Abstract
Objectives
This study aims to examine the correlation between four distinct Epstein-Barr virus (EBV) antibodies (EA-D, EBNA-1, VCA-p18, and ZEBRA) and the likelihood of developing prostate cancer (PCa) using the Mendelian Randomization (MR) technique. The primary objective is to determine whether a causal relationship exists between these EBV antibodies and prostate cancer.
Methods
Genome-wide association study (GWAS) data for EBV antibodies were sourced from the UK Biobank cohort, and prostate cancer data were obtained from the PRACTICAL consortium, which includes 79148 cases and 61106 controls. Univariable Mendelian Randomization (MR) analysis was conducted to evaluate the associations, while reverse Mendelian Randomization was employed to assess causality. Additionally, Multivariable Mendelian Randomization analysis was performed to identify independent risk factors.
Results
Univariable MR analysis revealed significant associations between EBV EA-D (OR = 1.084, 95% CI = 1.012-1.160, IVW_P = 0.021) and EBNA-1 (OR = 1.086, 95% CI = 1.025-1.150, IVW_P = 0.005) antibodies and an increased risk of prostate cancer. Reverse MR analysis did not establish a causal relationship. Multivariable MR analysis identified the EBV EBNA-1 antibody as an independent risk factor for prostate cancer (OR = 1.095, 95% CI = 1.042-1.151, IVW_P = 0.00036).
Conclusion
The study highlights the association between EBV antibody levels, particularly EBNA-1, and prostate cancer risk, suggesting EBNA-1 as an independent risk factor. Future research is needed to elucidate the biological pathways linking EBV antibody levels to prostate cancer. These insights could be instrumental in developing targeted prevention strategies and therapeutic interventions for prostate cancer.
Keywords: prostate cancer, epstein-barr virus, mendelian randomization, genetic epidemiology
Introduction
Prostate cancer is a prevalent type of cancer globally and contributes significantly to cancer-related mortality.1,2 PCa is a complicated condition that is influenced by a variety of environmental, physiological, immunological, and genetic factors. 3 Nonmodifiable risk factors for PCa include age, race, family history, and germline mutations, while possible modifiable risk factors are metabolic syndrome, obesity, and smoking. 4 Various environmental, lifestyle, infectious, and dietary factors could contribute to the development of PCa, but the evidence backing this claim is typically lacking. 4 Notably, among environmental factors, viral infections emerge as potential triggers for prostate cancer. Multiple studies have demonstrated a positive association between human papillomavirus (HPV) and overall PCa.5-8 There may be an association between Epstein-Barr virus, herpes simplex 1 and 2, Neisseria gonorrhea and PCa, but results were conflicting. 9
Epstein-Barr virus-associated diseases pose significant global health concerns. Epstein-Barr Virus (EBV), identified in 1964 as the initial human cancer-causing virus, is a highly prevalent virus among humans, with an impact on almost 90% of the global population.10-12 Belonging to the human herpes virus family with a double-strand DNA genome, EBV is primarily transmitted through saliva and has the capacity to infect both B cells and epithelial cells. 13 This virus typically maintains a lifelong latent asymptomatic infection. Under certain conditions that are still not well understood, EBV plays an etiological and pathogenic role in various cancers, encompassing both epithelial- and lymphatic-originated tumors.14-17 Upon infection with EBV, the human immune system responds by producing antibodies that specifically target EBV antigens. These antibodies play a crucial role in diagnosing EBV infection and determining its stage. After the initial infection, antibodies against viral capsid antigen (VCA) appear quickly, peak at 3 weeks, and then decrease but remain present indefinitely. Antibodies against EBV nuclear antigen (EBNA) are absent in the early stages of infection, but emerge 2-4 months later and remain as indicators of previous exposure. Notably, EBNA-1 is associated with infection, and EBNA-2 is linked to cell transformation and immortality.16,18 Levels of antibodies against early antigen (EA) increase during initial infection and in cases of EBV reactivation. 16 The activation of replication by ZEBRA (BamH1 Z encoded activator) occurs at the same time as the transition of EBV from the dormant to active phase. 19 Thus, increased amounts of anti-ZEBRA antibodies could be a sign of recent EBV reactivation.
Extensive literature has documented the association between the presence of antibodies against EBV proteins and the elevated risk of malignancies attributed to EBV. There is evidence suggesting that high levels of immunoglobulin A antibodies before diagnosis are linked to an increased risk of nasopharyngeal carcinoma. Additionally, patients with Hodgkin lymphoma tend to have significantly higher levels of immunoglobulin G antibodies compared to disease-free individuals.20-23 EBV related antibodies have been used as potential screening biomarkers in high-risk areas of nasopharyngeal carcinoma in China to improve early diagnosis.24-26 Nevertheless, the connection between the immune reaction to EBV and other cancers linked to EBV was not as well-defined, and its utilization in PCa is infrequent. Conventional research methods face obstacles from confounding factors and reverse causality, complicating the establishment of a direct link between EBV antibodies and prostate cancer. Given the documented links between EBV and various malignancies, further investigation into the association between EBV and prostate cancer is essential. Understanding the potential role of EBV reactivation and its antibodies in prostate cancer development could offer new insights into its etiology and support the identification of novel biomarkers for early detection and prevention.
Over the past decade, the application of Mendelian randomization (MR) has become a prominent strategy within the domain of epidemiological research. This epidemiological approach capitalizes on the genetic architecture, particularly focusing on single nucleotide polymorphisms (SNPs), to elucidate the causal pathways linking risk factors to disease development. 27 Phenotype-related genetic variants are used as instrumental variables (IV) in MR to investigate the causal inference of exposure-outcome associations.27-29 In particular, MR analysis is less susceptible to bias from confounding or reverse causality since the genotype is not influenced by the disease. Thus, MR analysis is more resistant to confounding, reverse causality, and measurement error compared to traditional observational studies. 30 In this study, we have applied two-sample MR and Multivariable Mendelian Randomization (MVMR) analyses to rigorously evaluate the causal associations between four specific EBV antibodies and the risk of prostate cancer. This approach enables us to mitigate potential biases inherent in traditional observational studies, such as confounding and reverse causality, thereby providing more robust insights into the potential causal pathways linking EBV antibodies to prostate cancer risk.
Materials and methods
Study Design
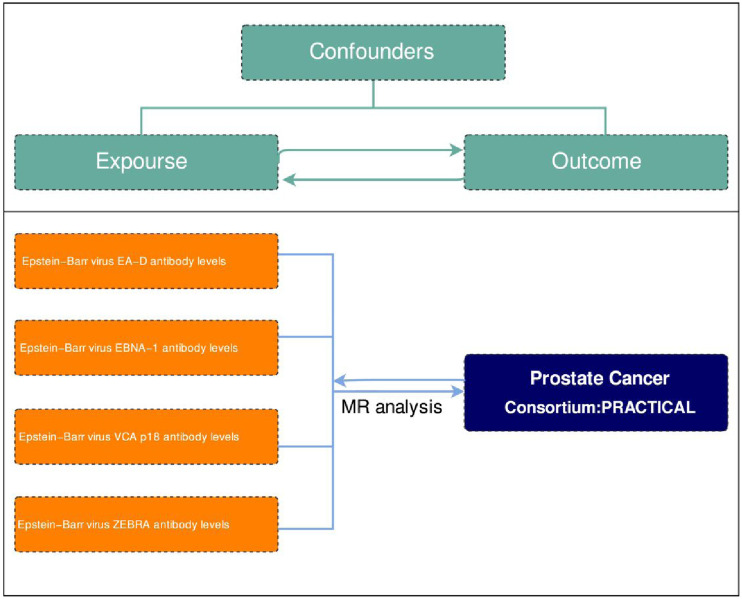
Genetic instrumental variables, in the form of SNPs reflecting phenotypic differences, were chosen to perform a two-sample MR analysis. The genetic instrumental variables used to study phenotypes must satisfy 3 key assumptions outlined in Figure 1: 1. They must have a strong association with the phenotype in question; 2. They should not be influenced by any potential confounders that could affect the relationship between exposure and outcome; 3. There should be no direct connections between the instrumental variables and the outcome being studied. 31 MR analysis was conducted to evaluate bidirectional causality between Prostate cancer and Epstein-Barr virus antibody (EA-D, EBNA-1, VCA p18, ZEBRA). Ethical approval for this study was deemed unnecessary as it exclusively utilized publicly accessible genome-wide association study (GWAS) data from the FinnGen database and the UK Biobank. Our research was meticulously conducted in strict accordance with the most recent Strengthening the Reporting of Observational Studies in Epidemiology utilizing Mendelian randomization (STROBE-MR) guidelines, which serve as a comprehensive framework to enhance the transparency and reliability of observational studies employing MR techniques to infer causal relationships in epidemiological research. 32
Figure 1.
Two-sample Mendelian randomization analysis flow chart.
Data Source
In the present study, four types of serum EBV antibodies were evaluated as exposure traits. The GWAS summary data were sourced from the research conducted by Guillaume Butler-Laporte et al 33 (Supplement Table 1). This investigation utilized the UK Biobank cohort, which encompasses up to 10 000 serological measurements of infectious diseases along with genome-wide genotyping. Antibodies were quantified using fluorescent bead-based multiplex serology on the Luminex 100 platform (Luminex Corporation, Austin, TX, USA), with median fluorescence intensity (MFI) as the output. For the GWASs, the researchers selected pathogens with a seroprevalence >15% to ensure sufficient statistical power. In this study, the GWAS data for prostate cancer were derived from the PRACTICAL consortium, encompassing 79148 cases and 61106 control subjects 34 (Supplement Table 1). This study includes two meta-analyses: (1) an evaluation of early-onset prostate cancer risk (n = 51244), comprising 6988 cases diagnosed by age 55 and 44256 non-prostate cancer controls; and (2) an assessment of advanced prostate cancer risk (n = 73475), with 15167 cases of advanced disease and 58308 controls. Most studies involved both case and control groups, but clinical characteristics were not matched. Advanced cases were characterized by metastatic prostate cancer, Gleason scores of 8 or higher, prostate-specific antigen (PSA) levels above 100 ng/mL, or prostate cancer-related deaths. All GWAS datasets utilized in this research were sourced from the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/).
SNPs Selection
In this study, instrumental variables were constructed by SNPs associated with four EBV antibody levels. During our comprehensive genetic analysis, we specifically targeted genetic variants that exhibited a significant association with the exposure traits, as determined by a genome-wide significance level with a P-value threshold of less than 5 × 10−8. Furthermore, we imposed stringent conditions on linkage disequilibrium, selecting only those variants with an r2 value of less than or equal to 0.001, and we ensured that the variants were separated by a substantial physical distance of at least 10 megabases (Mb). Given that palindromic SNPs can introduce ambiguity, all analyses were performed again after excluding palindromic SNPs. Studies have shown that there is a relationship between smoking, BMI and PCa, and affect the survival prognosis of PCa patients.35-38 Therefore, to avoid potential confounding factors between EBV antibodies and prostate cancer due to the secondary characteristics of the selected SNPS, we retrieve the PhenoScannerV2 Website (https://www.phenoscanner.medschl.cam.ac.uk/)and remove with may potentially confounding factors related SNP (P for trait-associated SNPs< 5 × 10−8; r2 for LD >0.8 in EUR).
We also calculated the F statistic for the instrumental variable of each exposure trait. An F statistic exceeding 10 was considered sufficient to rule out weak instrument bias. Each SNP, the F statistic was calculated using the formula F = R2*(N-2)/(1-R2) where R2 = β2/(β2+SE2*N). Within the context of our study, the symbol ‘N' refers to the total number of subjects included in the exposure cohort. The parameter 'β' signifies the estimated genetic effect size attributable to the SNP in question, which is utilized to evaluate its predictive power concerning the outcome variable. Additionally, ‘Se’ denotes the standard error associated with the genetic effect estimate, providing a measure of the precision of the β parameter.39-41
Statistical Analysis
Adhering to the MR analysis guidelines, 42 the inverse variance weighting (IVW) technique was selected as the principal analytical approach to estimate the causal effect. This choice was predicated on the method’s enhanced statistical efficacy, which is particularly evident when the instrumental variables adhere to the 3 critical assumptions necessary for valid causal inference. To mitigate the potential influence of heterogeneity and horizontal pleiotropy, 43 3 supplementary MR methods were implemented: the MR-Egger regression, which is sensitive to the detection of pleiotropy; the Weighted median approach, designed to be robust against outliers; and the Weighted mode method, which offers an additional layer of statistical robustness. The findings derived from the MR analyses were articulated through the computation of odds ratios (ORs), accompanied by their respective 95% confidence intervals (CIs), thereby furnishing a quantifiable measure of the association’s precision and statistical reliability. Furthermore, to ascertain the robustness of the MR outcomes, we conducted a series of sensitivity analyses. These included the Cochran’s Q statistic to assess heterogeneity, the MR-Egger regression intercept test for detecting pleiotropy, the MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) method for identifying and adjusting for potential outliers, and the Leave-One-Out sensitivity analysis to evaluate the directional consistency of the results. The presence of heterogeneity among the estimated effects of each genetic variant was assessed using Cochran’s Q test in the analysis. 44 A P value greater than 0.05 indicates the absence of heterogeneity.44,45 Horizontal pleiotropy was evaluated through MR-Egger intercept analysis. 29 When instances of heterogeneity or pleiotropy were observed within the MR findings, we employed the MR-PRESSO method to discern and address potential outliers, thereby ensuring the integrity and reliability of our MR analysis. 43 We conducted Leave-One-Out sensitivity analysis to assess how individual genetic variants affect the overall causal estimate. The process includes eliminating a single genetic variation from the examination and reevaluating the causal estimation to assess the influence of each specific variation. 46 Additionally, we conducted Multivariable Mendelian Randomization (MVMR). For MVMR analyses, instruments were constructed using SNPs from each GWAS satisfying our previously described single-variable MR selection criteria. To mitigate the potential for reverse causation in Mendelian randomization, this study conducted bidirectional MR analyses and employed the Steiger test. 46 The R software (version 4.2.2) was used for all analyses in this study, employing the “TwoSampleMR” (version 0.5.6) and “MRPRESSO” (version 1.0) packages.
Results
Causal Effects of Four Epstein-Barr Virus Antibodies on the Risk of Prostate Cancer
All SNPs exhibited F statistics surpassing 10 (Supplement Table 2), thus minimizing the potential influence of weak instrument bias on the study outcomes. Cochran’s Q test P values were all greater than 0.05, we used fixed-effect IVW model. Figure 2 illustrates the estimated effects of MR analysis on the association between four EBV antibody levels and prostate cancer. The results indicate a positive causal relationship between high levels of EBV EA-D antibodies and the occurrence of prostate cancer (OR = 1.084, 95%CI = 1.012-1.160, P = 0.021). Meanwhile, the beta direction of MR-Egger, Weighted Median and Weighted Mode were consistent with IVW, and the P values were< 0.05. Similarly, based on the IVW method, it is indicated that elevated levels of EBNA-1 antibodies are significantly associated with an increased risk of prostate cancer (OR = 1.086, 95% CI = 1.025-1.150, P = 0.005). The other 3 methods also demonstrate similar results: MR-Egger (OR = 1.172,95% CI = 1.046-1.314,P = 0.018),Weighted Median (OR = 1.149, 95%CI = 1.068-1.236, P < 0.001), Weighted Mode (OR = 1.155, 95% CI = 1.064-1.254, P = 0.004) (Figure 2). However, it was evident that Epstein-Barr virus VCA p18 antibody levels or Epstein-Barr virus ZEBRA antibody levels had no causal relationship with prostate cancer (P value for IVW: 0.803 and 0.847, respectively) (Figure 2).
Figure 2.
Mendelian randomization analysis of four EBV antibodies and prostate cancer.
In the sensitivity analysis, there was no heterogeneity among the SNPs for the exposure traits according to Cochran’s Q test results. Intercept value of MR-Egger regression P < 0.05, demonstrated the absence of horizontal pleiotropy. MR-PRESSO did not find outliers, and the global test also proved the absence of horizontal pleiotropy (Supplement Table 5). Leave-one-out sensitivity analysis showed that no single SNP strongly drove the overall effect of each exposure on prostate cancer. The leave-one-out plots are shown in Supplement Figure 1.
Causal Effects of Prostate Cancer on Four Epstein-Barr Virus Antibodies
To avoid the potential impact of reverse causation, an analysis was conducted considering prostate cancer as the exposure factor, specifically examining the reverse causal relationship with four Epstein-Barr virus antibody levels. With F statistics exceeding 10 for all SNPs analyzed (Supplement Table 2), the potential bias from weak instrumental variables on the study’s findings can be considered negligible.
Based on the IVW approach, prostate cancer did not show causal effects on four Epstein-Barr virus antibody levels (P value: 0.690 to 0.814) (Figure 3). We found no evidence linking prostate cancer with Epstein-Barr virus EA-D antibody levels (OR = 1.008, 95%CI = 0.971-1.046, P = 0.692). Prostate cancer and Epstein-Barr virus EBNA-1 antibody levels were also not relevant (OR = 0.992, 95%CI = 0.955-1.029, P = 0.658) (Figure 3). And we did not find strong evidence that genetically predicted prostate cancer has a causal relationship with Epstein-Barr virus VCA p18 antibody levels (OR = 1.007, 95%CI = 0.972-1.044, P = 0.690). Epstein-Barr virus ZEBRA antibody levels and PCa were also not relevant (OR = 1.004, 95%CI = 0.969-1.041, P = 0.814) (Figure 3). The leave-one-out plots are shown in Supplement Figure 2. The results were consistent with estimates made using MR-Egger, Weighted median or Weighted Mode. Upon examination of the MR-Egger intercept test outcomes, with P values ranging from 0.196 to 0.971, and the Cochran’s Q test results, with P values spanning from 0.859 to 0.999, our analysis yielded no statistically significant indications of pleiotropy or heterogeneity within the MR study (Supplement Table 5). The MR Steiger directionality test indicated the plausibility of all causal directions examined, while the MR-PRESSO test did not identify any outlying data points.
Figure 3.
Reverse Mendelian randomization results for four EBV antibodies and prostate cancer.
Multivariable Mendelian Randomization Between Epstein-Barr Virus Antibody Levels and Prostate Cancer
We conducted multivariable Mendelian randomization analysis to ascertain whether the levels of four Epstein-Barr virus antibodies are independent risk factors for prostate cancer. Based on the IVW approach, Epstein-Barr virus ENBA1 antibody levels showed a causal effect on PCa (P value for IVW: 0.00036, Supplement Table 7). The results were consistent with estimates made using MR-Egger (P value for MR-Egger: 0.00049). The remaining 3 antibodies and PCa were not relevant (P value for IVW: 0.320 to 0.959). Based on the results of the MR-Egger intercept test and Cochran’s Q test, we found no evidence for the presence of pleiotropy and heterogeneity.
Discussion
Our results demonstrated that genetically predicted Epstein‐Barr virus EA-D antibody levels was associated with an increased risk of Prostate cancer (IVW: OR = 1.084; P value = 0.021). Genetically predicted Epstein-Barr virus EBNA-1 antibody levels had a strong association with prostate cancer (IVW: OR = 1.086; P value = 0.005). Genetically predicted Epstein‐Barr virus VCA p18 antibody levels (IVW: OR = 1.007; P value = 0.803) or Epstein‐Barr virus ZEBRA antibody levels (IVW: OR = 0.992; P value = 0.847) had no causal association with prostate cancer. Furthermore, reverse Mendelian randomization analysis revealed no causal association between prostate cancer as an exposure trait and the studied outcomes. Subsequent multivariable MR analysis identified a causal relationship between Epstein-Barr virus ENBA1 antibody and prostate cancer. This suggests that the Epstein-Barr virus ENBA1 antibody may serve as a potential biomarker for prostate cancer, providing new evidence for research into the link between EBV and prostate cancer.
EBV encodes a variety of antigens, including VCA, EA, membrane antigen (MA), nuclear antigen (NA), etc. The body recognizes these EBV virus antigens, leading to the production of specific antibodies. Additionally, EBV infection can induce B cell activation, resulting in an increased production of IgM, IgG, and IgA antibodies. B lymphocytes are implicated as the principal viral reservoir, with asymptomatic carriers exhibiting remarkably low viral burdens, typically infecting only around 0.01% of B cells. 47 In EBV-associated malignancies, the virus exhibits a robust association, infecting virtually all neoplastic cells. These cancers predominantly manifest as B-cell lymphomas and epithelial-derived carcinomas. Examples include nasopharyngeal carcinoma, Hodgkin lymphoma, various subtypes of non-Hodgkin lymphoma, gastric cancer (EBV-associated gastric cancer), natural killer (NK)/T cell lymphoma, and leiomyosarcoma.48,49
We have observed that numerous studies in the existing literature have investigated antibodies against these four EBV proteins (EA-D, EBNA1, VCA and ZEBRA) in the context of EBV-related malignant tumors. In a retrospective study, all nasopharyngeal carcinoma cases showed typical reactivation patterns (IgG VCA+, IgG EA+, IgG EBNA+) in the serum, and IgA-EA-D antibodies were also present in 40% of cases. 50 Other studies have demonstrated that the combined detection of EBNA-1 and EA-D is more effective than the detection of other antibodies in identifying nasopharyngeal carcinoma. 51
Multiple antibody evaluations can enhance our understanding of the role of the EBV immune response in the development of various cancers. Literature reports indicate an increase in ZEBRA antibody titers among EBV patients with Hodgkin’s disease tumor cells. 52 Additionally, it has been observed that breast cancer patients with high titers of anti-ZEBRA antibodies experience poorer overall survival. 53 Two case-control studies have yielded consistent results regarding the association between EBV antibodies and the risk of gastric cancer. A population-based retrospective case-control study conducted in Spain revealed that elevated reactivity to EBNA-1 and VCA-p18 proteins is linked to an increased risk of gastric cancer. 54 Another population-based nested case-control study carried out in southern China discovered that higher pre-diagnostic serum levels of EBNA1-IgA and VCA-IgA were associated with a 2-fold increase in subsequent gastric cancer risk. Moreover, the combination of EBNA1-IgA and VCA-IgA indicated an almost 7-fold association between high serological risk and an elevated subsequent risk of gastric cancer. 55 Since EBV infection is so common, positive EBV antibodies may not serve as a reliable indicator of PCa risk, and the measurement of specific EBV antibodies might provide more informative insights. Nevertheless, the existing literature lacks sufficient discussion on this aspect. Based on our conclusion, it can be inferred that EA-D and EBNA1 antibiotics may function as potential biomarkers for prostate cancer.
The results of this study warrant further consideration of etiology: Elevated EA-D and EBNA1 antibodies before disease may support the direct carcinogenic role of EBV in prostate cancer, or changes in antibody levels near diagnosis may reflect a higher prevalence of impaired immunity in patients with PCa. In addition to etiological insights, the link between anti-EBV antibodies and PCa risk may eventually translate into biomarkers that can identify people who are more likely to develop PCa. It would be fascinating to investigate the role of antibodies produced by EBV infection in PCa progression. Altogether, it is still unclear whether EBV antibody levels play a promoting or bystander role in the development of prostate cancer. From the perspective of genetic etiology, elucidating the connection is crucial for enhancing treatment strategies and facilitating the development of diagnostic and prognostic markers.
In summary, our work reveals a positive association between EBNA-1 antibody levels and Epstein‐Barr virus EA-D antibody levels and risk of prostate cancer. We thus postulate that EBNA-1 antibody and Epstein‐Barr virus EA-D antibody may be potential markers for early detection or screening of prostate cancer. Specific EBV antibodies may advance our understanding of the role of humoral immune responses in the development of prostate cancer. Future work could examine a broader range of viral epitopes, antibody functional types, and immunoglobulin classes and subclasses. It is also possible to assess whether changes in specific EBV antibody types over time predict prostate cancer risk and whether it interacts with other possible cancer markers, such as prostate-specific antigens, as well as potential biological mechanisms by which EBV is carcinogenic in prostate cancer and other malignancies.
There are several strengths of our study. Firstly, one key strength of our work is the use of large-scale GWAS data and a two-sample MR approach to assess the bidirectional causal association between four Epstein-Barr virus antibody levels and prostate cancer. This method is less susceptible to the influence of confounding factors. Furthermore, we conducted rigorous identification of Epstein‐Barr virus antibody types to mitigate bias arising from the co-existence of four types. Additionally, we performed a series of sensitivity analyses to evaluate the robustness of our findings.
However, our study also has several limitations. Our study was constrained by the absence of data pertaining to the EBV status within the tumor samples, a critical parameter for ascertaining the specificity of the observed association between anti-EBV antibodies and EBV-positive prostate cancer. Moreover, we acknowledge the limitation of uncontrolled potential residual confounders, such as smoking, androgen levels, obesity, dietary factors, and family history of prostate cancer. Despite our diligent efforts to mitigate the influence of confounding factors by incorporating adjustments for recognized variables, the potential for residual confounding remains a consideration that cannot be fully discounted. Therefore, a prudent approach is warranted in the interpretation of our findings, and subsequent research endeavors may derive significant value from enhanced endeavors to quantify and account for these latent confounding elements.
Conclusions
Our investigation, utilizing bidirectional Mendelian Randomization, delved into the potential links between specific EBV antibodies and prostate cancer risk. While our analysis highlighted associations between EBV EA-D and EBNA-1 antibodies with an increased risk of prostate cancer. Additionally, our study identified EBV EBNA-1 antibody as an independent risk marker for prostate cancer in the multivariable analysis. These findings underscore the complexity of interpreting associations in epidemiological studies and highlight the need for cautious interpretation when inferring causality. The study contributes to the broader understanding of the potential role of viral infections in cancer etiology, particularly prostate cancer, and underscores the importance of comprehensive analyses to unravel the intricate nature of such associations.
Supplemental Material
Supplemental Material for A Bidirectional Mendelian Randomization Study on the Causal Relationship Between Epstein-Barr Virus Antibodies and Prostate Cancer Risk by Xiao-bo Ding Si-yan Ren, He-zhi Wen3, Zhi-bin Zhang, Jia-ang Ye, Wen-kai Pan and Jia-qi Ye in Cancer Control.
Supplemental Material for A Bidirectional Mendelian Randomization Study on the Causal Relationship Between Epstein-Barr Virus Antibodies and Prostate Cancer Risk by Xiao-bo Ding Si-yan Ren, He-zhi Wen3, Zhi-bin Zhang, Jia-ang Ye, Wen-kai Pan and Jia-qi Ye in Cancer Control.
Supplemental Material for A Bidirectional Mendelian Randomization Study on the Causal Relationship Between Epstein-Barr Virus Antibodies and Prostate Cancer Risk by Xiao-bo Ding Si-yan Ren, He-zhi Wen3, Zhi-bin Zhang, Jia-ang Ye, Wen-kai Pan and Jia-qi Ye in Cancer Control.
Appendix.
Abbreviations
- EBV
Epstein-Barr Virus
- NPC
Nasopharyngeal Carcinoma
- EBNA-1
Epstein-Barr Nuclear Antigen 1
- VCA
Viral Capsid Antigen
- SNP
Single-Nucleotide Polymorphisms
- MR
Mendelian Randomization
- IV
Instrumental Variable
- PCa
Prostate Cancer
- HPV
Human Papillomavirus
- GWAS
Genome-Wide Association Study
- MVMR
Multivariable Mendelian Randomization
- OR
Odds ratio
- CI
Confidence Interval
- STROBE-MR
Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization
Author’s Note: Xiao-bo Ding is also affiliated with Department of Ultrasound, Sir Run Run Shaw Hospital, Hangzhou, China, The First Affiliated Department of Oncology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China.
Si-yan Ren is also affiliated with the Department of Radiation Oncology, Sir Run Run Shaw Hospital, Hangzhou, China, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, China.
Wen-kai Pan is also affiliated with the Department of Nuclear Medicine, Sir Run Run Shaw Hospital, Hangzhou, China, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China.
Author Contributions: All authors have read and agreed to the published version of the manuscript. DXB, RSY, and WHZ conceptualized and designed experiments; ZZB and YJA wrote manuscript; PWK carried out experiments; PWK and YJQ analyzed experiments results. DXB, RSY, WHZ, PWK, and YJQ revised the manuscript, figures, and tables.
The authors affirm that this research has been executed free from any affiliations or financial interests that might be perceived as constituting conflicts of interest. The study’s integrity is thereby ensured, with no influence from external commercial entities.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
Ethics Statement
Ethics Approval
Summary data were obtained from published studies with prior institutional approvals. Secondary analysis of these data did not require additional ethical permits.
ORCID iD
Jia-qi Ye https://orcid.org/0009-0008-9528-6617
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2.Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77(1):38-52. [DOI] [PubMed] [Google Scholar]
- 3.Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev. 2018;32(17-18):1105-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandaglia G, Leni R, Bray F, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. 2021;4(6):877-892. [DOI] [PubMed] [Google Scholar]
- 5.Russo GI, Calogero AE, Condorelli RA, Scalia G, Morgia G, La Vignera S. Human papillomavirus and risk of prostate cancer: a systematic review and meta-analysis. Aging Male. 2020;23(2):132-138. [DOI] [PubMed] [Google Scholar]
- 6.Yin B, Liu W, Yu P, et al. Association between human papillomavirus and prostate cancer: a meta-analysis. Oncol Lett. 2017;14(2):1855-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson JS, Glenn WK. Evidence for a causal role by human papillomaviruses in prostate cancer - a systematic review. Infect Agents Cancer. 2020;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghoofei M, Keshavarz M, Ghorbani S, et al. Association between human papillomavirus infection and prostate cancer: a global systematic review and meta-analysis. Asia Pac J Clin Oncol. 2019;15(5):e59-e67. [DOI] [PubMed] [Google Scholar]
- 9.Lawson JS, Glenn WK. Multiple pathogens and prostate cancer. Infect Agents Cancer. 2022;17(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343(7):481-492. [DOI] [PubMed] [Google Scholar]
- 11.Tsurumi T, Fujita M, Kudoh A. Latent and lytic Epstein-Barr virus replication strategies. Rev Med Virol. 2005;15(1):3-15. [DOI] [PubMed] [Google Scholar]
- 12.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757-768. [DOI] [PubMed] [Google Scholar]
- 13.Farrell PJ. Epstein-Barr virus and cancer. Annu Rev Pathol. 2019;14:29-53. [DOI] [PubMed] [Google Scholar]
- 14.Xu M, Zhang WL, Zhu Q, et al. Genome-wide profiling of Epstein-Barr virus integration by targeted sequencing in Epstein-Barr virus associated malignancies. Theranostics. 2019;9(4):1115-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes D. The cancer-virus cures. Nat Med. 2014;20(6):571. [DOI] [PubMed] [Google Scholar]
- 16.Young LS, Yap LF, Murray PG. Epstein-Barr virus: more than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16(12):789-802. [DOI] [PubMed] [Google Scholar]
- 17.Klein G. Tumor associations of EBV--historical perspectives. Curr Top Microbiol Immunol. 2015;390(Pt 1):17-22. [DOI] [PubMed] [Google Scholar]
- 18.Jiang S, Zhou H, Liang J, et al. The epstein-barr virus regulome in lymphoblastoid cells. Cell Host Microbe. 2017;22(4):561-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaeffner M, Mrozek-Gorska P, Buschle A, et al. BZLF1 interacts with chromatin remodelers promoting escape from latent infections with EBV. Life Sci Alliance. 2019;2(2):e201800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao SM, Liu Z, Jia WH, et al. Fluctuations of epstein-barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PLoS One. 2011;6(4):e19100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chien YC, Chen JY, Liu MY, et al. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345(26):1877-1882. [DOI] [PubMed] [Google Scholar]
- 22.Ji MF, Wang DK, Yu YL, et al. Sustained elevation of Epstein-Barr virus antibody levels preceding clinical onset of nasopharyngeal carcinoma. Br J Cancer. 2007;96(4):623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu KJ, Hsu WL, Pfeiffer RM, et al. Prognostic utility of anti-EBV antibody testing for defining NPC risk among individuals from high-risk NPC families. Clin Cancer Res. 2011;17(7):1906-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildesheim A. Invited commentary: epstein-Barr virus-based screening for the early detection of nasopharyngeal carcinoma: a new frontier. Am J Epidemiol. 2013;177(3):251-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Ji MF, Huang QH, et al. Two Epstein-Barr virus-related serologic antibody tests in nasopharyngeal carcinoma screening: results from the initial phase of a cluster randomized controlled trial in Southern China. Am J Epidemiol. 2013;177(3):242-250. [DOI] [PubMed] [Google Scholar]
- 26.Ji MF, Sheng W, Cheng WM, et al. Incidence and mortality of nasopharyngeal carcinoma: interim analysis of a cluster randomized controlled screening trial (PRO-NPC-001) in southern China. Ann Oncol. 2019;30(10):1630-1637. [DOI] [PubMed] [Google Scholar]
- 27.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CH, Cook S, Lee JS, Han B. Comparison of two meta-analysis methods: inverse-variance-weighted average and weighted Sum of Z-scores. Genomics Inform. 2016;14(4):173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj. 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. Bmj. 2021;375:n2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler-Laporte G, Kreuzer D, Nakanishi T, Harroud A, Forgetta V, Richards JB. Genetic determinants of antibody-mediated immune responses to infectious diseases agents: a genome-wide and hla association study. Open Forum Infect Dis. 2020;7(11):ofaa450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumacher FR, Al Olama AA, Berndt SI, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergengren O, Pekala KR, Matsoukas K, et al. 2022 update on prostate cancer epidemiology and risk factors-A systematic review. Eur Urol. 2023;84(2):191-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darcey E, Boyle T. Tobacco smoking and survival after a prostate cancer diagnosis: a systematic review and meta-analysis. Cancer Treat Rev. 2018;70:30-40. [DOI] [PubMed] [Google Scholar]
- 37.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vidal AC, Oyekunle T, Howard LE, et al. Obesity, race, and long-term prostate cancer outcomes. Cancer. 2020;126(16):3733-3741. [DOI] [PubMed] [Google Scholar]
- 39.Palmer TM, Lawlor DA, Harbord RM, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21(3):223-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill D, Efstathiadou A, Cawood K, Tzoulaki I, Dehghan A. Education protects against coronary heart disease and stroke independently of cognitive function: evidence from Mendelian randomization. Int J Epidemiol. 2019;48(5):1468-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levin MG, Judy R, Gill D, et al. Genetics of height and risk of atrial fibrillation: a Mendelian randomization study. PLoS Med. 2020;17(10):e1003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowden J, Del Greco M F, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(11):e1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9(3):395-404. [DOI] [PubMed] [Google Scholar]
- 48.Jarrett AF, Armstrong AA, Alexander E. Epidemiology of EBV and Hodgkin's lymphoma. Ann Oncol. 1996;7 Suppl 4(Suppl 4):5-10. [DOI] [PubMed] [Google Scholar]
- 49.Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732):20160270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorusso F, Caleca MP, Bellavia C, et al. The EBV-DNA can be used as a diagnostic and follow-up parameter of the rhinopharyngeal tumors in the non-endemic population of the western sicily. Indian J Otolaryngol Head Neck Surg. 2019;71(3):396-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow KC, Ma J, Lin LS, et al. Serum responses to the combination of Epstein-Barr virus antigens from both latent and acute phases in nasopharyngeal carcinoma: complementary test of EBNA-1 with EA-D. Cancer Epidemiol Biomarkers Prev. 1997;6(5):363-368. [PubMed] [Google Scholar]
- 52.Drouet E, Brousset P, Fares F, et al. High Epstein-Barr virus serum load and elevated titers of anti-ZEBRA antibodies in patients with EBV-harboring tumor cells of Hodgkin's disease. J Med Virol. 1999;57(4):383-389. [DOI] [PubMed] [Google Scholar]
- 53.Marrão G, Habib M, Paiva A, et al. Epstein-Barr virus infection and clinical outcome in breast cancer patients correlate with immune cell TNF-α/IFN-γ response. BMC Cancer. 2014;14:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aragonés N, Fernández de Larrea N, Pastor-Barriuso R, et al. Epstein Barr virus antibody reactivity and gastric cancer: a population-based case-control study. Cancer Epidemiol. 2019;61:79-88. [DOI] [PubMed] [Google Scholar]
- 55.Du Y, Yu X, Chang ET, et al. EBV antibody and gastric cancer risk: a population-based nested case-control study in southern China. BMC Cancer. 2023;23(1):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for A Bidirectional Mendelian Randomization Study on the Causal Relationship Between Epstein-Barr Virus Antibodies and Prostate Cancer Risk by Xiao-bo Ding Si-yan Ren, He-zhi Wen3, Zhi-bin Zhang, Jia-ang Ye, Wen-kai Pan and Jia-qi Ye in Cancer Control.
Supplemental Material for A Bidirectional Mendelian Randomization Study on the Causal Relationship Between Epstein-Barr Virus Antibodies and Prostate Cancer Risk by Xiao-bo Ding Si-yan Ren, He-zhi Wen3, Zhi-bin Zhang, Jia-ang Ye, Wen-kai Pan and Jia-qi Ye in Cancer Control.
Supplemental Material for A Bidirectional Mendelian Randomization Study on the Causal Relationship Between Epstein-Barr Virus Antibodies and Prostate Cancer Risk by Xiao-bo Ding Si-yan Ren, He-zhi Wen3, Zhi-bin Zhang, Jia-ang Ye, Wen-kai Pan and Jia-qi Ye in Cancer Control.