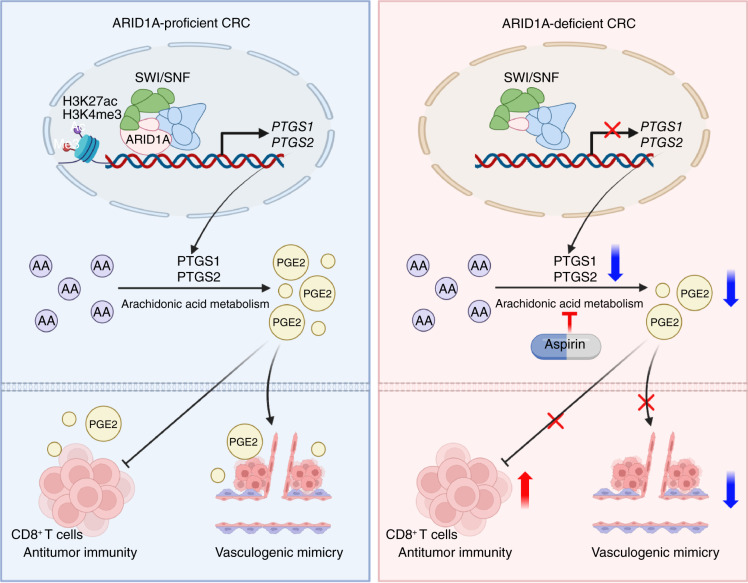
The arachidonic acid pathway is a metabolic vulnerability in ARID1A-deficient colorectal cancer that can be targeted with aspirin to suppress tumor growth and enhance sensitivity to immunotherapy, providing a promising therapeutic strategy.
Abstract
AT-rich interactive domain-containing protein 1A (ARID1A), a core constituent of the switch/sucrose nonfermentable (SWI/SNF) complex, is mutated in approximately 10% of colorectal cancers. Whereas ARID1A deficiency corresponds to heightened immune activity in colorectal cancer, immune checkpoint inhibitors (ICI) have shown limited efficacy in these tumors. The discovery of targetable vulnerabilities associated with ARID1A deficiency in colorectal cancer could expand treatment options for patients. In this study, we demonstrated that arachidonic acid (AA) metabolism inhibitors synergize with ICIs in ARID1A-deficient colorectal cancer by enhancing the activity of CD8+ T cells and inhibiting vasculogenic mimicry. Epigenetic analysis using ATAC-seq and ChIP–qPCR revealed that the lack of ARID1A results in reduced levels of PTGS1 and PTGS2, the key enzymes that control the AA pathway. Low PTGS1 and PTGS2 expression generated a reliance on the remaining functionality of the AA pathway in ARID1A-deficient cells. The AA pathway inhibitor aspirin selectively inhibited the growth of ARID1A-deficient colorectal cancer, and aspirin sensitized tumors lacking ARID1A to immunotherapy. Together, these findings suggest that blocking AA metabolism can enhance immune responses against tumors by activating CD8+ T cells and inhibiting vasculogenic mimicry, which synergizes with ICIs to improve treatment of ARID1A-deficient colorectal cancer.
Significance: The arachidonic acid pathway is a metabolic vulnerability in ARID1A-deficient colorectal cancer that can be targeted with aspirin to suppress tumor growth and enhance sensitivity to immunotherapy, providing a promising therapeutic strategy.
Graphical Abstract
Introduction
AT-rich interactive domain-containing protein 1A (ARID1A) is responsible for encoding a switch/sucrose nonfermentable (SWI/SNF) complex and mutated in approximately 10% of colorectal cancers (1, 2). Increasing evidence suggests that ARID1A deficiency impairs the interaction with MSH2 and was positively correlated with the mismatch repair (MMR) defective mutant phenotype in ovarian cancer (3, 4). ARID1A deficiency damages DNA damage repair, leading to a dominant C > T mutation pattern and increasing tumor mutation burden in ovarian and gastrointestinal cancers (3, 5). Absence of ARID1A activates the PI3K/AKT pathway and promotes the increase of PD-L1 expression in gastrointestinal cancer (6, 7). ARID1A deficiency characterizes a subgroup with heightened immune activity, marked by significant infiltration of CD8+ T cells in colorectal cancer (8, 9). These discoveries indicate that tumors lacking ARID1A have the potential to respond favorably to immune checkpoint inhibitor (ICI) therapy. Research has shown that the progression-free survival (PFS) of ARID1A-mutant tumors receiving immunotherapy is significantly longer than that of wild-type (WT) tumors (10.9 vs. 3.9 months, P = 0.006; ref. 10). However, the immunotherapy cohort of colorectal cancer showed that the PFS was 6.6 and 8.0 months in ARID1A-deficient and ARID1A-proficient groups, respectively. The ICI treatment showed limited therapeutic efficacy in mice with ARID1A-deficient tumors (11). This indicates that a combinatorial therapeutic strategy is essential to completely eliminate ARID1A-deficient colorectal cancer.
Tumor cells that contain harmful genetic mutations are easily inhibited by synthetic lethal targets (12). PARP inhibitors are aimed at treating breast and ovarian tumors harboring mutations in BRCA1 and BRCA2 (13, 14). PRMT5 inhibitors selectively treat methylthioadenosine phosphorylase mutant tumors (15, 16). Mutated tumor cells undergo metabolic reprogramming, resulting in their dependence on certain metabolites (17, 18). Restricting the uptake or production of key metabolites can selectively kill these tumor cells, which is a promising anticancer approach (19, 20). There is a suggestion that blocking the activity of glutamate–cysteine ligase catalytic subunit and disrupting glutathione metabolism may effectively target ARID1A-deficient tumor cells (21). ARID1A-deficient hepatocellular carcinoma produces dependence on genes associated with the tricarboxylic acid cycle (22). Hence, discovery of metabolic vulnerabilities associated with ARID1A deficiency in colorectal cancer could improve tumor therapy dramatically.
Arachidonic acid (AA) serves as a polyunsaturated fatty acid crucial for various cellular functions such as inflammation and immune responses (23–25). The COX pathway is vital for metabolizing AA and produces thromboxane A2 and prostaglandins (PG) by utilizing cyclooxygenase 1 (PTGS1) and cyclooxygenase 2 (PTGS2) enzymes (26, 27). Notably, PGE2 interacts with EP2 and EP4 receptors situated on CD8+ T cells, leading to suppression of CD8+ T-cell function, ultimately forming an inhibitory immune microenvironment (28–30). PGE2 can drive mitochondrial depolarization of CD8+ T cells, influencing their accumulation in the intestinal microenvironment (30). Additionally, PGE2 triggers the activation of protein kinase C (PKC)-dependent ERK1/2 pathways and the EGFR pathway, contributing to the process of vasculogenic mimicry (VM; refs. 31, 32). VM refers to a tubular formation encircled by tumor cells, exhibiting traits or functions akin to vascular cells. It significantly contributes to tumor progression, metastasis, and the modulation of immune cell infiltration (33–35). Hence, targeting the production of PGE2 or blocking its receptors could serve as supplementary approaches to enhance the effectiveness of immune-targeted medications (36–38). Nevertheless, the influence of ARID1A on the regulation of AA remains unexplored.
In this study, we demonstrate that ARID1A-deficient colorectal cancer exhibits particular susceptibility to inhibition of PTGS1 and PTGS2. Targeting the AA pathway works synergistically with ICIs by activating CD8+ T cells and inhibiting VM formation in ARID1A-deficient colorectal cancer.
Materials and Methods
Cell lines
Mouse colorectal cancer cell lines CT26 and CMT93 and human colorectal cancer cell lines SW620, DLD1, HCT15, SW480, HCT116, LOVO, LS174T, SW48, and RKO were obtained from the National Collection of Authenticated Cell Cultures. Colorectal cancer cell lines SW620, DLD1, SW480, HCT116, CT26, and CMT93 were grown in RPMI-1640 medium. Colorectal cancer cell lines HCT15, SW48, and RKO were grown in DMEM medium. Colorectal cancer cell line LOVO was grown in F12K, and LS174T was grown in Minimum Essential Medium. The culture medium was added 10% FBS and 1% penicillin/streptomycin. Cell lines are subcultured every 2 or 3 days. The authenticity of all cell lines was proved through short tandem repeat DNA profiling, whereas Mycoplasma contamination was ruled out by using a Mycoplasma PCR detection kit.
Mouse xenograft model
Mouse experiments adhered to Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for the care and use of laboratory animals and were approved by Harbin Medical University’s Institutional Animal Care and Use Committee. NYG, BALB/c, and C57BL/6 mice were obtained from Changsheng Biotechnology.
To examine tumor growth in vivo after ARID1A knockout (KO), 5 × 105 tumor cells in 100 μL of PBS were injected into the right flank of female BALB/c or C57BL/6 mice ages 6 weeks to establish the xenograft model. About 7 days after injection, a caliper was used to measure tumor size every 3 days. The formula was applied to estimate tumor volume: V = a2 × b/2, in which a represents the short axis and b denotes the long axis of the tumor mass. The mice were killed at a specified time point. For mouse treatment experiments, the mice were randomly divided and administered either control or drug treatment approximately 7 days after tumor cell inoculation. Aspirin was administered at a concentration of 600 mg/mL in drinking water and supplemented every 3 days. Anti–PD-1 mAb was given (10 mg/kg) every 3 days for 2 weeks. Tumor growth was monitored using a slide caliper and measured every 3 days. The tumors were evaluated by measuring tumor volume and weights.
Patient-derived xenograft (PDX) models were generated utilizing colorectal cancer tissue specimens obtained from patients who had undergone surgical resection and provided written informed consent. For the humanized colorectal cancer PDX model, 1 × 107 human peripheral blood mononuclear cells were administered into NYG mice via the tail vein. The tumor tissue was sliced into small fragments and then implanted subcutaneously into female NYG mice ages 6 weeks. Aspirin was administered in the drinking water (600 mg/mL), and anti–PD-1 mAb was given (10 mg/kg) every 3 days for 2 weeks. The tumors were evaluated by measuring tumor volume and weights.
Patient samples and information collection
We collected the basic and prognostic information of 136 patients with colorectal cancer and established three clinical treatment cohorts (Supplementary Table S1). In addition, tissue samples were collected from 75 patients with colorectal cancer. Treatment response was assessed according to RECIST version 1.1. Complete response (CR) means that total target lesions disappear and the short diameter of all pathologic lymph nodes must be reduced to less than 10 mm. Partial response (PR) means that the sum of the target lesion diameter has at least 30% reduction from baseline. Progressive disease means that the sum of the target lesion diameter has at least 20% increase or one or more new lesions appear. Stable disease is a state between progressive disease and PR. Objective response rate (ORR) refers to the proportion of patients whose tumors demonstrate a specified reduction in size, including those with achieve CR or PR. Disease control rate (DCR) refers to the proportion of patients whose tumors reach either CR, PR, or stable disease for a designated period. PFS refers to the period from the initiation of treatment until either disease progression or death occurs. Overall survival (OS) refers to the duration from the beginning of treatment until death from any cause or until the study concludes for patients who are still alive. All participants have signed written informed consent, and the study followed the guidelines of the Ethics Committee. This study received approval from the Ethics Committee of Harbin Medical University Cancer Hospital (KY-2022-37).
ARID1A KO using CRISPR/Cas9
The plasmids that knocked out ARID1A were constructed from the predicted sequence of the website [Eukaryotic Pathogen CRISPR guide RNA/DNA Design Tool (EuPaGDT)] and linked to the pGEMT vector to construct ARID1A–single-guide RNA (sgRNA) plasmids. The ARID1A sgRNA plasmids and Cas9 plasmid were transfected into CT26 or CMT93 cell lines with jetPRIME transfection reagent (Polyplus, #101000046). After 48 hours, the single-cell suspension was spread to 96-well plates. When the cells reached a certain density, they were passaged. Finally, ARID1A KO was verified by Western blot and IHC. The ARID1A sgRNA sequence is as follows: sgRNA1:5′-GCTGGGGATGAGGACTTTGC-3′; sgRNA2: 5′-GTGGTTGTGAGTAGGGGGGC-3′.
Gene knockdown using siRNA
The PTGS1 or PTGS2 siRNA were purchased from General Biol. The PTGS1 or PTGS2 siRNA were transfected using jetPRIME transfection reagent (Polyplus, #101000046). Western blot was used to evaluate the efficiency of knockdown. The PTGS1 siRNA sequence was “GGAGAAAGUAUGAUAGAGATT,” and the PTGS2 siRNA sequence was “UGAAAGGACUUAUGGGAAATT.”
Western blotting
Cultured cells and tissues were lysed using protease inhibitor (Beyotime, #P1005) containing RIPA buffer (Thermo Fisher Scientific, #89001) on ice for 30 minutes. The supernatant was obtained by centrifugation at 12,000 rpm for 10 minutes, and SDS loading buffer (Beyotime, #P0015F) was added. Proteins undergo electrophoresis, membrane transfer and blocking, followed by the application of antibodies overnight at 4°C. The antibodies were as follows: PTGS1 (Abcam, #ab109025, RRID: AB_10865291), PTGS2 (Abcam, #ab179800, RRID: AB_2894871), actin (Abcam, #ab8226, RRID: AB_306371), and ARID1A (Abcam, #ab182560, RRID: AB_3096240).
qRT-PCR
The RNA was isolated using TRIzol reagents (Thermo Fisher Scientific, #15596026) following guidelines. Subsequently, RNA was reverse transcribed into cDNA for fluorescence quantification. Target gene changes in the control and experimental groups were evaluated using 2-ΔΔCt method. Below are the primer sequences utilized for qRT-PCR:
ARID1A-F: 5′-CTTCCCCAACCACCAGTACAA-3′
ARID1A-R: 5′- CTGTGCGAAGGACGAAGAC-3′
PTGS1-F: 5′-ATGAGTCGAAGGAGTCTCTCG-3′
PTGS1-R: 5′-GCACGGATAGTAACAACAGGGA-3′
PTGS2-F: 5′- TGCACTATGGTTACAAAAGCTGG -3′
PTGS2-R:5′-TCAGGAAGCTCCTTATTTCCCTT -3′
ACTIN-F: 5′-GTGACGTTGACATCCGTAAAGA-3′
ACTIN-R: 5′- GCCGGACTCATCGTACTCC-3′.
Chromatin immunoprecipitation qPCR
Cells were treated with 1% formaldehyde (Sigma, #F8775) at room temperature for 10 minutes to induce cross-linking, and the reaction was then quenched using 0.125 mol/L glycine (Sigma, #G8790). The cells were lysed ultrasonically, and chromatin was incubated overnight with the following antibodies: SNF5 (Cell Signaling Technology, #91735, RRID: AB_2800172), ARID1A (Cell Signaling Technology, #12354, RRID: AB_2637010), H3K27ac (Cell Signaling Technology, #4353, RRID: AB_10545273), H3K4me3 (Cell Signaling Technology, #9751, RRID: AB_2616028), or IgG (Cell Signaling Technology, #2729). The agarose (Santa Cruz Biotechnology, #sc2003) was incorporated into the reaction for 4 hours, and then chromatin was eluted. Phenol/chloroform was used to purify DNA, and qPCR was used with the following primers:
PTGS1-F: 5′-AAGCTCACAGGCGAATCTCC-3′
PTGS1-R: 5′-CACGGCCCTTTAGAGACAGG-3′
PTGS2-F: 5′-AGTTTCCTAAGTGCTGCTTCTCA-3′
PTGS2-F: 5′-AGACCCCTGCTCAAAACAAA-3′.
IHC
Formalin-fixed colorectal cancer clinical specimens and cell line mouse s.c. grafts were dehydrated, paraffin-embedded, and sectioned into 4-mm-thick slices for IHC analysis. The sections were treated with the following antibodies overnight at 4°C: PTGS1 (Abcam, # ab109025, RRID: AB_10865291), PTGS2 (Abcam, #ab179800, RRID: AB_2894871), ARID1A (Abcam, #ab182560, RRID: AB_3096240), anti-CD8 (Abcam, #ab316778), and anti-CD31 (Abcam, #ab182981, RRID: AB_2920881). Next, sections were treated with secondary antibodies (OriGene, #PV6001). Cell nucleus was stained with Mayer’s hematoxylin (Beyotime, #C0107). A histologic score (H-score) was used to access IHC staining of samples. The intensity of staining (graded as 1+, 2+, 3+, or 4+) was assessed for each individual cell. The H-score was calculated with following formula: [1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+) + 4 × (% cells 3+)]. The ultimate score falls within the range of 0 to 400. The percentages of PTGS1- and PTGS2-positive cells in each slide were quantified.
For a double-staining assay, slides were stained with CD31 through IHC and then counterstained with periodic acid (Sigma, # 100482) and Schiff reagent (Sigma, # 3952016). The presence of VM refers to the tubular structure and patterned matrix formed by periodic acid Schiff (PAS)+/CD31−-stained colorectal cancer cells.
Luciferase reporter assay
For reporter plasmids, we synthesized separate WT or mutant target sequences containing predicted binding sites for PTGS1 or PTGS2. These sequences were then cloned and inserted into the pGL4.20 (luc2 Puro) vector (General Biol). Subsequently, the WT or mutant PTGS1 or PTGS2 plasmids were transfected into cells. The luciferase activity in each well was assessed using a dual-luciferase reporter assay system (Promega, #E1910). This allowed us to evaluate the influence of ARID1A deficiency on the regulation of PTGS1 or PTGS2.
T-cell–mediated cancer cell–killing analysis
For T-cell–mediated cancer cell–killing analysis, tumor cells were inoculated into 6-well plates and cocultured with T cells with or without aspirin. The cells were fixed using methanol and stained using crystal violet 48 hours later (39).
Flow cytometry
To analyze immune cells infiltrated by tumor tissue, the tumor tissue was first cut and placed in empty medium containing collagenase IV and DNase and digested in a 37°C shaking table for 1 hour. The cell suspension obtained from digestion was used for flow cytometry and was stained with the following antibodies: mCD45-APC/Fire 750 (BioLegend, #103154, RRID: AB_2572116), mCD3-FITC (BioLegend, #100204, RRID: AB_312661), mCD3-PerCP/Cyanine5.5 (BioLegend, #100218, RRID: AB_1595492), mCD8a-APC (BioLegend, #100712, RRID: AB_312750), mCD8a-PE (BioLegend, #100708, RRID: AB_3312747), mGZMB-PE (BioLegend, #372208, RRID: AB_2687032), mIFNγ-PE/Cyanine7 (BioLegend, #505826, RRID: AB_2295770), mPD-1-FITC (BioLegend, #135214, RRID: AB_10680238), mTim3-APC (BioLegend, #134008, RRID: AB_2562998), hCD45-APC (BioLegend, #368512, RRID: AB_2566372), hCD45-APC/Cyanine7 (BioLegend, #304014, RRID: AB_314402), hCD3-FITC (BioLegend, #300406, RRID: AB_314060), hCD3-APC (BioLegend, #300312, RRID: AB_314048), hCD8a-PE (BioLegend, #300908, RRID: AB_314112), hCD8-FITC (BioLegend, #344704, RRID: AB_1877178), hIFNγ-PerCP (BioLegend, #502524, RRID: AB_2616613), hPD-1-PerCP (BioLegend, #329937, RRID: AB_2563595), and hTim3-PE/Cyanine7 (BioLegend, #345014, RRID: AB_2561720). To detect intracellular cytokines, Cell Stimulation Cocktail (BioLegend, #423304) was used to stimulate cells for 4 hours. The BD flow cytometer (BD FACSMelody) was used to analyze stained cells. FlowJo software was used to analyze data.
Gene set enrichment analysis and single-sample gene set enrichment analysis
Molecular Signatures Database (MSigDB; https://www.gsea-msigdb.org/gsea/msigdb) provides the gene set enrichment analysis (GSEA) gene sets. The R software “clusterProfiler” package was adopted for GSEA between CT26 ARID1AKO–IgG and CT26 ARID1AKO–anti–PD-1 groups, whereas the R software “GseaVis” package was utilized for visualization. Single-sample GSEA (ssGSEA) analysis was conducted using the IBOR package by calling the calculate_sig_score function with method = “ssgsea.” We utilized the package’s built-in gene sets and quantified the relative levels of immune cell infiltration in tumors. P < 0.05 was deemed statistically significant (40).
Assay for transposase-accessible chromatin with high-throughput sequencing
For assay for transposase-accessible chromatin with high-throughput sequencing (ATAC-seq; NOVOGENE), 5 × 104 cells were collected for nuclear extraction, and after qualified quality control, the transposase was added to mix (mix contains transposase and two equimolar connectors, adapter 1 and adapter 2). Incubated at 37°C for 30 minutes, the end joint can be attached while the transposition is achieved. Sequencing libraries were constructed by primer amplification and purification and then sequenced by Illumina. We used Burrows-Wheeler Aligner for comparison analysis of the reference genome (GRCm38/mm10) of ATAC-seq data and used Integrative Genomics Viewer to visualize the peak distribution on genes of interest.
RNA sequencing
RNA was isolated from tissues or cells using standard extraction methods, and its integrity was detected using the Agilent 2100 bioanalyzer. The mRNA fragments were randomly fragmented with bivalent cations in New England Biolabs (NEB) fragment buffer, and a cDNA library was constructed. Upon library construction, the Qubit 2.0 fluorometer was used to quantify, and insert sizes in the library were determined using the Agilent 2100 bioanalyzer. qRT-PCR was used to precisely measure the library’s effective concentration, ensuring its quality. After qualification, Illumina sequencing was performed. Clean reads were matched to reference genomes using HISAT2 software. Differential gene analysis was performed using criteria of |log2fold change| ≥ 1 and FDR < 0.05.
Metabolomics analysis
For nontargeted metabolomics (METWARE), the samples were completely suspended after thawing by adding 80% methanol water internal standard extractor 500 μL and swirling for 3 minutes. The centrifuge tube was quickly frozen in liquid nitrogen for 5 minutes, defrosted on dry ice for 5 minutes and on ice for 5 minutes, and swirled for 2 minutes. After centrifugation, 300 μL supernatant was transferred to another centrifuge tube and kept for 30 minutes in the refrigerator at −20°C. After centrifugation, 200 μL supernatant was removed into the inner liner of the corresponding sample bottle for on-machine testing. The software Analyst 1.6.3 was used to process mass spectrum data. The screening criteria for differential metabolites were VIP >1 and P value <0.05. Metabolite set enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were performed to evaluate the enrichment of differential abundance metabolites.
Statistical analysis
Quantitative data are presented as the mean ± SEM. Fisher exact test, Fisher ANOVA, and Student t test were used to compare differences among samples. Spearman correlation analysis was utilized to explore associations between two variables. Survival distributions among experimental groups were compared using the log-rank test. Statistical significance was indicated as ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001; and ∗∗∗∗, P < 0.0001. Statistical analyses were performed using GraphPad Prism software (version 10.1.0).
Data availability
The data analyzed in this study were obtained from Gene Expression Omnibus at GSE120060. The data generated in this study are publicly available in Gene Expression Omnibus at GSE278081 (ATAC-seq), GSE278082 [chromatin immunoprecipitation sequencing (ChIP-seq)], and GSE278083 [RNA sequencing (RNA-seq)]. The raw metabolomics data and metabolomics analysis data (Supplementary Table S2) are provided as supplementary materials. All other raw data are available upon request from the corresponding author.
Results
ARID1A-deficient colorectal cancer cells have limited immunotherapy efficacy
To investigate the clinical status of treatment for patients with ARID1A mutations, we established three clinical cohorts based on three widely used treatment regimens for colorectal cancer. The majority of ARID1A mutation is truncating mutation in colorectal cancer, resulting in the loss of ARID1A protein expression. Therefore, patients were divided into a ARID1A-deficient group with deletion of ARID1A expression and a ARID1A-proficient group with ARID1A expression based to IHC. The ICI treatment cohort showed different therapeutic effects between ARID1A-proficient and -deficient groups. In the ARID1A-deficient group, the ORR was 33.3% (5 of 15) and the DCR was 93.3% (14 of 15), surpassing those of the ARID1A- proficient group (ORR, 10.3%, 3 of 29; DCR, 58.6%, 17 of 29; P < 0.01; Fig. 1A). The CT scans of representative cases illustrated tumor shrinkage (decrease 66.69%) in ARID1A-deficient tumors following ICI therapy, contrasting with the persistent progression (59.76% increase and appearance of new lesions) observed in ARID1A-proficient tumors (Fig. 1B). Furthermore, we noted that patients in the ARID1A-deficient group exhibited improved prognosis, accompanied by much longer PFS (P < 0.01) and OS (P < 0.05), compared with ARID1A-proficient patients (median PFS: 19.30 ± 5.22 vs. 3.70 ± 0.19; P = 0.0025; median OS: 45.83 ± 3.09 vs. 14.83 ± 0.32; P = 0.0245; Fig. 1C), indicating that ARID1A-deficient patients benefit from ICI treatment. Consistent with the ICI cohort, the ARID1A-deficient group had better treatment outcomes than the ARID1A-proficient group in the bevacizumab treatment cohort with higher ORR and DCR and longer PFS and OS, and the CT scan showed that the ARID1A-deficient group was stable (0% change) after bevacizumab treatment, whereas ARID1A-proficient tumors showed continuous progression (increase 21.31%; Fig. 1D–F). In contrast, the ARID1A-proficient and -deficient cohorts showed similar therapeutic efficacy in the cetuximab treatment cohort (31.88% and 36.47% increase, respectively; Fig. 1G–I). Next, we focused on immunotherapy of the ARID1A-deficient group.
Figure 1.
ARID1A-deficient colorectal cancer cells have limited immunotherapy efficacy. A, Tables and histograms recapitulating the outcomes of patients with colorectal cancer in the ICI cohort. B, Representative CT images showing tumor change at the baseline and the best response after ICI therapy. Red circles, lesions. The ARID1A status and therapeutic evaluation are depicted. C, PFS and OS of patients with colorectal cancer who received ICI therapy. D, Tables and histograms recapitulating the outcomes of patients with colorectal cancer in the bevacizumab cohort. E, Representative CT images showing tumor change at the baseline and the best response after bevacizumab therapy. Red circles, lesions. The ARID1A status and therapeutic evaluation are depicted. F, PFS and OS of patients with colorectal cancer who received bevacizumab therapy. G, Tables and histograms recapitulating the outcomes of patients with colorectal cancer in the cetuximab cohort. H, Representative CT images showing tumor change at the baseline and the best response after cetuximab therapy. Red circles, lesions. The ARID1A status and therapeutic evaluation are depicted. I, PFS and OS of patients with colorectal cancer who received cetuximab therapy. J and K, IHC images (J) and quantitation (K) of ARID1A and CD8 in CT26 and CT26 ARID1AKO cell–derived s.c. tumors. Scale bar, 120 μm. L and M, IHC images (L) and quantitation (M) of ARID1A and CD8 in colorectal cancer samples (n = 75). Scale bar, 200 μm. N, Gross images of CT26 ARID1AKO cell–derived s.c. tumors. The tumor-bearing BALB/c mice received 2 weeks of i.p. injection with anti–PD-1 or IgG. O–Q, Tumor growth curves (O), tumor volume (P), and tumor weight (Q) of CT26 ARID1AKO cell–derived s.c. tumors treated with anti–PD-1 or IgG (n = 6). ns, not significant; *, P < 0.05; ***, P < 0.001. PD, progressive disease; SD, stable disease.
To better simulate the situation of ARID1A mutation in clinical patients, we used CRISPR-Cas9–mediated KO technology to construct a CT26 and CMT93 cell line model with ARID1A gene KO. We confirmed ARID1A KO (ARID1AKO) status through both immunoblot analysis and IHC (Fig. 1J; Supplementary Fig. S1A). ARID1A mutation can lead to impaired DNA damage repair function, resulting in accumulation of double-strand break and the production of tumor neoantigens, enhancing tumor cell immunogenicity and promoting antitumor immune response (41). To validate the construction of the ARID1A KO model and its impact on DNA repair function, we assessed the expression of γH2AX, a DNA damage marker, and found that a significantly higher accumulation with γH2AX was displayed in the ARID1AKO cells (Supplementary Fig. S1B). ARID1A KO did not affect proliferation ability in vitro (Supplementary Fig. S1C–S1F). Further studies showed that transplanted tumors of the ARID1AKO cell line grew more slowly and infiltrated more CD8+ T cells than transplanted tumors of the ARID1A-WT cell line (Fig. 1J and K; Supplementary Fig. S2A–S2H). Our own clinical cohort and the The Cancer Genome Atlas (TCGA)–colorectal cancer database confirmed that colorectal cancer with ARID1A mutation has more CD8 infiltration (Fig. 1L and M; Supplementary Fig. S2I). Subsequently, we evaluated the responsiveness of ARID1A-deficient cells to anti–PD-1 in CT26 and CMT93 mouse transplanted tumor model. Surprisingly, there was no significant improvement in tumor size and tumor weight in ARID1AKO tumor cells with anti–PD-1 treatment (Fig. 1N–Q; Supplementary Fig. S2J–S2M). This forced us to take a closer look at ARID1A-deficient tumors.
Efficient immunotherapy heavily relies on the function of CD8+ T cells in the tumor microenvironment (TME). Decreased effector activity or increased exhaustion levels of these cells can result in immunotherapy failure. To identify the factors contributing to the restricted effectiveness of ICI therapy in ARID1AKO cell mouse grafted tumors, we first used flow cytometry to analyze the quantity and functionality of CD8+ T cells in CT26 ARID1AKO grafted tumors. We observed that the ratio of CD8+ T cells did not exhibit significant enhancement before and after ICI therapy (Supplementary Fig. S3A). The proportion of granzyme B (GZMB)+CD8+ and IFNγ+CD8+ was not significantly improved, and the proportion of PD-1+CD8+ and Tim3+CD8+ T cells remained high (Supplementary Fig. S3B–S3E). We performed the same experiment in CMT93 ARID1AKO mouse tumor transplants and obtained consistent results (Supplementary Fig. S3F–S3J).
To better examine the effect of TME immune activity induced by anti–PD-1 treatment on ARID1AKO mouse transplanted tumors, we conducted RNA-seq analysis on transplanted tumors of CT26 ARID1AKO cells mice treated with either IgG or anti–PD-1. Gene Ontology (GO) analysis of differential genes indicated that the immune response–related pathways remained stable (Supplementary Fig. S3K). Furthermore, GSEA and ssGSEA of the transcriptional differences between IgG and anti–PD-1 groups revealed no difference in pathways linked to immunotherapy response and the infiltration levels of immune cells (Supplementary Fig. S3L and S3M). To determine the functional and transcriptional heterogeneity within CD8+ TILs, we generated effector gene signatures including Ifng, Il10, Gzma, Gzmb, Prf1, Tnfsf10, Fasl, Tnf, Il2, Id2, Batf, Prdm1, Runx3, Klra2, Klrc2, and Nkg7 and exhaustion transcription factors such as Bhlhe40, Nr4a1, Nr4a2, Tox, Eomes, Nfil3, and Tcf7 (Supplementary Fig. S3N and S3O). Unfortunately, there was no significant improvement in effector factors and exhaustion factors in the anti–PD-1 treatment group. GSEA analysis of exhaustion T cells showed no difference (Supplementary Fig. S3P). Next, we used a variety of analytic methods to analyze the differences in T-cell infiltration levels and immune-related gene signatures, finding no notable disparities between the two groups (Supplementary Fig. S3Q and S3R).
ARID1A deficiency reprogrammed AA metabolism to generated susceptibility
As ARID1A governs chromatin accessibility, influencing the transcriptional activity of specific genes, we utilized ATAC-seq to investigate and contrast alterations in chromatin accessibility between CT26 and CT26 ARID1AKO cells (Fig. 2A). We discovered 14,504 sites exhibiting heightened accessibility and 69,041 sites displaying reduced accessibility upon ARID1A KO by ATAC-seq (Fig. 2B). These altered sites were further analyzed by GO and KEGG pathway analyses. GO analysis of biological processes indicated that ARID1A deficiency could impact various tumor metabolism–related processes, including metabolic process, cellular metabolic process, primary metabolic process, and organic substance metabolic process, among others (Fig. 2C). Notably, we observed strong enrichment of altered sites in pathways, including PI3K-AKT, Hippo, and mTOR signaling pathways, all of which, contribute to tumor metabolism (Fig. 2D). These findings strongly indicate that ARID1A deficiency is likely involved in regulating tumor metabolism.
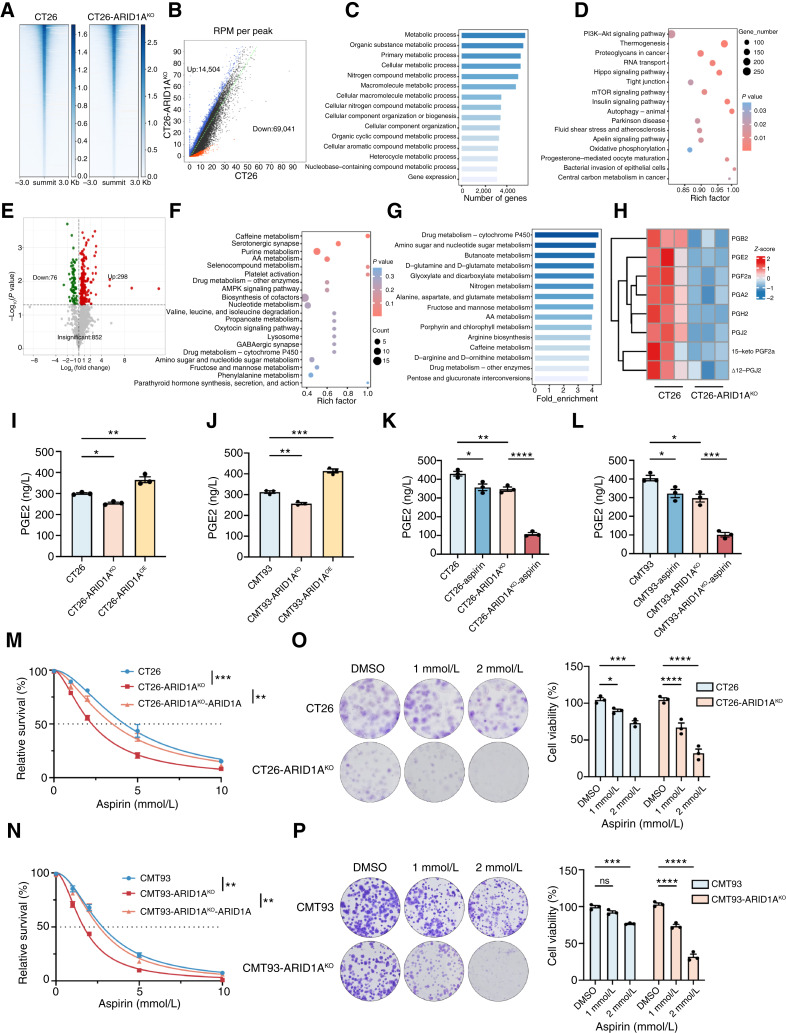
Figure 2.
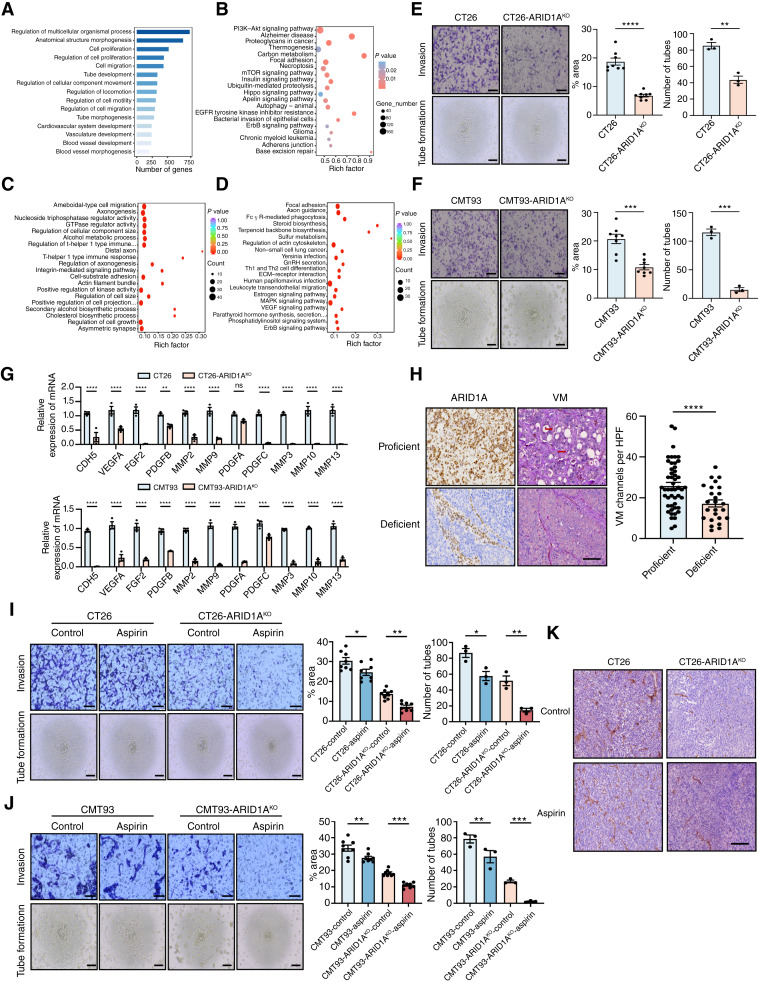
ARID1A deficiency reprogrammed AA metabolism to generate susceptibility. A, Heatmap shows the average signal distribution in the 3 kb region upstream and downstream of summit in CT26 and CT26 ARID1AKO cells. B, Differential peak calls of ATAC-seq in CT26 and CT26 ARID1AKO cells. Red and blue dots depict peaks with decreased or increased read density, respectively. The number in the bottom right or top left imply the count of called peaks showing decreased or increased accessibility. RPM, reads per million. C, GO analysis of differential genes explains the enrichment of metabolism-related signaling pathways. D, KEGG analysis of differentially expressed genes revealed signaling pathways involved in metabolic processes. E, Volcanic map of differential metabolites in CT26 and CT26 ARID1AKO cell lines. Red and green points represent upregulated and downregulated metabolites, respectively, whereas gray dots represent detected but not significantly different metabolites. F, KEGG analysis of differential metabolites uncovered alterations in metabolic pathways. G, Metabolite set enrichment analysis of differential metabolites revealed changes in metabolic pathways. H, Heatmap of differential metabolism of the AA metabolic pathway. I and J, The levels of PGE2 in CT26, CT26 ARID1AKO, and CT26 ARID1AOE (I) or CMT93, CMT93 ARID1AKO, and CTMT93 ARID1AOE (J) cells were detected by ELISA. K and L, The levels of PGE2 in CT26 and CT26 ARID1AKO cells (K) or CMT93 and CMT93 ARID1AKO cells (L) were detected by ELISA following treatment with or without aspirin (2 mmol/L) for 48 hours. M and N, Dose–response curves for CT26, CT26 ARID1AKO, and CT26 ARID1AKO cells with ARID1A restoration (M) or CMT93, CMT93 ARID1AKO, and CMT93 ARID1AKO cells with ARID1A restoration (N) to aspirin were detected by CCK8 assay. O and P, Relative cell viability rates for CT26 and CT26 ARID1AKO cells (O) or CMT93 and CMT93 ARID1AKO cells (P) to aspirin were detected by colony formation assay. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Based on the data provided by ATAC sequencing and previous reports on the metabolic vulnerability of ARID1A mutation, we performed metabolomics analysis, which detected 374 metabolites (Fig. 2E). The KEGG enrichment analysis and metabolite set enrichment analysis of differential metabolites all indicated enrichment of AA metabolism (Fig. 2F and G). We further screened the products related to AA metabolism and found that PGB2, PGE2, PGF2α, PGA2, PGH2, PGJ2, and other metabolites were downregulated in CT26 ARID1AKO cells (Fig. 2H).
Next, we performed RNA-seq of CT26 and CT26 ARID1AKO cell lines, identifying 1,530 increased genes and 1,161 decreased genes (Supplementary Fig. S4A). We observed that the accessibility of ATAC-seq was highly correlated with gene expression of RNA-seq (Supplementary Fig. S4B and S4C). We combined ATAC-seq and RNA-seq data to analyze the accessibility changes and corresponding changes on gene expression and identified 677 differential genes (Supplementary Fig. S4D and S4E). KEGG analysis of differential genes showed enrichment of AA metabolism and other related pathways (Supplementary Fig. S4F).
Subsequently we confirmed that ARID1A KO reduced PGE2 levels, whereas ARID1A overexpression increased the PGE2 content in CT26 and CMT93 cells (Fig. 2I and J; Supplementary Fig. S4G). We found consistent results in the TCGA–colorectal cancer dataset, with GSEA analysis indicating downregulation of the AA metabolism pathway and some key AA metabolism genes in ARID1A low-expression patients (Supplementary Fig. S4H and S4I). Because there is evidence to suggest that AA metabolism and PGE2 triggers changes in drug sensitivity, we exposed ARID1A-WT and ARID1AKO cells to aspirin, an AA pathway inhibitor. The ARID1AKO cells treated with aspirin showed a significant decrease in PGE2 levels, and the IC50 value of aspirin was markedly reduced in ARID1AKO cells, which was different from the ARID1A-WT cell lines (Fig. 2K–N). Colony formation assays obtained similar results that ARID1A-KO cells have lower cell viability than ARID1A-WT cells (Fig. 2O and P). We analyzed the drug sensitivity of multiple colorectal cancer cell lines to aspirin; the IC50 value of the ARID1A-low group was significantly lower than that of the ARID1A-high group (Supplementary Fig. S4J–S4L). Collectively, these results validate that ARID1A deficiency triggers a shift in AA metabolism, rendering cells more susceptible to certain interventions.
ARID1A deficiency leads to a reliance on PTGS1 and PTGS2 genes in colorectal cancer
The heightened susceptibility of ARID1A-deficient tumor cells to aspirin led us to propose that ARID1A potentially governs the transcription of genes linked to AA metabolism. To pinpoint potential treatment targets for ARID1A-deficient cancers within the AA metabolic pathway, we listed the key genes on the pathway and screened them (Fig. 3A and B; Supplementary Fig. S5A). In the end, we focused on PTGS1 and PTGS2 because they are rate-limiting enzymes in AA acid metabolism and noted for a striking decrease upon ARID1A deficiency. We validated that the protein and mRNA levels of Ptgs1 and Ptgs2 were reduced in ARID1AKO cells compared with ARID1A-WT cells (Fig. 3B and C; Supplementary Fig. S5A). Consistently, immunoblot analysis on multiple colorectal cancer cell lines proved a correlation between the ARID1A and PTGS1 or PTGS2 expression (Fig. 3D). The TCGA–colorectal cancer dataset showed a positive relationship between ARID1A and PTGS1 or PTGS2 expression (Supplementary Fig. S5B). Subsequently, we explored the correlation between the ARID1A and PTGS1 or PTGS2 expression in a panel of 75 cases of human colorectal cancer (Fig. 3E). Our analysis revealed that ARID1A-deficient colorectal cancer exhibited notably reduced expression levels of both PTGS1 and PTGS2. Furthermore, there was a positive correlation among PTGS1 and PTGS2. We further analyzed the drug sensitivity of TFAP (a selective PTGS1 inhibitor) and celecoxib (a selective PTGS2 inhibitor) and found that ARID1AKO cells had a lower IC50 value (Fig. 3F–I).
Figure 3.
ARID1A deficiency leads to a reliance on PTGS1 and PTGS2 genes in colorectal cancer. A, Diagram illustrating the molecules implicated in the AA metabolic pathway and inhibitors targeting their products. B, Histogram of genes associated with AA metabolism in CT26 and CT26 ARID1AKO cells. C, Immunoblotting for validation of PTGS1 and PTGS2 expression in CT26 and CT26 ARID1AKO cells or CMT93 and CMT93 ARID1AKO cells. D, Immunoblotting for validation of correlation between ARID1A and PTGS1 and PTGS2 expression in multiple colorectal cancer cells. E, IHC images and quantitation of ARID1A, PTGS1, and PTGS2 in colorectal cancer samples (n = 75). Scale bar, 200 μm. F and G, Dose–response curves for CT26 and CT26 ARID1AKO cells to TFAP (F) or celecoxib (G) were detected by CCK8 assay. H and I, Dose–response curves for CMT93 and CMT93 ARID1AKO cells to TFAP (H) or celecoxib (I) were detected by CCK8 assay. J, Immunoblotting for validation of PTGS1 silencing by siRNA in CT26 and CT26 ARID1AKO cells. K, Relative cell viability rates were assessed after siPTGS1 transfection in CT26 and CT26 ARID1AKO cells by CCK8 assay. L, Immunoblotting for validation of PTGS2 silencing by siRNA in CT26 and CT26 ARID1AKO cells. M, Relative cell viability rates were assessed after siPTGS2 transfection in CT26 and CT26 ARID1AKO cells by CCK8 assay. N and O, Relative cell viability rates for CT26 and CT26 ARID1AKO cells with or without siPTGS1 (N) or siPTGS2 (O) were detected by colony formation assay. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Next, we investigated whether the observed growth suppression in ARID1A-deficient cells treated with aspirin relies on the presence of PTGS1 and PTGS2. To achieve this objective, we rescued PTGS1 or PTGS2 in ARID1AKO cells, which resulted in an elevated IC50 value of aspirin (Supplementary Fig. S5C–S5F). Additionally, inhibition of PTGS1 or PTGS2 by siRNA in ARID1A-WT cells decreased the IC50 value of aspirin (Supplementary Fig. S5G–S5J). These findings validate that the growth suppression of ARID1AKO cells resulting from blocking the AA pathway relies on the presence of PTGS1 or PTGS2.
Subsequently, we further revealed that silencing PTGS1 (Fig. 3J and K; Supplementary Fig. S5K and S5L) or PTGS2 (Fig. 3L and M; Supplementary Fig. S5M and S5N) with siRNA notably inhibited the growth of ARID1AKO colorectal cancer cells. We monitored colony formation assays to evaluate the synthetic lethality phenotypes linked to PTGS1 or PTGS2 (Fig. 3N and O; Supplementary Fig. S5O and S5P). The results showed that silencing either PTGS1 (Fig. 3N; Supplementary Fig. S5O) or PTGS2 (Fig. 3O; Supplementary Fig. S5P) led to significant suppression of proliferation in ARID1AKO colorectal cancer cells. Together, these findings validate the combined lethal effect of PTGS1 and PTGS2 genes in ARID1A-deficient tumor cells.
PTGS1 and PTGS2 are target genes of the SWI/SNF complex containing ARID1A
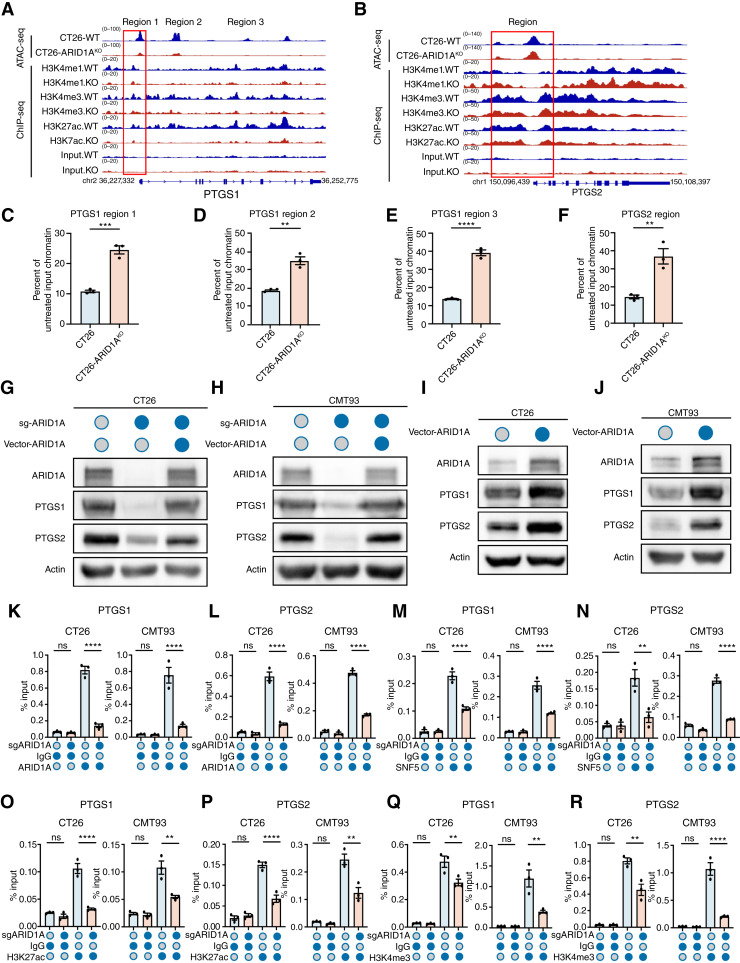
Subsequently, we investigated how ARID1A modulates the AA metabolic pathway. ATAC-seq and ChIP-seq of CT26 and CT26 ARID1AKO cells demonstrated reductions in chromatin accessibility at the promoters of PTGS1 and PTGS2 in ARID1AKO cells, and ARID1A KO decreased the association of lysine 27 on histone H3 (H3K27ac) and lysine 4 on histone H3 (H3K4m3) with the promoters of PTGS1 and PTGS2 genes (Fig. 4A and B). In addition, we reexamined a previous ChIP-seq analysis in ARID1A-WT and ARID1A-KO cells (Supplementary Fig. S6A and S6B) and specifically investigated genes that encode elements of the AA metabolic pathway. The ChIP-seq showed that ARID1A interacts with the promoters of PTGS1 and PTGS2 and that ARID1A KO reduced the association of SNF5, H3K27ac, and H3K4m3 with the promoters of these genes (Supplementary Fig. S6A and S6B). Compared with CT26 cells, the CT26 ARID1AKO cell line has less chromatin accessibility, which reduces the digestion of DNase I, leading to more substrates available for qPCR (Fig. 4C–F). In addition, we confirmed that ARID1A KO in CT26 and CMT93 cells led to decreased levels of both PTGS1 and PTGS2 mRNA and protein. The reintroduction of WT ARID1A effectively reversed the decrease in PTGS1 and PTGS2 expression caused by ARID1A KO (Fig. 4G and H; Supplementary Fig. S6C and S6D). Similarly, introducing WT ARID1A in CT26 and CMT93 cell lines resulted in increased expression of both PTGS1 and PTGS2 (Fig. 4I and J; Supplementary Fig. S6E and S6F). Knockdown of SWI/SNF complex components SNF5, BRG1, or ARID1B also decreased the expression of PTGS1 and PTGS2 (Supplementary Fig. S6G–S6I). Consistently, the activity of PTGS1 and PTGS2 was decreased in ARID1A-KO cells, and this reduction was further amplified by aspirin treatment (Supplementary Fig. S6J–S6M).
Figure 4.
PTGS1 and PTGS2 are target genes of the SWI/SNF complex containing ARID1A. A and B, Representative browser track of ATAC-seq and ChIP-seq on the PTGS1 (A) and PTGS2 (B) locus in CT26 and CT26 ARID1AKO cells. C–F, qPCR was performed on DNase I–treated chromatin samples. G and H, Immunoblotting for validation of PTGS1 and PTGS2 expression in CT26, CT26 ARID1AKO, and CT26 ARID1AKO with ARID1A restoration cells (G) or CMT93, CMT93 ARID1AKO, and CMT93 ARID1AKO with ARID1A restoration cells (H). I and J, Immunoblotting for validation of PTGS1 and PTGS2 expression in CT26 and CT26 ARID1AOE cells (I) or CMT93 and CMT93 ARID1AOE cells (J). K–N, The association of ARID1A (K and L) or SNF5 (M and N) with the PTGS1 and PTGS2 gene promoters in CT26 and CT26 ARID1AKO cells or CMT93 and CMT93 ARID1AKO cells was assessed by ChIP-qPCR. O–R, The association of H3K27ac (O and P) or H3K4me3 (Q and R) with the PTGS1 and PTGS2 gene promoters in CT26 and CT26 ARID1AKO cells or CMT93 and CMT93 ARID1AKO cells was assessed by ChIP-qPCR. ns, not significant; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Additionally, we investigated the binding of ARID1A and SNF5 to the promoters of PTGS1 and PTGS2 through ChIP-qPCR analysis (Fig. 4K–N; Supplementary Fig. S6N–S6Q). The ChIP-qPCR analysis confirmed that ARID1A KO led to a decrease in the binding of SNF5 to the promoters of PTGS1 and PTGS2, indicating that the activation of PTGS1 and PTGS2 by ARID1A is dependent on the SWI/SNF complex (Fig. 4K–N). This was related to the decreased enrichment of H3K27ac and H3K4me3 in their promoters (Fig. 4O–R). On the other hand, introducing WT ARID1A into CT26 and CMT93 cells increased the binding of SNF5 to promoters of both PTGS1 and PTGS2 (Supplementary Fig. S6N–S6Q), which correlated with their upregulation. To investigate whether ARID1A regulates PTGS1 or PTGS2 transcription through association with the promoter, we used the JASPAR website to predict the binding motif (Supplementary Fig. S7A) and then constructed WT or mutant (MUT) promoter-luciferase reporter plasmids (Supplementary Fig. S7B). Double luciferase reports experimentally confirmed that transfection of PTGS1-WT or PTGS2-WT significantly decreased luciferase activities in ARID1AKO cells, whereas transfection of PTGS1-MUT or PTGS2-MUT had no effect on luciferase activities compared with the control group (Supplementary Fig. S7C–S7F). In other words, these findings indicate that the SWI/SNF complex, which includes ARID1A, adjusts PTGS1 and PTGS2 expression.
Targeting AA metabolism promotes further amplification of CD8+ T-cell signaling
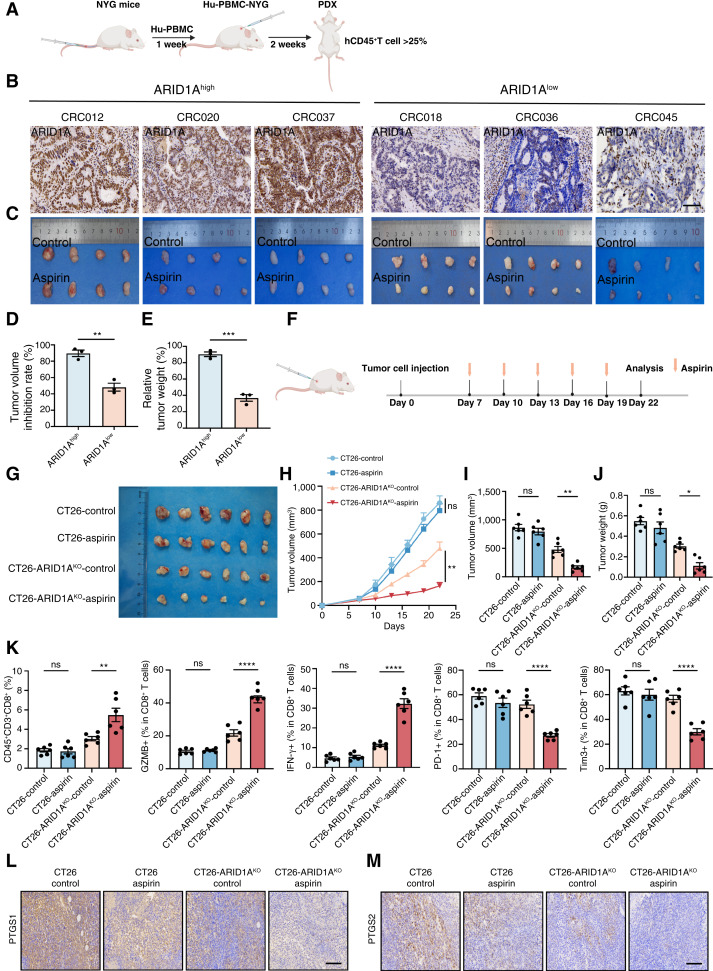
Based on the promising outcomes seen in vitro, we proceeded to access the therapeutic effectiveness of aspirin in treating ARID1A-deficient colorectal cancer in vivo. First, we investigated whether ARID1A deficiency exhibit a corresponding reliance on the AA metabolism pathway using a humanized PDX mouse model (Fig. 5A). To create an immune reconstitution model, human peripheral blood mononuclear cells were administered via the tail vein of female NYG mice. Three weeks after infusion, flow cytometry was utilized to assess the presence of human-derived T cells in the peripheral blood of mice, indicating the extent of immunologic reconstitution (Supplementary Fig. S8A and S8B). IHC was used to verify the ARID1A status in PDXs (Fig. 5B). The results suggested that aspirin decreased the expression of PTGS1 and PTGS2 (Supplementary Fig. S8C–S8E), and ARID1Alow PDXs were more susceptible to aspirin than ARID1Ahigh PDXs (Fig. 5C). Due to variations in heterogeneity and growth rates, we assessed treatment susceptibility across these models by calculating the relative inhibition rates of tumor volume and tumor weight (Fig. 5D and E). These findings validate that ARID1Alow group is more likely to benefit from treatment with aspirin.
Figure 5.
Targeting AA metabolism promotes further amplification of CD8+ T-cell signaling. A, Schematic illustration of Hu-NYG PDX mouse model establishment and validation of immune cells. B, The levels of ARID1A expression were analyzed by IHC in colorectal cancer PDX models and divided into ARID1Ahigh and ARID1Alow. Scale bar, 100 μm. C, ARID1Ahigh and ARID1Alow PDX tumor sizes are shown after grouping and treatment for 2 weeks. D and E, The tumor volume inhibition rates (D) and relative tumor weight (E) after the indicated treatments in ARID1Ahigh and ARID1Alow PDX models. F, Schematic depiction of tumor cell inoculation into mouse xenograft models, followed by treatment with or without aspirin. G, Gross images of CT26 and CT26 ARID1AKO cell–derived s.c. tumors. BALB/c mice bearing tumors were treated with or without aspirin (n = 6). H–J, Tumor growth curves (H), tumor volume (I), and tumor weight (J) of CT26 and CT26 ARID1AKO cell–derived s.c. tumors treated with or without aspirin (n = 6). K, Quantification of CD45+CD3+CD8+, GZMB+CD8+, IFNγ+CD8+, PD-1+CD8+, and Tim3+CD8+ T cells in CT26 and CT26 ARID1AKO cell–derived s.c. tumor treated with or without aspirin. L and M, Representative images for PTGS1 (L) and PTGS2 (M) in CT26 and CT26 ARID1AKO cell–derived s.c. tumors treated with or without aspirin. Scale bar, 120 μm. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. A and F, Created in BioRender. Cui, L. (2024) https://BioRender.com/p39x013.
Next, the effectiveness of aspirin in inhibiting tumor growth was assessed in vivo by implanting mice with s.c. tumors derived from CT26 or CT26 ARID1AKO cells (Fig. 5F). Aspirin effectively suppressed the growth of CT26 ARID1AKO tumors while showing minimal impact on the growth of CT26 tumors (Fig. 5G–J). Subsequently, flow cytometry of transplanted tumors in mice revealed that aspirin notably elevated the proportion of CD8+ T cells in ARID1AKO tumors, increased the proportion of IFNγ+ or GZMB+ CD8+ T cells, and decreased the proportion of PD-1+ or Tim3+ CD8+ T cells (Fig. 5K). In this model, aspirin decreased the expression of PTGS1 and PTGS2 (Fig. 5L and M) and the level of PGE2 (Supplementary Fig. S9A). Aspirin increased the levels of GZMB, TNFα, and IFNγ in ARID1AKO tumors (Supplementary Fig. S9B). S.c. transplantation of CMT93 ARID1AKO cells also showed susceptibility to aspirin by enhancing CD8+ T-cell function (Supplementary Fig. S9C–S9K). Together, these data validate the pronounced selectivity of this treatment approach for addressing ARID1A-deficient colorectal cancer, with observed enhanced activity in CD8+ T cells within the ARID1AKO group. Subsequently, we conducted T-cell–mediated cancer cell–killing assay and observed that ARID1AKO cells with aspirin were more vulnerable to T-cell–induced cytotoxicity (Supplementary Fig. S10A and S10B). Coculture of tumor cells with CD8+ T cells demonstrated ARID1AKO cells with aspirin had more tumor cell apoptosis and more GZMB+, IFNγ, and TNFα CD8+ T cells (Supplementary Fig. S10C and S10D). We examined the cell supernatant, and ARID1AKO cells with aspirin had a higher level of GZMB+, IFNγ, and TNFα (Supplementary Fig. S10E and S10F).
Targeting AA metabolism further inhibits VM formation
Targeting AA metabolism is known to contribute to inhibition of VEGF signaling, which is a key mediator in VM. To reveal the potential effects of ARID1A in regulating VM, we examined ATAC-seq results of the CT26-WT and CT26 ARID1A-KO cell lines (Fig 6A and B). The GO analysis suggested that ARID1A deficiency may decrease tumor VM-related biological processes, such as tube morphogenesis, tube development, vasculature development, and blood vessel morphogenesis, among others (Fig. 6A). The KEGG analysis also showed downregulation of the PI3K-AKT and EGFR signaling pathways, among others (Fig. 6B). Next, we performed RNA-seq analysis. The GO analysis showed downregulation of regulation of cell growth, and the KEGG analysis showed downregulation of MAPK signaling and VEGF signaling pathways, which was consistent with previous ATAC-seq results (Fig. 6C and D). As expected, we proved ARID1A deficiency decreased VM formation and cell invasion (Fig. 6E and F). Then we evaluated VM-related markers. qPCR analysis showed that the KO of ARID1A suppressed expression of Cdh5, Vegfa, Mmp2, Mmp10, and other genes in CT26 and CMT93 cells (Fig. 6G). To confirm the relevance between ARID1A and VM formation, we performed CD31/PAS staining on pathologic sections of 75 patients with clinical colorectal cancer and found that the ARID1A-deficient group had less VM formation (Fig. 6H). Examination of gene expression in the TCGA–colorectal cancer database revealed a notable relationship between VM-related genes and ARID1A expression (Supplementary Fig. S11A). GSEA analysis conducted on TCGA–colorectal cancer and TCGA-PANCAN datasets further demonstrated a strong positive association between ARID1A expression and VM (Supplementary Fig. S11B and S11C). In summary, these findings indicate that ARID1A deficiency may be involved in modulating VM.
Figure 6.
Targeting AA metabolism further inhibits vascular mimicry formation. A and B, GO (A) and KEGG (B) analysis of downregulated differential genes by ATAC-seq explained the enrichment of VM-related signaling pathways in CT26 and CT26 ARID1AKO cells. C and D, GO (C) and KEGG (D) analysis of differential genes in RNA-seq of CT26 and CT26 ARID1AKO cells. E and F, The tube formation and transwell invasion analysis of CT26 and CT26 ARID1AKO cells (E) or CMT93 and CMT93 ARID1AKO cells (F). Top, scale bar, 100 μm. Bottom, scale bar, 200 μm. G, Heatmaps of genes associated with VM formation in CT26 and CT26 ARID1AKO cells or CMT93 and CMT93 ARID1AKO cells. H, IHC images and quantitation of ARID1A and VM in colorectal cancer samples (n = 75). Scale bar, 120 μm. HPF, high-power field. I and J, The tube formation and transwell invasion analysis of CT26 and CT26 ARID1AKO cells (I) or CMT93 and CMT93 ARID1AKO cells (J) treated with or without aspirin. Top, scale bar, 100 μm. Bottom, scale bar, 200 μm. K, Representative images of immunostaining for VM in CT26 and CT26 ARID1AKO cell–derived s.c. tumors treated with or without aspirin. Scale bar, 120 μm. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To further determine the effects of targeting AA on VM, we treated cells with aspirin to analyze the effects on tube formation and cell invasion (Fig. 6I and J). The findings showed that tube formation and cell invasion were notably reduced in ARID1AKO cells compared with WT cells after treatment with aspirin. We performed IHC staining of CD31/PAS in aspirin-treated mouse grafts and found that aspirin treatment further reduced the amount of VM formation in ARID1AKO cell line grafts and ARID1Alow PDXs, which was not evident in ARID1A-WT cell line grafts and ARID1Ahigh PDXs (Fig. 6K; Supplementary Fig. S11D–S11F). These results suggest that targeting AA metabolism to further inhibits VM formation.
The AA pathway inhibitor and ICIs are synergistic in suppressing ARID1A-deficient colorectal cancer
Based on the findings that targeting AA metabolism can increase the quantity and function of CD8+ T cells and inhibit VM formation and that tumors with ARID1A mutations are more sensitive to aspirin, we propose that combining inhibition of the AA pathway with ICI treatment may synergistically suppress ARID1A-deficient colorectal cancers.
Consequently, we first investigated whether aspirin works synergistically with ICIs in the ARID1A-deficient humanized PDX mouse model (Fig. 7A). In this model, we observed downregulation of PTGS1 and PTGS2 expression in the aspirin and combination treatment groups (Supplementary Fig. S12A and S12B). Notably, the combination therapy of aspirin and ICIs demonstrated significantly greater efficacy in decreasing tumor volume and weight compared with individual treatment (Fig. 7B–D). Consistently, the combination therapy showed notable enhancements in infiltration of CD8+ T cells, along with improved CD8+ T-cell functionality, indicated by an increase in GZMB+ or IFNγ+ CD8+ T cells and a decrease in PD-1+ or Tim3+ CD8+ T cells (Fig. 7E; Supplementary Fig. S12C and S12D). The combination therapy increased the levels of GZMB, TNFα, and IFNγ (Supplementary Fig. S12E).
Figure 7.
The AA metabolic inhibitor and ICIs are synergistic in suppressing ARID1A-deficient colorectal cancer. A, Schematic illustration of the Hu-NYG PDX mouse model and cell line mouse transplanted tumor model treated with or without aspirin or anti–PD-1. B, Gross images of ARID1A-deficient colorectal cancer PDX model. NYG mice bearing tumors were treated with or without aspirin or anti–PD-1 (n = 3). C and D, Tumor volume (C) and tumor weight (D) of ARID1A-deficient colorectal cancer PDX model treated with or without aspirin or anti–PD-1 (n = 3). E, Quantification of CD45+CD3+CD8+, IFNγ+CD8+, and Tim3+CD8+ T cells in the ARID1A-deficient colorectal cancer PDX model treated with or without aspirin or anti–PD-1 (n = 3). F, Gross images of CT26 ARID1AKO cell–derived s.c. tumors. BALB/c mice bearing tumors were treated with or without aspirin or anti–PD-1 (n = 6). G–I, Tumor growth curves (G), tumor volume (H), and tumor weight (I) of CT26 ARID1AKO cell–derived s.c. tumors treated with or without aspirin or anti–PD-1 (n = 6). J, The secretion of PGE2 in CT26 ARID1AKO cell–derived s.c. tumors treated with or without aspirin or anti–PD-1 were detected by ELISA. K, Quantification of CD45+CD3+CD8+, GZMB+CD8+, IFNγ+CD8+, PD-1+CD8+, and Tim3+CD8+ T cells in CT26 ARID1AKO cell-derived subcutaneous tumors treated with or without aspirin or anti–PD-1 (n = 6). L, Gross images of CMT93 ARID1AKO cell–derived s.c. tumors. C57BL/6 mice bearing tumors were treated with or without aspirin or anti–PD-1 (n = 6). M–O, Tumor growth curves (M), tumor volume (N), and tumor weight (O) of CMT93 ARID1AKO cell–derived s.c. tumors treated with or without aspirin or anti–PD-1 (n = 6). P, The secretion of PGE2 in CMT93 ARID1AKO cell–derived subcutaneous tumors treated with or without aspirin or anti–PD-1 were detected by ELISA. Q, Quantification of CD45+CD3+CD8+, GZMB+CD8+, IFNγ+CD8+, PD-1+CD8+ and Tim3+CD8+ T cells in CMT93 ARID1AKO cell–derived s.c. tumors treated with or without aspirin or anti–PD-1 (n = 6). ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. A, Created in BioRender. Cui, L. (2024) https://BioRender.com/p39x013.
Next, we investigated the potential variation in the antitumor effectiveness of combining aspirin with ICIs in mice that had been subcutaneously injected with CT26 ARID1AKO cells. The combined therapy effectively suppressed the growth of ARID1AKO tumors compared with the other treatment groups (Fig. 7F and G). Mice treated with the combination of aspirin and ICIs exhibited even greater reductions in tumor volume and weight compared with the other treatment groups (Fig. 7H and I). Aspirin and combination treatment groups had lower PGE2 levels (Fig. 7J). Likewise, the combined treatment elevated the proportion of CD8+ T cells and markers linked to effector function, such as IFNG+ or GZMB+ CD8+ T cells, while decreasing the exhaustion markers, such as PD-1+ or Tim3+ CD8+ T cells (Fig. 7K; Supplementary Fig. S12F). In this model, we observed downregulation of PTGS1 and PTGS2 expression (Supplementary Fig. S12G). The combination therapy increased the levels of GZMB, TNFα, and IFNγ (Supplementary Fig. S12H). We observed a comparable synergy between aspirin and ICIs in reducing tumor burden in CMT93 models as well (Fig. 7L–Q; Supplementary Fig. S12I and S12J). Our results suggest that combining AA pathway inhibitor with ICIs effectively suppresses the progression of ARID1A-deficient colorectal cancer through synergistic effects.
Discussion
Increasing evidence has suggested that ARID1A can alter the TME and affect immunotherapy sensitivity (3, 8, 42). However, there are conflicting accounts of their relationship (11, 43). In our results, the clinical cohort suggested that patients with colorectal cancer harboring ARID1A mutations may benefit from ICI therapy, whereas mouse models of transplanted tumors have achieved only limited therapeutic efficacy. To explore this phenomenon, we performed flow cytometry and RNA-seq on the IgG and anti–PD-1 groups of transplanted tumors in CT26 ARID1AKO mice. RNA-seq demonstrated that immunotherapy response–related pathways remained stable. A previous study declared that ICI treatment alone did not improve the ratio of PD-1+ and TIM3+CD8+ T cells in mice with ARID1A-inactivated tumors (43). There have even been studies showing that ARID1A mutations lead to a decreased proportion of IFNG+CD8+ T cells, leading to failure of ICI therapy (11). So, these results indicate an urgent need to explore a combination regimen to treat ARID1A-deficient tumors.
Targeting metabolic vulnerability in ARID1A-deficient tumors is a promising therapeutic approach. A previous research has showed that ARID1A deficiency reduced levels of HMGCS1 and HMGCR, resulting in a dependency on mevalonate metabolism in ovarian carcinoma (44). Another study revealed that the downregulation of ARID1A leads to reduced levels of glutamate–cysteine ligase catalytic subunit and SLC7A11, resulting in a reliance on glutathione metabolism in cells (21). Another study has revealed that the loss of ARID1A reduces the levels of pyruvate kinase M, causing a shift in cellular glucose metabolism (22). In this study, we used ATAC-seq and ChIP-qPCR to demonstrate that ARID1A deficiency downregulates PTGS1 and PTGS2 expression by affecting chromatin accessibility. ARID1A deficiency increases tumor sensitivity to aspirin in vitro and in vivo. These findings proved that changes in PTGS1 and PTGS2, which decrease activity of the AA metabolism, result in a reliance on the remaining activity of this pathway.
The AA pathway shows protumorigenic activity and metabolizes to produce many PGs, especially PGE2, whose accumulation promotes inflammation and cancer progression (45, 46). Inhibition of PGE2 promotes the infiltration of CD8+ T cells, dendritic cells, and NK cells to inhibit tumor growth (30, 47). In addition, the reduction of PGE2 activates CD8+ T cells and reduces exhaustion (38, 48). Our results show that aspirin significantly promotes the activity of CD8+ T cells and reduces the ratio of exhausted CD8+ T cells by reducing PGE2 levels in ARID1A-deficient colorectal cancer. Previous studies have shown that a combination therapy strategy is necessary to treat colorectal cancer with ARID1A mutation, and a finding suggests that combination therapy with statins and ICIs synergically inhibits ARID1A mutant tumors by inhibiting the mevalonate pathway and modulating antitumor immunity in TME (44). Indeed, we noticed that aspirin and ICIs have a synergistic effect in inhibiting the progression of ARID1A-deficient colorectal cancer. In the combination therapy group of aspirin and ICIs, we observed a rise in the proportion of activated CD8+ T cells and a decline in exhausted CD8+ T cells, resulting in a significant decline in both tumor volume and tumor weight in the mice carrying tumors.
AA pathway significantly contributes to the activation of the VEGF pathway and vascular mimicry formation (32, 49). In this study, the analysis of clinical cohorts showed that ARID1A deficiency was significantly associated with reduced VM formation. ATAC-seq and RNA-seq results of CT26 and CT26 ARID1AKO cell lines revealed downregulation of the tube-forming capacity of ARID1AKO cell lines. We found the decreased tube formation ability and transwell invasion of the ARID1AKO cell lines. Blocking the AA pathway effectively hinders the growth of ARID1A-inactivated cells by impeding VM. GSEA analysis of TCGA–colorectal cancer and pan-cancer datasets proved a correlation between decreased ARID1A expression and the downregulation of VM signaling. Previous research has also indicated that ARID1A deficiency influences the expression of VM-related genes, including EGFR, VEGFA, PDGFA, and PIK3R1 (50). So, our findings suggest that ARID1A deficiency reduces VM formation by targeting the AA pathway.
In conclusion, our results demonstrate the correlation between ARID1A and the key enzymes of AA metabolism, PTGS1 and PTGS2. ARID1A-deficient tumors exhibit sensitivity to AA pathway inhibitors and the inhibition of PTGS1 and PTGS2. Thus, aspirin combined with ICIs is a promising therapeutic option for ARID1A-deficient colorectal cancer.
Supplementary Material
Supplementary Figure and Figure Legend
Supplementary Materials and Methods
Supplementary WB raw data
Clinical and prognostic information of ARID1A proficient and ARID1A deficient colorectal cancer patients receiving immune checkpoint inhibitors, bevacizumab or cetuximab therapy
Metabolomics analysis data of CT26 WT and CT26 ARID1A-KO cells
Supplementary raw metabolomics data
Acknowledgments
We thank all the researchers who supported our study. This study was supported by grants from the National Natural Science Foundation of China (nos. U22A20330, 82173233, 82373372, 82102858, and 82102988); the Key Project of Research and Development Plan in Heilongjiang Province (nos. 2022ZX06C01 and JD2023SJ40); and the Natural Science Funding of Heilongjiang (no. YQ2022H017).
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Authors’ Disclosures
No disclosures were reported.
Authors’ Contributions
L. Cui: Investigation, methodology, writing–original draft, writing–review and editing. R. Liu: Investigation. S. Han: Investigation. C. Zhang: Investigation. B. Wang: Investigation, methodology. Y. Ruan: Investigation. X. Yu: Investigation, methodology. Y. Li: Investigation, methodology. Y. Yao: Investigation. X. Guan: Investigation. Y. Liao: Investigation. D. Su: Investigation. Y. Ma: Investigation. S. Li: Conceptualization, supervision. C. Liu: Conceptualization, resources, supervision, funding acquisition, methodology, writing–original draft, writing–review and editing. Y. Zhang: Conceptualization, resources, supervision, funding acquisition.
References
- 1. Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem 2009;78:273–304. [DOI] [PubMed] [Google Scholar]
- 2. Tokunaga R, Xiu J, Goldberg RM, Philip PA, Seeber A, Battaglin F, et al. The impact of ARID1A mutation on molecular characteristics in colorectal cancer. Eur J Cancer 2020;140:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shen J, Ju Z, Zhao W, Wang L, Peng Y, Ge Z, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med 2018;24:556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosse T, ter Haar NT, Seeber LM, v Diest PJ, Hes FJ, Vasen HF, et al. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations, TP53 and microsatellite instability in endometrial cancer. Mod Pathol 2013;26:1525–35. [DOI] [PubMed] [Google Scholar]
- 5. Parikh AR, He Y, Hong TS, Corcoran RB, Clark JW, Ryan DP, et al. Analysis of DNA damage response gene alterations and tumor mutational burden across 17,486 tubular gastrointestinal carcinomas: implications for therapy. Oncologist 2019;24:1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li L, Li M, Jiang Z, Wang X. ARID1A mutations are associated with increased immune activity in gastrointestinal cancer. Cells 2019;8:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim YB, Ahn JM, Bae WJ, Sung CO, Lee D. Functional loss of ARID1A is tightly associated with high PD-L1 expression in gastric cancer. Int J Cancer 2019;145:916–26. [DOI] [PubMed] [Google Scholar]
- 8. Mehrvarz Sarshekeh A, Alshenaifi J, Roszik J, Manyam GC, Advani SM, Katkhuda R, et al. ARID1A mutation may define an immunologically active subgroup in patients with microsatellite stable colorectal cancer. Clin Cancer Res 2021;27:1663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathur R, Alver BH, San Roman AK, Wilson BG, Wang X, Agoston AT, et al. ARID1A loss impairs enhancer-mediated gene regulation and drives colon cancer in mice. Nat Genet 2017;49:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okamura R, Kato S, Lee S, Jimenez RE, Sicklick JK, Kurzrock R. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J Immunother Cancer 2020;8:e000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li J, Wang W, Zhang Y, Cieślik M, Guo J, Tan M, et al. Epigenetic driver mutations in ARID1A shape cancer immune phenotype and immunotherapy. J Clin Invest 2020;130:2712–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang A, Garraway LA, Ashworth A, Weber B. Synthetic lethality as an engine for cancer drug target discovery. Nat Rev Drug Discov 2020;19:23–38. [DOI] [PubMed] [Google Scholar]
- 13. Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123–34. [DOI] [PubMed] [Google Scholar]
- 14. Ponti G, De Angelis C, Ponti R, Pongetti L, Losi L, Sticchi A, et al. Hereditary breast and ovarian cancer: from genes to molecular targeted therapies. Crit Rev Clin Lab Sci 2023;60:640–50. [DOI] [PubMed] [Google Scholar]
- 15. Mavrakis KJ, McDonald ER III, Schlabach MR, Billy E, Hoffman GR, deWeck A, et al. Disordered methionine metabolism in MTAP/CDKN2A-deleted cancers leads to dependence on PRMT5. Science 2016;351:1208–13. [DOI] [PubMed] [Google Scholar]
- 16. Kryukov GV, Wilson FH, Ruth JR, Paulk J, Tsherniak A, Marlow SE, et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science 2016;351:1214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gai X, Liu Y, Lan X, Chen L, Yuan T, Xu J, et al. Oncogenic KRAS induces arginine auxotrophy and confers a therapeutic vulnerability to SLC7A1 inhibition in non-small cell lung cancer. Cancer Res 2024;84:1963–77. [DOI] [PubMed] [Google Scholar]
- 18. Bian Y, Li W, Kremer DM, Sajjakulnukit P, Li S, Crespo J, et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature 2020;585:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Zhang J, Zheng K, Du Q, Wang G, Huang J, et al. Discovering metabolic vulnerability using spatially resolved metabolomics for antitumor small molecule-drug conjugates development as a precise cancer therapy strategy. J Pharm Anal 2023;13:776–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merteroglu M, Santoro MM. Exploiting the metabolic vulnerability of circulating tumour cells. Trends Cancer 2024;10:541–56. [DOI] [PubMed] [Google Scholar]
- 21. Ogiwara H, Takahashi K, Sasaki M, Kuroda T, Yoshida H, Watanabe R, et al. Targeting the vulnerability of glutathione metabolism in ARID1A-deficient cancers. Cancer Cell 2019;35:177–90.e8. [DOI] [PubMed] [Google Scholar]
- 22. Xing T, Li L, Chen Y, Ju G, Li G, Zhu X, et al. Targeting the TCA cycle through cuproptosis confers synthetic lethality on ARID1A-deficient hepatocellular carcinoma. Cell Rep Med 2023;4:101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin SA, Brash AR, Murphy RC. The discovery and early structural studies of arachidonic acid. J Lipid Res 2016;57:1126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen X, Liu Y, Wang Y, Wang C, Chen X, Xiong Y, et al. CYP4F2-Catalyzed metabolism of arachidonic acid promotes stromal cell-mediated immunosuppression in non-small cell lung cancer. Cancer Res 2022;82:4016–30. [DOI] [PubMed] [Google Scholar]
- 25. Wu Q, Liu Z, Gao Z, Luo Y, Li F, Yang C, et al. KLF5 inhibition potentiates anti-PD1 efficacy by enhancing CD8+ T-cell-dependent antitumor immunity. Theranostics 2023;13:1381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang B, Wu L, Chen J, Dong L, Chen C, Wen Z, et al. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther 2021;6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Badimon L, Vilahur G, Rocca B, Patrono C. The key contribution of platelet and vascular arachidonic acid metabolism to the pathophysiology of atherothrombosis. Cardiovasc Res 2021;117:2001–15. [DOI] [PubMed] [Google Scholar]
- 28. Majima M, Amano H, Hayashi I. Prostanoid receptor signaling relevant to tumor growth and angiogenesis. Trends Pharmacol Sci 2003;24:524–9. [DOI] [PubMed] [Google Scholar]
- 29. Nikolos F, Hayashi K, Hoi XP, Alonzo ME, Mo Q, Kasabyan A, et al. Cell death-induced immunogenicity enhances chemoimmunotherapeutic response by converting immune-excluded into T-cell inflamed bladder tumors. Nat Commun 2022;13:1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Villa M, Sanin DE, Apostolova P, Corrado M, Kabat AM, Cristinzio C, et al. Prostaglandin E2 controls the metabolic adaptation of T cells to the intestinal microenvironment. Nat Commun 2024;15:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Treps L, Faure S, Clere N. Vasculogenic mimicry, a complex and devious process favoring tumorigenesis - interest in making it a therapeutic target. Pharmacol Ther 2021;223:107805. [DOI] [PubMed] [Google Scholar]
- 32. Rong X, Huang B, Qiu S, Li X, He L, Peng Y. Tumor-associated macrophages induce vasculogenic mimicry of glioblastoma multiforme through cyclooxygenase-2 activation. Oncotarget 2016;7:83976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999;155:739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hendrix MJC, Seftor EA, Seftor REB, Chao J-T, Chien D-S, Chu Y-W. Tumor cell vascular mimicry: novel targeting opportunity in melanoma. Pharmacol Ther 2016;159:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu Q, Zhao E, Geng B, Gao S, Yu H, He X, et al. Correction to: tumor-associated macrophage-derived exosomes transmitting miR-193a-5p promote the progression of renal cell carcinoma via TIMP2-dependent vasculogenic mimicry. Cell Death Dis 2022;13:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Renner K, Bruss C, Schnell A, Koehl G, Becker HM, Fante M, et al. Restricting glycolysis preserves T cell effector functions and augments checkpoint therapy. Cell Rep 2019;29:135–50.e9. [DOI] [PubMed] [Google Scholar]
- 37. Chirasani SR, Leukel P, Gottfried E, Hochrein J, Stadler K, Neumann B, et al. Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. Int J Cancer 2013;132:843–53. [DOI] [PubMed] [Google Scholar]
- 38. Pelly VS, Moeini A, Roelofsen LM, Bonavita E, Bell CR, Hutton C, et al. Anti-inflammatory drugs remodel the tumor immune environment to enhance immune checkpoint blockade efficacy. Cancer Discov 2021;11:2602–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer 2021;9:e001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zuo S, Wei M, Wang S, Dong J, Wei J. Pan-cancer analysis of immune cell infiltration identifies a prognostic immune-cell characteristic score (ICCS) in lung adenocarcinoma. Front Immunol 2020;11:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shen J, Peng Y, Wei L, Zhang W, Yang L, Lan L, et al. ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov 2015;5:752–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goswami S, Chen Y, Anandhan S, Szabo PM, Basu S, Blando JM, et al. ARID1A mutation plus CXCL13 expression act as combinatorial biomarkers to predict responses to immune checkpoint therapy in mUCC. Sci Transl Med 2020;12:eabc4220. [DOI] [PubMed] [Google Scholar]
- 43. Wu S, Fukumoto T, Lin J, Nacarelli T, Wang Y, Ong D, et al. Targeting glutamine dependence through GLS1 inhibition suppresses ARID1A-inactivated clear cell ovarian carcinoma. Nat Cancer 2021;2:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou W, Liu H, Yuan Z, Zundell J, Towers M, Lin J, et al. Targeting the mevalonate pathway suppresses ARID1A-inactivated cancers by promoting pyroptosis. Cancer Cell 2023;41:740–56.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jagusch H, Werner M, Werz O, Pohnert G. 15-Hydroperoxy-PGE2: intermediate in mammalian and algal prostaglandin biosynthesis. Angew Chem Int Ed Engl 2019;58:17641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu C, Gu L, Hu L, Jiang C, Li Q, Sun L, et al. FADS1-arachidonic acid axis enhances arachidonic acid metabolism by altering intestinal microecology in colorectal cancer. Nat Commun 2023;14:2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bayerl F, Meiser P, Donakonda S, Hirschberger A, Lacher SB, Pedde AM, et al. Tumor-derived prostaglandin E2 programs cDC1 dysfunction to impair intratumoral orchestration of anti-cancer T cell responses. Immunity 2023;56:1341–58.e11. [DOI] [PubMed] [Google Scholar]
- 48. Basingab FS, Ahmadi M, Morgan DJ. Ifnγ-dependent interactions between ICAM-1 and LFA-1 counteract prostaglandin E2-mediated inhibition of antitumor CTL responses. Cancer Immunol Res 2016;4:400–11. [DOI] [PubMed] [Google Scholar]
- 49. Konishi Y, Ichise H, Watabe T, Oki C, Tsukiji S, Hamazaki Y, et al. Intravital imaging identifies the VEGF-TXA2 Axis as a critical promoter of PGE2 secretion from tumor cells and immune evasion. Cancer Res 2021;81:4124–32. [DOI] [PubMed] [Google Scholar]
- 50. Kelso TWR, Porter DK, Amaral ML, Shokhirev MN, Benner C, Hargreaves DC. Chromatin accessibility underlies synthetic lethality of SWI/SNF subunits in ARID1A-mutant cancers. Elife 2017;6:e30506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure and Figure Legend
Supplementary Materials and Methods
Supplementary WB raw data
Clinical and prognostic information of ARID1A proficient and ARID1A deficient colorectal cancer patients receiving immune checkpoint inhibitors, bevacizumab or cetuximab therapy
Metabolomics analysis data of CT26 WT and CT26 ARID1A-KO cells
Supplementary raw metabolomics data
Data Availability Statement
The data analyzed in this study were obtained from Gene Expression Omnibus at GSE120060. The data generated in this study are publicly available in Gene Expression Omnibus at GSE278081 (ATAC-seq), GSE278082 [chromatin immunoprecipitation sequencing (ChIP-seq)], and GSE278083 [RNA sequencing (RNA-seq)]. The raw metabolomics data and metabolomics analysis data (Supplementary Table S2) are provided as supplementary materials. All other raw data are available upon request from the corresponding author.