Abstract
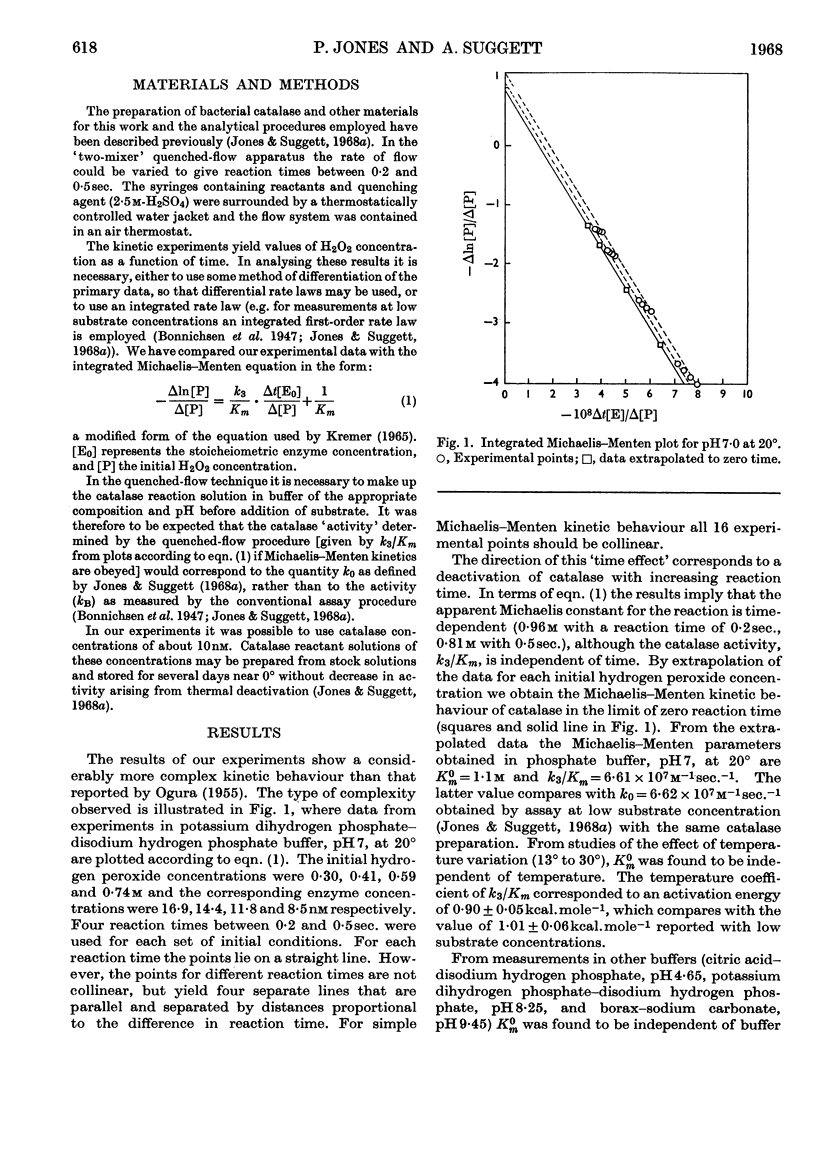
1. A re-examination of the catalase–hydrogen peroxide reaction at high substrate concentrations, by using the quenched-flow technique, reveals a more complex kinetic behaviour than that previously reported. At constant reaction time the catalatic process obeys Michaelis–Menten kinetics, but the apparent Michaelis constant is markedly time-dependent, whereas the conventional catalase activity is independent of time. 2. The kinetics of the `time effect' were analysed and it is suggested that the effect derives from the formation of an inactive species (thought to be catalase Compound II). The process shows Michaelis–Menten kinetics, with a Michaelis constant equal to that for the catalatic reaction in the limit of zero reaction time. 3. It has been confirmed that certain buffer components have marked inhibitory effects on the catalatic reaction and that, in unbuffered systems, catalatic activity is substantially independent of pH in the range 4·7–10·5.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- George P. The effect of the peroxide concentration and other factors on the decomposition of hydrogen peroxide by catalase. Biochem J. 1949;44(2):197–205. doi: 10.1042/bj0440197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P., Suggett A., Pain R. H. Sub-unit nature of catalase compound II. Nature. 1968 Mar 16;217(5133):1050–1050. doi: 10.1038/2171050a0. [DOI] [PubMed] [Google Scholar]
- Jones P., Suggett A. The catalase-hydrogen peroxide system. A theoretical appraisal of the mechanism of catalase action. Biochem J. 1968 Dec;110(4):621–629. doi: 10.1042/bj1100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P., Suggett A. The catalase-hydrogen peroxide system. Role of sub-units in the thermal deactivation of bacterial catalase in the absence of substrate. Biochem J. 1968 Aug;108(5):833–838. doi: 10.1042/bj1080833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGURA Y. Catalase activity at high concentration of hydrogen peroxide. Arch Biochem Biophys. 1955 Aug;57(2):288–300. doi: 10.1016/0003-9861(55)90291-5. [DOI] [PubMed] [Google Scholar]


