Abstract
Introduction
Query fever (Q fever), a zoonotic disease, caused by Coxiella burnetii, is an infectious disease that has long been considered a rare and regionally restricted disease. It can be responsible for endocarditis and endovascular infections. Systemic capillary leak syndrome (SCLS), a rare disease of unknown etiology that most commonly develops in adults 50–70 years of age, is diagnosed clinically based on a characteristic symptomatic triad of hypotension, hemoconcentration (elevated hemoglobin or hematocrit), and serum hypoalbuminemia resulting from fluid extravasation. Although Q fever has increasingly been recognized and reported in recent years, the treatment of Q fever complicated by SCLS, with an etiological diagnosis aided by metagenomic next-generation sequencing (mNGS), remains uncommon.
Case presentation
This report describes a case of acute Q fever with concurrent SCLS in a 54-year-old male who worked in a slaughterhouse. The patient presented with fever, chest tightness, and shortness of breath, accompanied by severe headache. His condition rapidly deteriorated, leading to acute fever, generalized weakness, and hypotension. Due to respiratory failure and shock, he was admitted to the intensive care unit (ICU) for treatment. Despite empirical antibiotic therapy along with fluid resuscitation, his blood pressure continued to decline, and metabolic acidosis and respiratory distress worsened. As his condition failed to improve, tracheal intubation was performed. mNGS detected both Coxiella burnetii in his BALF and blood samples. Based on the mNGS results, he was started on doxycycline, alongside penicillin antibiotics, vasopressors, and continuous renal replacement therapy (CRRT). The patient’s condition gradually improved, and he was discharged home after 12 days of treatment. At his 90-day follow-up, he had nearly fully recovered to his pre-illness status.
Conclusions
mNGS plays a crucial role in assisting the diagnosis of Q fever, which enables the timely treatment of the underlying disease triggering SCLS. This, combined with restrictive fluid resuscitation strategies, is essential for improving patient outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-10699-8.
Keywords: Q fever, Coxiella burnetii, Systematic capillary leakage syndrome, Metagenomic next-generation sequencing
Introduction
Coxiella burnetii, the causative agent of query fever (Q fever), is a gram-negative, intracellular, pleomorphic bacterium responsible for zoonotic infections globally [1], and can usually be isolated from domestic animals including cattle, sheep, goats, cats, and dogs [2]. The clinical presentation of Q fever can be acute or chronic, often with nonspecific symptoms. Common complications include endocarditis, hepatitis, pneumonia, chronic fatigue, and, in severe cases, vascular and neurological infections, which may lead to death [3]. Due to the nonspecific nature of its symptoms and the absence of specific conventional microbiological diagnostic methods [4], diagnosing Coxiella burnetii infection remains challenging, particularly in non-endemic regions.
In recent years, with the widespread application of metagenomic next-generation sequencing (mNGS) [5, 6], reported cases of Q fever have increased. By extracting and analyzing microbial genetic information from a variety of clinical samples, mNGS performs well in identifying rare, novel, difficult-to-detect and coinfected pathogens directly from clinical samples, especially in cases with negative serological or culture results [7].
In this particular case, the Q fever caused by Coxiella burnetii was confirmed through mNGS, which proved to be highly valuable in guiding clinical decision-making and treatment strategies, including the management of the rare complication of systemic capillary leak syndrome (SCLS).
Case presentation
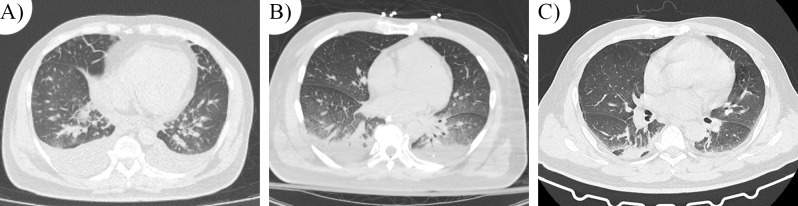
A 54-year-old male was admitted due to recurrent fever and fatigue for 7 days, and chest tightness for 3 days. The patient experienced fever for 7 days prior to admission, with a persistent temperature around 38 °C and generalized weakness. There were no chills, muscle aches, chest tightness, or pain. He developed a sudden onset of dyspnea, becoming unable to lie flat at night, accompanied by a dry cough for 3 days before admission. During this time, the patient did not receive formal treatment, and the symptoms recurred. On the day of admission, his dyspnea worsened, accompanied by generalized edema, leading to his transport to a local hospital for treatment. Chest CT revealed pulmonary edema and pleural effusion (Fig. 1A). Following right-sided thoracentesis and symptomatic treatment with diuretics, the patient did not show significant improvement. Consequently, he was transferred to our hospital at night. During a routine examination in the emergency department, his condition rapidly deteriorated, presenting with hypotension (88/52 mmHg), severe hypoxemia (SpO2 of 89% on 3 L nasal cannula), mild generalized edema, and drowsiness. After treatment with intubation, mechanical ventilation, and vasopressors, he was transferred to the intensive care unit (ICU) for further management.
Fig. 1.
Changes in pulmonary lesions on CT imaging. (A) (Day.1): The initial CT scan depicts bilateral ground-glass opacities in the lung periphery with areas of consolidation. Pleural effusion is confirmed by thoracentesis in the areas of consolidation. (B) (Day.3): The follow-up CT scan shows increased bilateral ground-glass opacities, suggesting a progression of pulmonary edema. (C) (Day.6): The subsequent CT scan after treatment demonstrates a significant improvement with a marked reduction in ground-glass opacities and resolution of pleural effusion, indicating a response to therapy and resolution of pulmonary edema
The patient has had diabetes for 5 years and regularly takes metformin (Glucophage 0.5 g/d). He also has a 10-year history of hypertension, for which he takes amlodipine besylate (Norvasc 5 mg/d). He has no history of hepatitis, peripheral edema, or chronic kidney disease. Prior to his illness, he worked at a slaughterhouse and was involved in the slaughtering of livestock.
On the day of ICU admission (Day 1), the patient developed a persistent high fever (around 39 °C) during sedation and analgesia, accompanied by tachycardia (heart rate: 150 bpm) and somnolence. Auscultation revealed pronounced dry and wet rales in both lungs, with diminished breath sounds on the left side. The patient’s skin was cold and clammy, and there was mild generalized edema. Laboratory findings showed a white blood cell (WBC) count of 35.4 × 10⁹/L, C-reactive protein (CRP) level of 84.6 mg/L, procalcitonin (PCT) at 3.345 ng/mL, lactate at 3.7 mmol/L, albumin at 31.9 g/L, aspartate aminotransferase (AST) at 42 U/L, alanine aminotransferase (ALT) at 117 U/L, and creatinine at 74.5 µmol/L. Arterial blood gas analysis revealed: pH 7.35, PO₂ 66.7 mmHg, PCO₂ 26.1 mmHg, and an oxygenation index of 111. Tests for influenza A and B, cytomegalovirus, Epstein-Barr virus, Toxoplasma, rubella virus, herpes simplex virus, cryptococcal antigen, galactomannan, and β-D-glucan were all negative. A preliminary clinical diagnosis of pulmonary infection was made, and empirical anti-infective therapy was initiated with piperacillin-tazobactam (4.5 g IV q8h). Norepinephrine [1.3 µg (kg·min) IV pump] and Ringer’s solution (500 ml/h IV qd) were administered to correct hypotension. Considering the possibility of atypical pathogen infection, bronchoalveolar lavage fluid (BALF) and blood samples were collected for mNGS testing via bronchoscopy.
However, the patient’s condition did not improve after treatment (Day 2). Blood pressure continued to drop, body temperature rose further, and the oxygenation index further deteriorated. Repeat tests showed increasing trends in white blood count (WBC), CRP, and procalcitonin (PCT) levels (Fig. 2; Table 1), and generalized edema worsened. To alleviate the oxygenation index, a closed left-sided thoracic drainage procedure was performed. Pleural fluid analysis showed an albumin level of 16 g/L. Further laboratory tests indicated hemoglobin at 192 g/L, hematocrit at 56.8%, albumin at 19 g/L, creatinine at 167 µmol/L, blood urea nitrogen at 15.73 mmol/L, and alanine aminotransferase at 71.1 U/L. Given the suspicion of SCLS, crystalloid infusion was discontinued, and fresh frozen plasma, hydroxyethyl starch, and albumin were administered for volume resuscitation. Terlipressin [0.025 µg (kg·min) IV pump] was added to manage hypotension, and continuous renal replacement therapy (CRRT) was initiated to maintain fluid balance. Additionally, oral oseltamivir (75 mg, PO, q12h) was given to cover atypical viral infections.
Fig. 2.
Change in inflammatory markers, temperature, blood pressure, serum albumin and PO2/FiO2 during ICU hospitalization. Abbreviations: CRP: C-reactive protein; WBC: White blood count; PCT: Procalcitonin; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; IV: Intravenous injection
Table 1.
The timeline of laboratory and clinical data of the patient
| Test | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 |
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin, g/L | 188 | 192 | 133 | 131 | 110 | 107 | 112 | 115 | 110 |
| WBC, ×109/L | 35.4 | 35.5 | 39.1 | 37.4 | 25.3 | 23.2 | 12.3 | 17.0 | 15.4 |
| Neutrophil% | 72.7 | 83.0 | 73.8 | 78.6 | 78.5 | 70.3 | 75.2 | 84.7 | 82.5 |
| Platelets, ×109/L | 175 | 228 | 168 | 159 | 110 | 107 | 137 | 161 | 236 |
| Albumin, g/L | 31.9 | 18.0 | 19.0 | 23.0 | 26.8 | 26.3 | 28.7 | 30.6 | 29.9 |
| ALT, U/L | 117.0 | 67.4 | 71.1 | 59.2 | 39.9 | 26.1 | 38.3 | 56.5 | 45.3 |
| AST, U/L | 42.0 | 27.4 | 27.4 | 21.5 | 32.9 | 24.5 | 28.6 | 39.1 | 17.3 |
| Creatinine, µmmol/L | 74.5 | 166.0 | 78.2 | 72.2 | 56.1 | 80.9 | 63.7 | 48.3 | 54.4 |
| Urea, mmol/L | 9.61 | 16.0 | 7.1 | 6.3 | 4.9 | 6.3 | 5.3 | 4.4 | 7.9 |
| CK, U/L | 38 | 33 | 12.2 | 1076 | 353 | 254.8 | / | / | / |
| CKMB, U/L | 13.0 | 13.4 | 13.4 | 54.1 | 38.2 | 22.6 | / | / | / |
| CRP, mg/L | 84.6 | 75.1 | 70.4 | 36.0 | 36.7 | 40.2 | 36.7 | 10.1 | 10.8 |
| Troponin-I, ng/L | 0.02 | 0.01 | / | 0.01 | / | 0.01 | / | / | / |
| Procalcitonin, ng/L | 3.35 | 5.58 | 5.17 | 1.8 | 1.01 | 0.57 | 0.47 | 0.41 | 0.40 |
| BNP, ng/L | < 10 | 211.5 | 170.4 | / | < 10 | 23.56 | / | / | / |
| HbA1c, % | 11.5 | / | / | / | / | / | / | / | 10.9 |
| Arterial blood gas | |||||||||
| PH | 7.36 | 7.28 | 7.34 | 7.35 | 7.46 | 7.47 | 7.42 | 7.46 | 7.46 |
| PO2, mmHg | 66.7 | 90.4 | 82.3 | 86.4 | 110 | 104 | 98.7 | 94.1 | 95.1 |
| PCO3, mmHg | 26.1 | 35.5 | 38.1 | 35.8 | 38.0 | 40.3 | 45.1 | 44.2 | 44.0 |
| HCO3−, mmol/L | 14.6 | 16.6 | 23.2 | 26.2 | 27.0 | 30.0 | 30.6 | 31.4 | 31.5 |
| Lactate, mmol/L | 3.7 | 1.8 | 0.9 | 1.1 | 1.0 | 0.8 | 0.9 | 1.1 | 1.3 |
| Oxygen support | |||||||||
| PEEP, cmH2O | 12 | 12 | 12 | 12 | 8 | 5 | / | / | / |
| HFOT, L/min | / | / | / | / | / | / | 45 | 40 | 35 |
| FiO2, % | 60 | 60 | 60 | 45 | 35 | 30 | 30 | 30 | 30 |
| NE, µg (kg·min) | 1.3 | 1.3 | 0.75 | 0.375 | 0.187 | / | / | / | / |
| Terlipressin, µg (kg·min) | / | 0.025 | 0.025 | 0.025 | 0.025 | / | / | / | / |
Abbreviations: WBC, white blood count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CKMB, creatine kinase muscle/brain; CRP: c-reactive protein; BNP, b-type natriuretic peptide; PEEP, positive end-expiratory pressure; HFOT: high-flow oxygen therapy; NE: norepinephrine
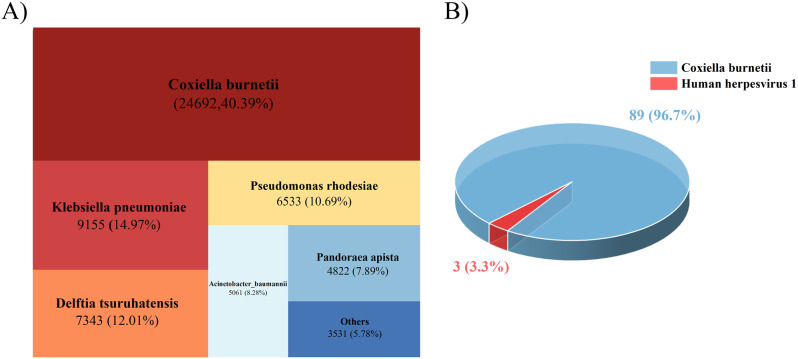
Despite aggressive fluid resuscitation and CRRT support, the patient’s generalized edema and blood pressure improved, but the fever persisted, and WBC, CRP, and PCT levels continued to rise (Day 3). A follow-up chest CT revealed multiple patchy opacities in both lungs and increased bilateral pleural effusion compared to the previous scan (Fig. 1B). The results of mNGS from both blood and BALF samples subsequently identified Coxiella burnetii infection. The BALF mNGS results revealed 24,692 Coxiella burnetii sequences out of 61,137 total reads, accounting for 40.38%, indicating it as the dominant pathogen (Fig. 3). Similarly, the blood mNGS results showed 89 out of 90 reads (98.89%) matched Coxiella burnetii, with 1 read (1.11%) corresponding to HSV-1. The patient’s diagnosis of acute Q fever, a rare disease in this region, was ultimately made based on the epidemiological history and pathogen detection results, in conjunction with the current European Union (EU) case definition for Q fever [8] (Table 2). Targeted treatment for Coxiella burnetii infection was initiated with doxycycline (100 mg IV, every 12 h).
Fig. 3.
Distribution chart of pathogens detected in BALF and blood samples. (A) Distribution of pathogens in BALF and (B) blood samples
Table 2.
European union case definition since 2008 (revised in 2012)
| Criteria | Definition |
|---|---|
| Laboratory | • Isolation of Coxiella burnetii from a clinical specimen |
| • Detection of C. burnetii nucleic acid in a clinical specimen | |
| • C. burnetii specific antibody response (IgG or IgM phase II) | |
| Clinical | • Fever |
| • Pneumonia | |
| • Hepatitis | |
| Epidemiological | • Exposure to a common source |
| • Animal to human transmission |
On Day 4, following the adjustment of antibiotic therapy, the patient’s temperature and inflammatory markers began to decrease. On Day 5, hemodynamic stability was achieved, leading to the discontinuation of vasopressor support and CRRT. On Day 6, the extubation of the endotracheal tube was successfully performed. A follow-up chest CT scan demonstrated a reduction in pleural effusion and an improvement in the patchy opacities (Fig. 1C). On Day 9, the patient exhibited significant clinical improvement, prompting the cessation of antibiotic therapy and subsequent transfer to the general ward. The patient was discharged in stable condition on Day 12.
Discussion
Q fever is a global zoonotic disease caused by Coxiella burnetii [1, 3]. Humans are typically infected through the inhalation of contaminated dust particles. As a result, acute Q fever commonly manifests as a pulmonary disease, characterized by pneumonia and influenza-like symptoms, with the potential to progress to hepatitis, endocarditis, acute aneurysms, or heart failure [9, 10]. Traditional methods for diagnosing Q fever include microbial culture, serological testing, and nucleic acid detection via polymerase chain reaction. However, these procedures require a Biosafety Level 3 laboratory [11], and in China, most tertiary hospitals lack such facilities.
mNGS, a culture-independent and assumption-free genomic method, enables the unbiased detection of microbial nucleic acids in clinical specimens. These nucleic acids are then matched against microbial gene databases to identify pathogens, greatly enhancing the diagnostic and therapeutic capabilities of medical institutions for rare and challenging infectious diseases [12, 13]. In the study by He et al. [14], 31 patients with psittacosis pneumonia were included. The results showed that the application of mNGS improved the diagnostic accuracy of Chlamydia psittaci pneumonia, reduced unnecessary antibiotic use, shortened the duration of illness, and ultimately enhanced patient prognosis. Similarly, in a large multicenter study, Qu et al. [15] also reported that mNGS demonstrated significant advantages in detecting atypical pathogens, including Chlamydia psittaci and Leptospira species.
In addition to its application in diagnosing atypical pathogens like Chlamydia psittaci and Leptospira, mNGS has also shown significant value in outbreak settings. For instance, during the coronavirus disease 2019 (COVID-19) pandemic, mNGS emerged as a powerful diagnostic tool, demonstrating tremendous value in identifying concurrent fungal, viral, or bacterial infections in affected patients [16]. Building on its success in detecting atypical pathogens and co-infections during COVID-19, mNGS has also proven highly effective in diagnosing rare infections like Q fever. Huang et al. [17] demonstrated the utility of mNGS during a Q fever outbreak in Zhuhai, China, where it was used to identify Coxiella burnetii in patients and trace the infection source back to livestock in a slaughterhouse, highlighting its role in both diagnosis and epidemiological tracing. In our case, similar to previous reports, the patient had a history of working in a slaughterhouse and a clear epidemiological background. However, due to the rarity of Q fever in China and the clinicians’ lack of experience, a timely diagnosis was not made, resulting in a delay in treatment. Subsequently, mNGS detected Coxiella burnetii in both BALF and blood samples, confirming the diagnosis of Q fever. Based on the treatment guidelines [18], the patient then received targeted doxycycline therapy (200 mg/day), which led to significant improvement in his condition.
SCLS, first described by Clarkson in 1960 [19], has a high acute-phase mortality rate of approximately 20-30% [20], is a rare condition characterized by endothelial dysfunction and the extravasation of fluid from the vascular to the interstitial space, leading to shock, hemoconcentration, hypoalbuminemia, and organ failure. While it is most often idiopathic, SCLS can also occur secondary to a range of underlying causes, including infections, allergic reactions, and autoimmune diseases, all of which increase vascular permeability [21]. The prodromal symptoms of SCLS are nonspecific, including fatigue, dizziness, and flu-like symptoms. The condition is marked by a rapid onset of fluid leakage into the interstitial space, leading to edema, pleural and abdominal effusions, and organ dysfunction. Severe hypovolemia may lead to shock and multiple organ failure, with fatal pulmonary edema occurring in the recovery phase due to iatrogenic fluid overload.
In our case, the patient’s early aggressive fluid resuscitation exacerbated pleural effusion and pulmonary edema, with no improvement in blood pressure, which is consistent with SCLS characteristics in response to fluid treatment [22]. The patient subsequently developed the classic “3Hs” triad of SCLS: hypotension, hypoalbuminemia, and hemoconcentration, which confirmed the diagnosis of SCLS. As seen in previous studies [23], infections such as severe acute respiratory syndrome coronavirus 2 can damage endothelial cells, triggering a systemic inflammatory response and increasing capillary permeability, resulting in the leakage of fluid from the vascular compartment and the development of SCLS [24]. Similarly, this case also reveals SCLS may as a rare complication of Q fever caused by Coxiella burnetii.
Importantly, SCLS is not confined to Q fever and can manifest in association with various other infections, particularly those that cause systemic inflammation and increased vascular permeability. For example, during the COVID-19 pandemic, SCLS was reported as a rare but serious complication, especially in patients with severe inflammatory responses [23, 25]. Similar cases have been documented in patients with influenza virus infections [26, 27]. These examples further highlight that SCLS can occur in a variety of infectious diseases, emphasizing the importance of early recognition and prompt management of the underlying infection to improve patient outcomes.
In this case, our treatment approach focused on both maintaining the patient’s blood pressure through fluid resuscitation to ensure adequate tissue perfusion and carefully balancing the risk of worsening systemic edema due to excessive fluid administration, while actively addressing the underlying condition. We limited crystalloid fluid supplementation, utilized colloids, and increased the dose of vasopressors to maintain lower blood pressure while monitoring tissue perfusion levels with lactate. Controlled fluid balance through CRRT and employed PEEP to alleviate pulmonary edema. This treatment strategy bought time for addressing the underlying condition, and ultimately, as the Q fever improved, the symptoms associated with SCLS also resolved.
To our knowledge, there have been no reported cases of SCLS triggered by Q fever. As a rare complication of Q fever, SCLS should command significant attention from clinicians. Early recognition, appropriate fluid resuscitation strategies, and targeted treatment of the underlying condition are crucial for improving patient outcomes.
However, there are limitations, including the rarity of SCLS as a Q fever complication, which may affect generalizability. Relying on a single case can introduce bias due to varied patient responses. Another important challenge is the accessibility of mNGS in resource-limited settings. The high cost of mNGS, along with the need for specialized equipment, trained personnel, and advanced bioinformatics capabilities, may restrict its implementation in low-resource environments. In such settings, traditional diagnostic methods, while limited in their sensitivity, may still be the mainstay of clinical practice.
The next-generation sequencing performed in this study was only for the identification of the causative agent, and no new gene sequence was generated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- Q fever
Query fever
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BALF
Bronchoalveolar lavage fluid
- CRP
C-reactive protein
- CRRT
Continuous renal replacement therapy
- ICU
Intensive care unit
- IV
Intravenous injection
- mNGS
Metagenomic next-generation sequencing
- PEEP
Positive end-expiratory pressure
- SCLS
Systemic capillary leak syndrome
- EU
European Union
- COVID-19
Coronavirus disease 2019
- NE
Norepinephrine
- HFOT
High-flow oxygen therapy
- BNP
B-type natriuretic peptide
Author contributions
Zhao.JJ and Wang.KW carried out the studies, participated in collecting data, and drafted the manuscript. Zhao.JJ performed the statistical analysis and participated in its design and prepared Figs. 1, 2 and 3. Zhao.JJ, Wang.KW, Fang.HL, Zhang.WW and Luo.J participated in acquisition data, and revised the manuscript. All authors read and approved the final version.
Funding
This work was supported in part by grants from Medical and Health Research Program of Zhejiang Province (No. 2023KY1296, HL Fang).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
As a case report, our paper did not require any referral to our institutional clinical ethics committee.
Consent for publication
Written informed consent was obtained from the patient for publication of this report and any accompanying images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Honglong Fang, Email: fang124113@163.com.
Kaiyu Wang, Email: linjia240@gmail.com.
References
- 1.Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017;30:115–90. 10.1128/CMR.00045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celina SS, Cerný J. Coxiella burnetii in ticks, livestock, pets and wildlife: A mini-review. Front Vet Sci. 2022;9:1068129. 10.3389/fvets.2022.1068129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ullah Q, Jamil T, Saqib M, et al. Q Fever—A Neglected Zoonosis Microorganisms. 2022;10:1530. 10.3390/microorganisms10081530. [DOI] [PMC free article] [PubMed]
- 4.Luce-Fedrow A, Mullins K, Kostik AP, et al. Strategies for detecting rickettsiae and diagnosing rickettsial diseases. Future Microbiol. 2015;10:537–64. 10.2217/fmb.14.141. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Hao Y, Wang Z, et al. Diagnosis of Coxiella burnetii infection via metagenomic next-generation sequencing: a case report. BMC Infect Dis. 2022;22:373. 10.1186/s12879-022-07309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Chen H, Han D, et al. Clinical usefulness of metagenomic next-generation sequencing for Rickettsia and Coxiella burnetii diagnosis. Eur J Clin Microbiol Infect Dis. 2023;42:681–9. 10.1007/s10096-023-04586-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han D, Li Z, Li R, et al. mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. 2019;45:668–85. 10.1080/1040841X.2019.1681933. [DOI] [PubMed] [Google Scholar]
- 8.Commission E. Commission implementing decision of 8 August 2012 amending decision 2002/253/EC laying down case definitions for reporting communicable diseases to the community network under decision 2119/98/EC of the European Parliament and of the Council (notified under document C (2012) 5538). Official J Eur Union. 2012;262:21. [Google Scholar]
- 9.Van Roeden SE, Wever PC, Kampschreur LM, et al. Chronic Q fever-related complications and mortality: data from a nationwide cohort. Clin Microbiol Infect. 2019;25:1390–8. 10.1016/j.cmi.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Yakovenko V, Brauner R, Votinov E, et al. Infective endocarditis and thromboses due to antiphospholipid syndrome following acute Q fever. Lancet. 2022;399:1154. 10.1016/S0140-6736(22)00376-2. [DOI] [PubMed] [Google Scholar]
- 11.Gikas A, Kokkini S, Tsioutis C. Q fever: clinical manifestations and treatment. Expert Rev Anti-infective Therapy. 2010;8:529–39. 10.1586/eri.10.29. [DOI] [PubMed] [Google Scholar]
- 12.Gu W, Miller S, Chiu CY. Clinical metagenomic Next-Generation sequencing for pathogen detection. Annu Rev Pathol Mech Dis. 2019;14:319–38. 10.1146/annurev-pathmechdis-012418-012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Sun Y, Tang J, et al. The clinical application of metagenomic next-generation sequencing in immunocompromised patients with severe respiratory infections in the ICU. Respir Res. 2024;25:360. 10.1186/s12931-024-02991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He L, Yang H, Liu S, et al. Physiological analysis of severe chlamydia psittaci pneumonia and clinical diagnosis after doxycycline-based treatment. Front Physiol. 2023;14:1132724. 10.3389/fphys.2023.1132724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu J, Zhang J, Chen Y, et al. Aetiology of severe community acquired pneumonia in adults identified by combined detection methods: a multi-centre prospective study in China. Emerg Microbes Infections. 2022;11:556–66. 10.1080/22221751.2022.2035194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Chang S, Ma R, et al. COVID-19 in pulmonary critically ill patients: metagenomic identification of fungi and characterization of pathogenic microorganisms. Front Cell Infect Microbiol. 2024;13:1220012. 10.3389/fcimb.2023.1220012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang M, Ma J, Jiao J, et al. The epidemic of Q fever in 2018 to 2019 in Zhuhai City of China determined by metagenomic next-generation sequencing. PLoS Negl Trop Dis. 2021;15:e0009520. 10.1371/journal.pntd.0009520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson A, Bijlmer H, Fournier P-E, et al. Diagnosis and management of Q fever–United States, 2013: recommendations from CDC and the Q fever working group. MMWR Recomm Rep. 2013;62:1–30. [PubMed] [Google Scholar]
- 19.Druey KM, Parikh SM. Idiopathic systemic capillary leak syndrome (Clarkson disease). J Allergy Clin Immunol. 2017;140:663–70. 10.1016/j.jaci.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eo TS, Chun KJ, Hong SJ, et al. Clinical presentation, management, and prognostic factors of idiopathic systemic capillary leak syndrome: A systematic review. J Allergy Clin Immunology: Pract. 2018;6:609–18. 10.1016/j.jaip.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Guffroy A, Dervieux B, Gravier S, et al. Systemic capillary leak syndrome and autoimmune diseases: A case series. Semin Arthritis Rheum. 2017;46:509–12. 10.1016/j.semarthrit.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017;92:37–46. 10.1016/j.kint.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Cheung PC, Eisch AR, Maleque N, et al. Fatal exacerbations of systemic capillary leak syndrome complicating coronavirus disease. Emerg Infect Dis. 2021;27:2529–34. 10.3201/eid2710.211155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Z, Ghosh CC, Patel R, et al. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome). Blood. 2012;119:4321–32. 10.1182/blood-2011-08-375816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Case R, Ramaniuk A, Martin P, et al. Systemic capillary leak syndrome secondary to coronavirus disease 2019. Chest. 2020;158:e267–8. 10.1016/j.chest.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karatzios C, Gauvin F, Egerszegi EP, et al. Systemic capillary leak syndrome presenting as recurrent shock. Pediatr Crit Care Med. 2006;7:377–9. 10.1097/01.PCC.0000227120.61949.FB. [DOI] [PubMed] [Google Scholar]
- 27.Ling SK, Fong NM, Chan MS. A case of recurrent systemic capillary leak syndrome triggered by influenza A infection associated with cardiogenic shock supported by veno-arterial extracorporeal membrane oxygenation. Perfusion. 2023;38:428–31. 10.1177/02676591211057510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.