Abstract
Triple-negative breast cancer (TNBC) is an aggressive subtype with poor prognosis and limited treatment options. Chimeric antigen receptor (CAR)-T cell therapy holds promise, but its efficacy is hindered by tumor antigen escape and heterogeneity. To address these challenges, we developed a novel bispecific T cell engagers CAR-T (BiTEs CAR-T) targeting Mesothelin (MSLN) and secreting NKG2D-Bispecific T cell Engagers (BiTEs) to engage NKG2D ligands (NKG2DL). Analysis of TNBC tissues using The Cancer Genome Atlas and tumor microarrays revealed high but weakly correlated expression of MSLN and NKG2DL, making them ideal targets for dual engagement. To reduce immunogenicity and enhance stability, we used a nanobody and the natural receptor NKG2D as antigen-binding domains instead of traditional scFvs in the CAR construct. The secreted BiTEs could promote the cytotoxicity of untransduced T cells against NKG2DL + tumor cells. In vitro, BiTEs CAR-T cells exhibited superior cytotoxicity, T cell activation, and cytokines production against heterogeneous target cells compared to MSLN CAR-T. In vivo, BiTEs CAR-T cells demonstrated potent antitumor activity in zebrafish and murine TNBC models, significantly reducing tumor burden and prolonging survival without detectable toxicity. These findings suggest that BiTE CAR-T cells offer a highly promising therapeutic strategy for TNBC by addressing antigen heterogeneity and immune escape mechanisms, with promising translational potential for clinical application.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40164-025-00621-y.
Keywords: CAR-T therapy, BiTEs, Triple-negative breast cancer, Mesothelin, NKG2D ligands
To the editor,
Triple-negative breast cancer (TNBC) is the most malignant subtype of breast cancer with a poor prognosis [1]. Although chimeric antigen receptor (CAR)-T cell therapy has shown promise, its efficacy in TNBC is hampered by tumor antigen escape and heterogeneity [2, 3]. Targeting multiple tumor-associated antigens simultaneously could address these limitations. Bispecific T cell Engagers (BiTEs) redirect bystander T cells to tumor cells and was shown to circumvent antigen escape without detectable toxicity [4]. Therefore, we developed a novel BiTE-secreting CAR-T for TNBC treatment.
Single-target CAR-T cells targeting Mesothelin (MSLN) or NKG2D ligands (NKG2DL) show good safety in clinic, but limited efficacy [5, 6]. Our analysis of TCGA [7], showed high but independent expression of MSLN and NKG2DL in TNBC tissues (Fig. S1A, S1B). Immunohistochemistry validated these findings showing that 71.05% and 55.26% of TNBC samples were positive for MSLN and MICA/B, respectively, with only 15.79% negative for both (Fig. 1A, S1C). MSLN appears to be more specific than NKG2DL, as the latter can be induced by acute infection and chemotherapeutics [8]. Then we designed MSLN-targeted CAR-T cells secreting NKG2D-BiTEs (BiTEs CAR-T). To avoid possible immunogenicity and aggregation risks of scFvs, we used nanobody (VHH) with small, stable structure and low immunogenicity to target MSLN [9, 10], while the extracellular domain of NKG2D was used to target NKG2DL (Fig. 1B). Our BiTEs CAR-T possess advantages of low immunogenicity and high stability.
Fig. 1.
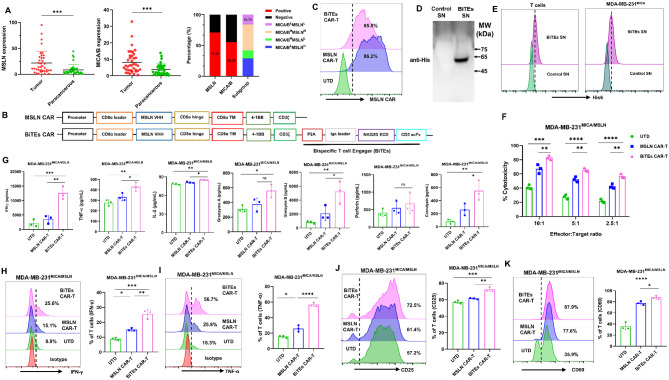
BiTEs CAR-T cells are efficacious against TNBC cells with heterogeneous antigens in vitro. (A) Expression (H-score) of Mesothelin (MSLN) and MICA/B in TNBC tissues and proportion of MSLN/MICA/B-positive patients in clinical cohort (n = 38). Subgroup analysis showed that the percentages of MICA/Blow/MSLNhigh, MICA/BhighMSLNlow, MICA/Bhigh/MSLNhigh, and MICA/Blow/MSLNlow patients were 28.95%, 13.16%, 42.11%, and 15.79% respectively. (B) Schematic diagrams of MSLN CAR and BiTEs CAR constructs. (C) MSLN and BiTEs CAR expression of T cells on day 7 using FACS. (D) Western blot analysis for concentrated BiTEs from culture supernatants of T cells transduced with BiTEs CAR or control. (E) Flow cytometry analysis demonstrating his-tag detection of concentrated BiTEs binding to NKG2DL on MDA-MB-231MICA and CD3 on T cells after incubation with BiTEs supernatant (SN). (F) The cytotoxic activity of UTD, MSLN CAR-T, and BiTEs CAR-T cells against MDA-MB-231MICA/MSLN (a mixture of 80% MDA-MB-231MICA and 20% MDA-MB-231MSLN) cells at E: T = 2.5, 5 and 10 using RTCA (n = 3). (G) MDA-MB-231MICA/MSLN cells were cultured with UTD, MSLN CAR-T, or BiTEs CAR-T cells at an E: T ratio of 5:1 for 24 h. IFN-γ, TNF-α, IL-2, granzyme A, granzyme B, perforin and granulysin were measured using a LEGENDplex multi-analyte Flow Assay Kit (n = 3). (H) Intracellular flow cytometry analysis of IFN-γ secreting CD3+ T cells after co-culturing UTD, MSLN CAR-T, or BiTEs CAR-T cells and MDA-MB-231MICA/MSLN cells at an E: T ratio of 5:1 for 24 h (n = 3). (I) Intracellular flow cytometry analysis of TNF-α secreting CD3+ T cells after co-culturing UTD, MSLN CAR-T, or BiTEs CAR-T cells and MDA-MB-231MICA/MSLN cells at an E: T ratio of 5:1 for 24 h (n = 3). (J) Flow cytometry analysis of expression of CD25 on different CD3+ T cells after co-culture with MDA-MB-231MICA/MSLN cells at an E: T ratio of 5:1 for 24 h (n = 3). (K) Flow cytometry analysis of expression of CD69 on different CD3+ T cells after co-culture with MDA-MB-231MICA/MSLN cells at an E: T ratio of 5:1 for 24 h (n = 3). Each experiment was repeated at least twice with similar results. Representative data are shown. Statistical significance was considered as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, ns, not significant
BiTEs CAR-T was generated as observed by MSLN CAR expression (Fig. 1C) and BiTEs secretion in culture supernatants using Western blot and ELISA (Fig. 1D, S2A). Transduction efficiency of BiTEs CAR-T was lower than MSLN CAR-T (Fig. S2B), CD4/CD8 ratios remain unchanged (Fig. S2C). Secreted BiTEs efficiently bind to CD3 on T cells and NKG2DL on tumor cells (Fig. 1E). To evaluate cytotoxicity, MICA-overexpressing MDA-MB-231 (MDA-MB-231MICA, Fig. S2D) cells were generated. BiTEs-containing supernatant enhanced untransduced T (UTD) cells cytotoxicity and activation, as indicated by increased CD69 expression and cytokine secretion (Fig. S2E). BiTEs CAR-T showed higher cytotoxicity against MDA-MB-231MICA and higher CD69 expression than MSLN CAR-T (Fig. S3A, B). The secreted IFN-γ, TNF-α, granzyme A, granzyme B, perforin and granulysin were elevated (Fig. S3C). Further assessment using MICA-overexpressing 293T (293TMICA) and MSLN-overexpressing 4T1 (4T1MSLN) cells (Fig. S3D, E) demonstrated that BiTEs CAR-T was cytotoxic only against NKG2DL + cells (Fig. S3F, G). BiTEs CAR-T also showed higher cytotoxicity against MDA-MB-231MICA compared to wild-type MDA-MB-231 (Fig. S3A, H).
We then evaluated BiTEs CAR-T activity in tumor cells with varying levels of MSLN and NKG2DL expression, including MDA-MB-468, HCT116, HeLa and MSLN-overexpressing MDA-MB-231 (MDA-MB-231MSLN) cells (Fig. S4A–C). BiTEs CAR-T exhibited higher cytotoxicity than MSLN CAR-T (Fig. S4D-G), and increased IFN-γ and TNF-α secretion (Fig. S4H, I). To simulate antigen heterogeneity, we mixed 20% MDA-MB-231MSLN and 80% MDA-MB-231MICA (MDA-MB-231MICA/MSLN). BiTEs CAR-T exhibited superior cytotoxicity across different E: T ratio (Fig. 1F) and induced higher levels of IFN-γ, TNF-α, IL-2, granzyme B and granulysin than MSLN CAR-T (Fig. 1G), confirmed by intracellular staining (Fig. 1H, I). CD25 and CD69 expression indicated enhanced T cell activation (Fig. 1J, K). Similar results were observed using 1:1 mixture of MDA-MB-231MSLN and MDA-MB-231MICA cells (Fig. S5).
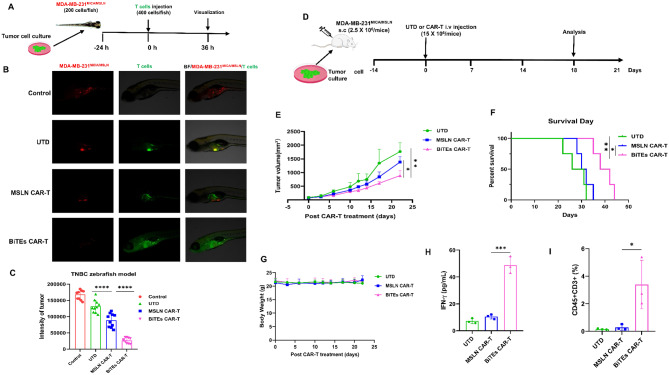
A zebrafish xenograft MDA-MB-231MICA/MSLN model was used to evaluate BiTEs CAR-T effect in vivo due to its efficiency and low cost [11]. BiTEs CAR-T significantly reduced tumor fluorescence intensity, confirming tumor regression (Fig. 2A–C). For the first time, we evaluated the efficacy of BiTEs CAR-T in a zebrafish model. We further assessed BiTEs CAR-T in an immunocompromised mouse model (Fig. 2D). BiTEs CAR-T effectively reduced tumor volume and prolonged survival compared to MSLN CAR-T (Fig. 2E, F), without significant weight loss (Fig. 2G). BiTEs CAR-T-treated mice showed higher levels of IFN-γ and increased CD3+ T cell percentages in blood samples (Fig. 2H, I, S6), indicating strong persistence and potency.
Fig. 2.
In vivo antitumor activity of BiTEs CAR-T cells against TNBC with heterogeneous antigens in zebrafish and mice models. (A) Schematic diagram of the zebrafish TNBC xenograft model. MDA-MB-231MICA/MSLN (a mixture of 80% MDA-MB-231MICA and 20% MDA-MB-231MSLN) cells were used to establish the TNBC model with heterogeneous antigens. (B) Cell fluorescence was visualized using fluorescent stereomicroscopy 36 h post T cells injection. (C) Statistical analysis of the fluorescence intensity of MDA-MB-231MICA/MSLN tumor treated with UTD, MSLN CAR-T or BiTEs CAR-T cells in zebrafish model at 36 h post-treatment (n = 10). (D) Schematic diagram of the murine TNBC xenograft model. MDA-MB-231MICA/MSLN cells were used to establish the TNBC model with heterogeneous antigens. (E) Growth curve of MDA-MB-231MICA/MSLN xenograft treated with UTD, MSLN CAR-T or BiTEs CAR-T cells. (F) Kaplan-Meier survival curves of MDA-MB-231MICA/MSLN tumor bearing-mice treated with UTD, MSLN CAR-T or BiTEs CAR-T cells (n = 4). (G) Body weight of MDA-MB-231MICA/MSLN tumor bearing-mice treated with UTD, MSLN CAR-T or BiTEs CAR-T cells (n = 4). (H) The concentration of IFN-γ in blood samples of MDA-MB-231MICA/MSLN tumor bearing-mice treated with UTD, MSLN CAR-T or BiTEs CAR-T cells (n = 3). (I) Percentage of transferred CD45+ CD3+ T cells in peripheral blood samples from tumor bearing-mice treated with UTD, MSLN CAR-T or BiTEs CAR-T cells (n = 3). Each experiment was repeated at least twice with similar results. Representative data are shown. Statistical significance was considered as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, ns, not significant
Despite promising results, BiTEs CAR-T efficacy in the mouse model was limited, likely due to tumor aggressiveness or CAR-T dosage [12]. Combining BiTEs CAR-T with chemotherapy or UTD may enhance the efficacy. Given the dual-targeting nature of BiTEs CAR-T, further toxicity assessments, including on-target off-tumor effects and cytokine release syndrome are essential. Overall, BiTEs CAR-T, with low immunogenicity and high stability, demonstrated superior antitumor activity against heterogeneous TNBC, highlighting its therapeutic potential.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- BiTEs
Bi-specific T cells Engagers
- CAR
Chimeric Antigen Receptor
- MSLN
Mesothelin
- NKG2DL
Natural Killer Group 2 member D Ligands
- scFv
Single Chain Variable Fragments
- TCGA
The Cancer Genome Atlas
- TNBC
Triple Negative Breast Cancer
- UTD
Untransduced T cells
- VHH
Variable Heavy domain of Heavy Chain
Author contributions
QW, ML, and XW were involved in the conception and design of the work. MAS, QW, MDS, RBS, YR, PZ, BC and YX prepared Fig. 1 and Fig. S1. MAS, QW, MDS, and XL prepared Fig. 2. MAS, PZ, YX, and XL prepared Figs. S2–S6. MAS and ML wrote the main manuscript. FL, XW revised the manuscript. XW and ML provided supervision for the work as well as funding acquisition. All authors reviewed and approved the final manuscript draft.
Funding
This study was supported by National Key R&D Program of China (2019YFA0906100), Natural Science Foundation of China (82204265, 82311530690), Guangdong Basic and Applied Basic Research Foundation (2021A1515110054), the Science and Technology Innovation Fund of Shenzhen (JCYJ20210324101400001, JCYJ20190807160013654), Key R&D Program of Hunan Province (2023SK2082).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All mouse experiments were approved by the Ethics Committee and Institutional Review Board of Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences (SIAT-IACUC-20190730-YYS-DBYWZX-XCG-A0784-01). The maximal tumor size permitted by the ethics committee is 2000 mm3 and this threshold was not exceeded in this study. The zebrafish experiments were approved by the Institutional Animal Care and Use Committee (lACUC) of Hunter Biotechnology (IACUC-2023-6592). All the studies involving human subjects were approved by the Institutional Review Board at Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences (SIAT-IRB-190715-H0363). Blood samples were collected from healthy donors with written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maoxuan Liu, Email: mx.liu@siat.ac.cn.
Xiaochun Wan, Email: xc.wan@siat.ac.cn.
References
- 1.Li Y, Zhang H, Merkher Y, Chen L, Liu N, Leonov S, et al. Recent advances in therapeutic strategies for triple-negative breast cancer. J Hematol Oncol. 2022;15(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdou Y, Goudarzi A, Yu JX, Upadhaya S, Vincent B, Carey LA. Immunotherapy in triple negative breast cancer: beyond checkpoint inhibitors. Npj Breast Cancer. 2022;8(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ai K, Liu B, Chen X, Huang C, Yang l, Zhang W, et al. Optimizing CAR-T cell therapy for solid tumors: current challenges and potential strategies. J Hematol Oncol. 2024;17(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, et al. CAR-T cells secreting bites circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37(9):1049–58. [DOI] [PubMed] [Google Scholar]
- 5.Adusumilli PS, Zauderer MG, Rivière I, Solomon SB, Rusch VW, O’Cearbhaill RE, et al. A phase I trial of regional Mesothelin-Targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the Anti-PD-1 agent pembrolizumab. Cancer Discov. 2021;11(11):2748–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallman DA, Kerre T, Havelange V, Poiré X, Lewalle P, Wang ES, et al. CYAD-01, an autologous NKG2D-based CAR T-cell therapy, in relapsed or refractory acute myeloid leukaemia and myelodysplastic syndromes or multiple myeloma (THINK): haematological cohorts of the dose escalation segment of a phase 1 trial. Lancet Haematol. 2023;10(3):e191–202. [DOI] [PubMed] [Google Scholar]
- 7.Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molfetta R, Quatrini L, Zitti B, Capuano C, Galandrini R, Santoni A, et al. Regulation of NKG2D expression and signaling by endocytosis. Trends Immunol. 2016;37(11):790–802. [DOI] [PubMed] [Google Scholar]
- 9.Wagner DL, Fritsche E, Pulsipher MA, Ahmed N, Hamieh M, Hegde M, et al. Immunogenicity of CAR T cells in cancer therapy. Nat Rev Clin Oncol. 2021;18(6):379–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren Q, Zu Y, Su H, Lu Q, Xiang B, Luo Y, et al. Single VHH-directed BCMA CAR-NK cells for multiple myeloma. Experimental Hematol Oncol. 2023;12(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X, Yin X, Wu J, Wickström SL, Duo Y, Du Q, et al. Visualization of human T lymphocyte-mediated eradication of cancer cells in vivo. Proc Natl Acad Sci U S A. 2020;117(37):22910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bandara V, Foeng J, Gundsambuu B, Norton TS, Napoli S, McPeake DJ, et al. Pre-clinical validation of a pan-cancer CAR-T cell immunotherapy targeting nfP2X7. Nat Commun. 2023;14(1):5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.