inhibits LAT1 transcription, consequently leading to reduced branched-chain amino acid transport in colonic epithelial cells. Subsequently, the reduction of amino acid transport triggers the activation of autophagy, which promotes the dissociation of FAK from FIP200 and activates FAK. Activation of FAK accelerates focal adhesion turnover and colonic epithelial cell migration, ultimately facilitating colonic wound healing. Moreover, the ERβ−/− colitis mice exhibited impaired wound healing compared to their wild-type colitis littermates, attesting to the crucial role of ERβ. Importantly, the combination of ERβ-agonist DPN with the first-line anti-UC agent 5-ASA exhibited a synergistic effect, enhancing the therapeutic action of 5-ASA against colitis in mice.
Author contributions
Yilei Guo: Writing – original draft, Formal analysis, Data curation, Conceptualization. Yanrong Zhu: Formal analysis, Data curation. Jing Zhang: Formal analysis. Yue He: Formal analysis. Mianjiang Zhao: Methodology. Haochang Lin: Methodology. Zhifeng Wei: Supervision, Resources, Methodology. Yufeng Xia: Supervision, Methodology, Funding acquisition. Yue Dai: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by National Natural Science Foundation of China (Nos. 82174049 and 82073861). We would like to extend our sincere gratitude to Dr. Yu Tao of Jiangsu Province Hospital of Traditional Chinese Medicine for providing human samples, Dr. John Gray for his insightful suggestions on our article writing, and Ms. Yajing Zhang for help with our flow cytometry experiments.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2024.11.014.
Contributor Information
Yufeng Xia, Email: yfxiacpu@126.com.
Yue Dai, Email: yuedaicpu@cpu.edu.cn.
Appendix A. Supporting information
The following is the Supporting Information to this article:
References
- 1.Le Berre C., Honap S., Peyrin-Biroulet L. Ulcerative colitis. Lancet. 2023;402:571–584. doi: 10.1016/S0140-6736(23)00966-2. [DOI] [PubMed] [Google Scholar]
- 2.Mehandru S., Colombel J.F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat Rev Gastroenterol Hepatol. 2021;18:83–84. doi: 10.1038/s41575-020-00399-w. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi T., Siegmund B., Le Berre C., Wei S.C., Ferrante M., Shen B., et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. doi: 10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 4.Neurath M.F. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19. doi: 10.1038/mi.2013.73. [DOI] [PubMed] [Google Scholar]
- 5.Neurath M.F., Vieth M. Different levels of healing in inflammatory bowel diseases: mucosal, histological, transmural, barrier and complete healing. Gut. 2023;72:2164–2183. doi: 10.1136/gutjnl-2023-329964. [DOI] [PubMed] [Google Scholar]
- 6.Mak W.Y., Zhao M., Ng S.C., Burisch J. The epidemiology of inflammatory bowel disease: east meets west. J Gastroenterol Hepatol. 2020;35:380–389. doi: 10.1111/jgh.14872. [DOI] [PubMed] [Google Scholar]
- 7.Shah S.C., Khalili H., Chen C.Y., Ahn H.S., Ng S.C., Burisch J., et al. Sex-based differences in the incidence of inflammatory bowel diseases-pooled analysis of population-based studies from the Asia-Pacific region. Aliment Pharmacol Ther. 2019;49:904–911. doi: 10.1111/apt.15178. [DOI] [PubMed] [Google Scholar]
- 8.Armuzzi A., Bortoli A., Castiglione F., Contaldo A., Daperno M., D'Incà R., et al. Female reproductive health and inflammatory bowel disease: a practice-based review. Dig Liver Dis. 2022;54:19–29. doi: 10.1016/j.dld.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Kane S.V., Reddy D. Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Am J Gastroenterol. 2008;103:1193–1196. doi: 10.1111/j.1572-0241.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- 10.Campbell-Thompson M., Lynch I.J., Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERβ isoforms in colon cancer. Cancer Res. 2001;61:632–640. [PubMed] [Google Scholar]
- 11.Garcia-Villatoro E.L., Allred C.D. Estrogen receptor actions in colitis. Essays Biochem. 2021;65:1003–1013. doi: 10.1042/EBC20210010. [DOI] [PubMed] [Google Scholar]
- 12.Fan W., Ding C., Liu S., Gao X., Shen X., De Boevre M., et al. Estrogen receptor β activation inhibits colitis by promoting NLRP6-mediated autophagy. Cell Rep. 2022;41 doi: 10.1016/j.celrep.2022.111454. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A., Priyamvada S., Ge Y., Jayawardena D., Singhal M., Anbazhagan A.N., et al. A novel role of SLC26A3 in the maintenance of intestinal epithelial barrier integrity. Gastroenterology. 2021;160:1240–1255. doi: 10.1053/j.gastro.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miranda J., Brazil J.C., Morris A.H., Parkos C.A., Quiros M., Nusrat A. Maresin-2 promotes mucosal repair and has therapeutic potential when encapsulated in thermostable nanoparticles. Proc Natl Acad Sci U S A. 2023;120 doi: 10.1073/pnas.2218162120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S., He T.L., Zhong Y.M., Chen M.J., Yao Q., Chen D., et al. Roles of focal adhesion proteins in skeleton and diseases. Acta Pharm Sin B. 2023;13:998–1013. doi: 10.1016/j.apsb.2022.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posor Y., Kampyli C., Bilanges B., Ganguli S., Koch P.A., Wallroth A., et al. Local synthesis of the phosphatidylinositol-3,4-bisphosphate lipid drives focal adhesion turnover. Dev Cel. 2022;57:1694–1711. doi: 10.1016/j.devcel.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain U., Lai C.W., Xiong S., Goodwin V.M., Lu Q., Muegge B.D., et al. Temporal regulation of the bacterial metabolite deoxycholate during colonic repair is critical for crypt regeneration. Cell Host Microbe. 2018;24:353–363. doi: 10.1016/j.chom.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SenGupta S., Parent C.A., Bear J.E. The principles of directed cell migration. Nat Rev Mol Cel Biol. 2021;22:529–547. doi: 10.1038/s41580-021-00366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Combs J.D., 3rd, Salaita K., Shu X. Polarized focal adhesion kinase activity within a focal adhesion during cell migration. Nat Chem Biol. 2023;19:1458–1468. doi: 10.1038/s41589-023-01353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rana N., Privitera G., Kondolf H.C., Bulek K., Lechuga S., De Salvo C., et al. GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell. 2022;185:283–298. doi: 10.1016/j.cell.2021.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Q., Chen Y., Chen D., Zhao H., Feng Y., Meng Q., et al. Calcium transients on the ER surface trigger liquid-liquid phase separation of FIP200 to specify autophagosome initiation sites. Cell. 2022;185:4082–4098. doi: 10.1016/j.cell.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Wen J., Zellner A., Braun N.C., Bajaj T., Gassen N.C., Peitz M., et al. Loss of function of FIP200 in human pluripotent stem cell-derived neurons leads to axonal pathology and hyperactivity. Transl Psychiatry. 2023;13:143. doi: 10.1038/s41398-023-02432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrnes K., Blessinger S., Bailey N.T., Scaife R., Liu G., Khambu B. Therapeutic regulation of autophagy in hepatic metabolism. Acta Pharm Sin B. 2022;12:33–49. doi: 10.1016/j.apsb.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B., Chen Y., Shi X., Zhou M., Bao L., Hatanpaa K.J., et al. Regulation of branched-chain amino acid metabolism by hypoxia-inducible factor in glioblastoma. Cell Mol Life Sci. 2021;78:195–206. doi: 10.1007/s00018-020-03483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao C., Dahlman-Wright K., Gustafsson J.Å. Estrogen signaling via estrogen receptor β. J Biol Chem. 2010;285:39575–39579. doi: 10.1074/jbc.R110.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pineton de Chambrun G., Peyrin-Biroulet L., Lémann M., Colombel J.F. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 28.Greuter T., Manser C., Pittet V., Vavricka S.R., Biedermann L. Gender differences in inflammatory bowel disease. Digestion. 2020;101:98–104. doi: 10.1159/000504701. [DOI] [PubMed] [Google Scholar]
- 29.Bábíčková J., Tóthová Ľ., Lengyelová E., Bartoňová A., Hodosy J., Gardlík R., et al. Sex differences in experimentally induced colitis in mice: a role for estrogens. Inflammation. 2015;38:1996–2006. doi: 10.1007/s10753-015-0180-7. [DOI] [PubMed] [Google Scholar]
- 30.Liu F., Nong X., Qu W., Li X. Pharmacokinetics and tissue distribution of 12 major active components in normal and chronic gastritis rats after oral administration of Weikangling capsules. J Ethnopharmacol. 2023;316 doi: 10.1016/j.jep.2023.116722. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Q., Wang Z., Wang X., Yan X., Guo Q., Yue Y., et al. The bioaccessibility, bioavailability, bioactivity, and prebiotic effects of phenolic compounds from raw and solid-fermented mulberry leaves during in vitro digestion and colonic fermentation. Food Res Int. 2023;165 doi: 10.1016/j.foodres.2023.112493. [DOI] [PubMed] [Google Scholar]
- 32.Melkoumian Z.K., Peng X., Gan B., Wu X., Guan J.L. Mechanism of cell cycle regulation by FIP200 in human breast cancer cells. Cancer Res. 2005;65:6676–6684. doi: 10.1158/0008-5472.CAN-04-4142. [DOI] [PubMed] [Google Scholar]
- 33.Mowers E.E., Sharifi M.N., Macleod K.F. Novel insights into how autophagy regulates tumor cell motility. Autophagy. 2016;12:1679–1680. doi: 10.1080/15548627.2016.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenific C.M., Stehbens S.J., Goldsmith J., Leidal A.M., Faure N., Ye J., et al. NBR1 enables autophagy-dependent focal adhesion turnover. J Cel Biol. 2016;212:577–590. doi: 10.1083/jcb.201503075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim K.H., Lee M.S. Autophagy—a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10:322–337. doi: 10.1038/nrendo.2014.35. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Wang X., Hu C.A. Therapeutic potential of amino acids in inflammatory bowel disease. Nutrients. 2017;9:920. doi: 10.3390/nu9090920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Holeček M. Side effects of amino acid supplements. Physiol Res. 2022;71:29–45. doi: 10.33549/physiolres.934790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang T.Q., Chen Y.X., Zeng S.L., Lin Y., Li F., Jiang Z.M., et al. Bergenin alleviates ulcerative colitis by decreasing gut commensal Bacteroides vulgatus-mediated elevated branched-chain amino acids. J Agric Food Chem. 2024;72:3606–3621. doi: 10.1021/acs.jafc.3c09448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.