Abstract
Objective
This study aimed to analyze the profiles and evolution of Staphylococcus aureus in the pediatric intensive care units (PICUs) of 17 hospitals in China from 2016 to 2022.
Methods
Susceptibility testing was performed to bacterial strains with a uniform monitoring protocol, which was provided by the US Clinical and Laboratory Standards Institute (CLSI) and used by the China Antimicrobial Surveillance Network (CHINET). The results were interpreted in accordance with the performance standards for antimicrobial susceptibility testing issued by the US Clinical and Laboratory Standards Institute.
Results
Twenty-six thousand six hundred thirteen bacterial strains were isolated from 17 PICUs in China from 2016 to 2022, 3,147 of which were Staphylococcus aureus, ranking second among etiological agents of infections from PICUs. In 2022, Staphylococcus aureus had the highest detection rate, being 36.19%. And in 2021, MRSA had the highest detection rate, being 10.35% in Staphylococcus aureus. There were statistically significant differences in the annual detection rate of gram-positive bacteria, Staphylococcus aureus and MRSA between the years from 2016 to 2022 (P < 0.05). More males were found with Staphylococcus aureus or methicillin-resistant Staphylococcus aureus, but there were no statistical differences in gender distribution between any two years (P < 0.05). The top 3 highest detection rate of Staphylococcus aureus in age groups were infants (1244, 39.7%), toddlers (741, 23.7%), and children at school age and older (731, 23.4%). For MRSA, The top 3 in age groups were infants (91, 38.9%), children at school age and older (87, 29.1%), and toddlers (48, 20.5%). The detection rate of Staphylococcus aureus was statistically different in the distribution of age stratification (P < 0.05). There was no statistically significant difference in these two aspects of MRSA (P > 0.05). The top 3 highest detection rate of Staphylococcus aureus in infected sites were the lower respiratory tract (2,552, 81.7%), bloodstream (217, 6.5%), and skin wounds (110, 3.9%). For MRSA, The top 3 in infected sites were the lower respiratory tract (156, 77.9%), skin wounds (47, 8.8%), and bloodstream (15, 6.6%). The detection rate of Staphylococcus aureus and MRSA was statistically different in the distribution of infected sites (P < 0.05). All the strains of Staphylococcus aureus were sensitive to tigecycline, nitrofurantoin, vancomycin, and linezolid. The resistant rate of Staphylococcus aureus, to penicillin G was as high as 87.5% at least, to erythromycin was as high as 51.8% at least, to benzocillin was as high as 38.0% at least, to cefoxitin was as high as 35.5% at least, and to clindamycin was as high as 32.7% at least. All the strains of MRSA were sensitive to vancomycin, linezolid, quinupristin/dalfopristin, and tigecycline. Of these 234 strains of MRSA, 179 (76.5%) were resistant to erythromycin, 116 (49.6%) to clindamycin, 39 (16.7%) to tetracycline, 29 (12.4%) to levofloxacin, 27 (11.5%) to ciprofloxacin, 27 (11.5%) to moxifloxacin, 14 (6.0%) to TMP-SMX, eight (3.4%) to rifampicin, and six (2.6%) to gentamicin.
Conclusions
Staphylococcus aureus is the most common gram-positive bacterium in PICUs. Infants are most likely to be infected by Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. And the lower respiratory tract is the most common infected site of Staphylococcus aureus. Staphylococcus aureus has a high resistant rates to commonly used antimicriobials in pediatrics, but no strains resistant to vancomycin and/or linezolid were found. When considering Staphylococcus aureus infection clinically, it is necessary to select antimicrobials reasonably based on the patient's age, infected site and local epidemiological characteristics.
Keywords: Pediatric intensive-care unit, Staphylococcus aureus, Antimicrobial resistance, Evolution
Background
As a major cause of community-acquired and nosocomial infections, Staphylococcus aureus (S. aureu) can trigger a variety of infectious diseases, including skin and soft tissue infections, endocarditis, osteomyelitis, septicemia, pneumonia, and many other serious and even fatal conditions [1]. Antimicrobial resistance (AMR) refers to the ability of microorganisms to have intrinsic resistance to antimicrobial drugs or to develop extrinsic resistance through acquired mechanisms. Unfortunately, the overuse of broad-spectrum antimicrobials has led to the development of AMR in S. aureus, resulting in poor anti-infective efficacy, and thus has become a major challenge in clinical settings [2]. S. aureus can be categorized into two groups according to its resistance to benzoxacillin: methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus(MRSA). MRSA has rapidly become the most common drug-resistant pathogen in many countries around the world, including Europe, the United States, North Africa, the Middle East, and East Asia [3, 4]. In China, the proportion of MRSA obtained by hospitals has reached 50.4% [5]. Children suffering from skin and soft tissue infections with the detection rate of S. aureus was 62.57%, among which MRSA accounted for 14.79% [6]. Very immature preterm infants, especially those with a birth weight less than 1,500 g may account for approximately 80% of all cases of neonatal MRSA infections [5–7]. These make it become a focus of global public health. Therefore, monitoring the epidemiological characteristics of S. aureus can help us cope with the harm better.
Children in PICUs often experience critical conditions with Immunosuppressive state, frequent invasive procedures, intensive and prolonged antimicrobial use, and poor infection prevention and control, which are all associated with the development of AMR [8–10]. Dynamically monitoring the prevalence, clinical distribution, and AMR profiles of S. aureus infections in PICUs is crucial for the diagnosis and treatment of these infections. Here, we retrospectively analyzed clinical S. aureus isolates from the PICUs of 17 hospitals in the China paediatric Intensive care Unit Pathogen Surveillance Network (CHIPS) from 2016 to 2022, to provide evidence for the clinical treatment and infection control of this pathogen.
Subjects and methods
Specimen sources
The specimens sent for testing were collected from children admitted to 17 PICUs, including Children's Hospital of Fudan University, Shanghai Children’s Medical Center affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai Children’s Hospital affiliated to Shanghai Jiaotong University, Children’s Hospital Affiliated to Zhejiang University Medical School, Children’s Hospital of Soochow University, Ningbo Women and Children’s Hospital, Wuxi Children’s Hospital, The First Affiliated Hospital of Xiamen University, Xiamen Children's Hospital, Anhui Children’s Hospital, Shenzhen Children’s Hospital, Henan Children’s Hospital, Kaifeng Children's Hospital, Hunan Children's Hospital, Jiangxi Provincial Children’s Hospital, Kunming Children's Hospital and Guiyang Children’s Hospital, during the period from 1 January 2016 to 31 December 2022. All the PCUs belong to CHIPS member hospitals, that means they all basically the same in terms of level, disease diagnosis and treatment, specimen collection and testing. Blood, cerebrospinal fluid, urine, pus, exudates, tissue specimens from the respiratory tract and gastrointestinal tract, and specimens from catheter tips were examined. The inclusion criteria were: (i) age 30 days to 18 years; and (ii) culture specimens that were positive for S. aureus. Duplicate strains at the same site in the same patient were eliminated.
Age stratification of the specimen
The specimens were stratified according to age,including infant (30 days −1 year), toddler (1 year −3 years), preschool children (3 years−6 years), and children at school age and older (> 6 years).
Isolation and identification of bacterial strains and drug susceptibility testing (DST)
The bacterial strains were isolated and cultured with reference to the National Guide to Clinical Laboratory Procedures (4th edition) [11]. The Vitek 2 Compact fully automated system (Mérieux, France) was used for strain identification. The methods and standards of antimicrobial susceptibility test of CLSI in the United States are the standards followed by China. We took the performance standards for antimicrobial susceptibility testing issued by the US CLSI study. DST and the interpretation of its results were based on the US Clinical and Laboratory Standards Institute (CLSI) guidelines (2020 edition) [12]. DST was performed for the following drugs: cefoxitin, ceftaroline, benzoxicillin, ampicillin/sulbactam, penicillin G, sulfamethoxazole-trimethoprim (TMP-SMX), erythromycin, clindamycin, nitrofurantoin, vancomycin, rifampicin, teicoplanin, linezolid, tigecycline, levofloxacin, amikacin, ciprofloxacin, moxifloxacin, tetracycline, minocycline, chloramphenicol, amoxicillin/clavulanic acid, ceftriaxone, cefoperazone/sulbactam, tobramycin, clarithromycin, azithromycin, quinupristin/dalfopristin, fosfomycin, and gentamicin. The quality control strain, ATCC 25923 (S. aureus), was provided by Shanghai Municipal Centre for Disease Control and Prevention (Shanghai CDC) (Shanghai, China), and the quality control of DST was performed on a weekly basis.
Definition of susceptibility
S. aureus can be categorized into two groups, MSSA and MRSA. MSSA refers to S. aureus that is not resistant to methicillin, whereas MRSA refers to S. aureus that is resistant to all penicillins, including methicillin and other antibiotics that are resistant to β-lactamase.
Statistical analysis
Statistical analyses were performed with the WHONET 5.6 and SPSS 26.0 software, and graphs were drawn with the GraphPad software. The count data are presented as cases and percentages. Comparisons between multiple groups were analyzed by χ2 test. If the frequency of a single cell was less than 5, Fisher’s exact test was used for comparison between multiple groups. χ2 test or Fisher’s exact test was followed by Z-test, which was used for comparison between two groups within multiple groups. A two-tailed P-value < 0.05 was considered statistically significant.
Results
Overview of the distribution of bacterial strains
From 2016 to 2022, a total of 26,613 bacterial isolates were identified, 9,743 of which were gram-positive bacteria. 3147 strains of gram-positive bacteria were S. aureus, accounting for 32.30%. 234 strains of S. aureus were MRSA, accounting for 7.44% (Table 1). The top 10 bacterial isolates were shown in Fig. 1. S. aureus ranked second in all, and occupied the first place in gram-positive bacteria. From 2016 to 2019, with the increase of the total number of bacterial strains, the number and proportion of S. aureus showed an upward trend. From 2020 to 2022, the total number of strains detected fluctuated, as did the number and proportion of S. aureus (Table 1 and Fig. 2). The annual detection rate of S. aureus was shown in Table 1. In 2022, S. aureus had the highest detection rate, being 36.19%. And in 2021, MRSA had the highest detection rate, being 10.35% in S. aureus. There were statistically significant differences in the annual detection rate of gram-positive bacteria, S. aureus and MRSA between the years from 2016 to 2022 (P < 0.05), and the statistical differences between any two years were shown in Table 1 for details. The detection rate of MRSA differed significantly between 2020 and 2021 (P < 0.05), with the lowest rate being 4.99% in 2020 and the highest rate being 10.35% in 2021 (Table 1).
Table 1.
Isolation of related strains in PICUs of 17 hospitals in China from 2016 to 2022
| The year | Total number of bacterial isolates | Number of GM+ isolates (%) | Number of S. aureus isolates (%) | Number of MRSA isolates (%) |
|---|---|---|---|---|
| 2016 | 3,650 | 1,220 (33.42%)a | 302 (24.75%)a | 28(9.27%)a,b |
| 2017 | 3846 | 1315 (34.19%)a | 385 (29.28%)a,b | 20(5.19%)a,b |
| 2018 | 3734 | 1322 (35.40%)a,b | 420 (31.77%)b,c | 22(5.24%)a,b |
| 2019 | 4326 | 1584 (36.62%)a,b,c | 526 (33.21%)b,c | 49(9.32%)a,b |
| 2020 | 3697 | 1449 (39.19%)c | 521 (35.96%)c | 26(4.99%)b |
| 2021 | 4045 | 1604 (39.65%)c | 541 (33.73%)b,c | 56(10.35%)a |
| 2022 | 3315 | 1249 (37.68%)b,c | 452 (36.19%)c | 33(7.30%)a,b |
| Total number | 26,613 | 9743 (36.61%) | 3147 (32.30%) | 234 (7.44%) |
| χ2 value | *** | 56.404 | 57.018 | 21.155 |
| P value | *** | < 0.001 | < 0.001 | 0.002 |
GM+Gram-positive bacteria, MRSA methicillin-resistant Staphylococcus aureus
***The specific values were not calculated separately; please refer to Note 1 for specific statistical significance
a,b, c The same letters between any 2 years indicate P > 0.05, whereas different letters between any 2 years indicate P < 0.05
Fig. 1.
The top 10 strains of of bacteria from 2016 to 2022. Notes: Aba: Acinetobacter baumannii; Sau: Staphylococcus aureus; Kpn: Klebsiella pneumoniae; Pae: Pseudomonas aeruginosa; Eco: Escherichia coli; Spn: Streptococcus pneumoniae; Hin: Haemophilus influenzae; Sep: Staphylococcus epidermidis; Efm: Enterococcus faecium; Pce: Burkholderia cepacia
Fig. 2.
Related strains in PICUs of 17 hospitals in China from 2016 to 2022. A shows annual changes in the number of S. aureus strains; (B) shows annual changes in the proportion of S. aureus strains. Notes: GM + : Gram-positive bacteria; Sau: S. aureus; MRSA: methicillin-resistant S. aureus
Distribution of S. aureus and MRSA
From 2016 to 2022, more males than females (male:female = 1968:1179) were infected with S. aureus, but there was no statistically significant differences in the sex distributions overall and between any 2 years (P > 0.05), as shown in Table 2. With regard to MRSA, the above situation is similar to that of S. aureus in general, as shown in Table 3.
Table 2.
The distribution of S. aureus in gender, age and infected sites
| Characteristics | The year | Χ2 /Fisher value | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |||
| Gender No. (%) | 7.702 | 0.261 | |||||||
| Male | 185(61.3)a | 258(67.0)a | 253(60.2)a | 312(59.3)a | 325(62.4)a | 348(64.3)a | 287(63.5)a | *** | *** |
| Female | 117(38.7)a | 127(33)a | 167(39.8)a | 214(40.7)a | 196(37.6)a | 193(35.7)a | 165(36.5)a | *** | *** |
| Age No. (%) | 110.546 | < 0.001 | |||||||
| Infant | 143(48.1)a | 190(49.5)a | 173(41.7)a,b | 220(41.8)a,b | 183(35.6)b,c | 215(39.7)a,b | 120(26.5)c | *** | *** |
| Toddler | 70(23.6)a | 79(20.5)a | 108(26.0)a | 136(25.9)a | 135(26.3)a | 118(21.8)a | 95(21.1)a | *** | *** |
| Preschool children | 31(10.4)a | 41(10.6)a | 45(10.8)a | 50(9.5)a | 61(11.8)a | 70(12.9)a | 66(14.6)a | *** | *** |
| Children at school age and older | 49(16.5)a | 65(16.9)a | 78(18.8)a | 114(21.7)a | 128(24.9)a | 135(25.0)a | 162(35.8)b | *** | *** |
| Infected sites No. (%) | --- | 0.004 | |||||||
| Lower respiratory tract | 208(73.0)a | 308(80.0)a,b | 352(84.0)b | 448(85.0)b | 427(82.0)b | 443(82.0)a,b | 366(81.0)a,b | *** | *** |
| Bloodstream | 22(7.7)a | 23(6.0)a | 36(8.6)a | 31(5.9)a | 34(6.6)a | 35(6.5)a | 36(8.0)a | *** | *** |
| Skin wound | 19(6.7)a | 7(1.8)b | 6(1.4)b | 16(3.1)a,b | 26(5.0)a,b | 22(4.1)a,b | 14(3.1)a,b | *** | *** |
| Abscesses | 13(4.6)a,b | 22(5.7)b | 13(3.1)a,b | 9(1.7)a | 12(2.3)a,b | 17(3.1)a,b | 16(3.5)a,b | *** | *** |
| Serous effusion | 8(2.8)a | 9(2.3)a | 4(1.0)a | 7(1.3)a | 7(1.3)a | 10(1.8)a | 4(0.9)a | *** | *** |
| Upper respiratory tract | 6(2.1)a | 5(1.3)a | 2(0.5)a | 6(1.1)a | 2(0.4)a | 5(0.9)a | 4(0.9)a | *** | *** |
| Catheter tip | 4(1.4)a | 1(0.3)a | 2(0.5)a | 0(0)a | 2(0.4)a | 4(0.7)a | 1(0.2)a | *** | *** |
| Others | 3(1.1)a | 6(1.6)a | 3(0.7)a | 5(1.0)a | 3(0.6)a | 2(0.4)a | 3(0.7)a | *** | *** |
| Urinary tract | 1(0.4)a | 2(0.5)a | 2(0.5)a | 2(0.4)a | 6(1.3)a | 2(0.4)a | 7(1.5)a | *** | *** |
| Alimentary canal | 1(0.4)a | 1(0.3)a | 0(0)a | 0(0)a | 0(0)a | 1(0.2)a | 1(0.2)a | *** | *** |
a,b,cThe same letters between any 2 years indicate P > 0.05, whereas different letters between any 2 years indicate P < 0.05
***The specific values were not calculated separately; please refer to Note 1 for specific statistical significance
−−−The memory is insufficient to calculate
Table 3.
The distribution of MRSA in gender, age and infected sites
| Characteristics | The year | Χ2 /Fisher value | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |||
| Gender No. (%) | 4.791 | 0.578 | |||||||
| Male | 17(60.7)a | 12(60)a | 16(72.7)a | 32(65.4)a | 21(80.7)a | 38(67.8)a | 19(57.5)a | *** | *** |
| Female | 11(39.3)a | 8(40)a | 6(27.3)a | 17(34.6)a | 5(19.3)a | 18(32.2)a | 14(42.5)a | *** | *** |
| Age No. (%) | 30.070 | 0.182 | |||||||
| Infant | 12(42.8)a | 11(55.0)a | 9(40.9)a | 19(38.8)a | 7(26.9)a | 20(35.7)a | 13(39.4)a | *** | *** |
| Toddler | 5(17.9)a | 3(15.0)a | 5(22.7)a | 13(26.5)a | 6(23.1)a | 9(16.1)a | 7(21.2)a | *** | *** |
| Preschool children | 5(17.9)a | 1(5.0)a | 2(9.1)a | 7(14.3)a | 2(7.7)a | 3(5.4)a | 3(9.1)a | *** | *** |
| Children at school age and older | 6(21.4)a | 3(15.0)a | 5(22.7)a | 9(18.4)a | 11(42.3)a | 24(42.8)a | 10(30.3)a | *** | *** |
| Infected sites No. (%) | --- | < 0.001 | |||||||
| Lower respiratory tract | 13(65.0)a | 18(90.0)a | 3(14.0)b | 40(82.0)a | 23(88.0)a | 50(89.0)a | 29(88.0)a | *** | *** |
| Bloodstream | 3(15.0)a | 1(5.0)a | 3(14.0)a | 3(6.1)a | 1(3.8)a | 2(3.6)a | 2(6.1)a | *** | *** |
| Skin wound | 0(0)a | 0(0)a | 14(64.0)b | 2(4.1)a | 29(7.7)a | 2(3.6)a | 0(0)a | *** | *** |
| Abscesses | 0(0)a | 1(5.0)a | 1(4.5)a | 0(0)a | 0(0)a | 1(1.80 | 0(0)a | *** | *** |
| Serous effusion | 0(0)a | 0(0)a | 0(0)a | 2(4.1)a | 0(0)a | 0(0)a | 0(0)a | *** | *** |
| Upper respiratory tract | 1(5.0)a | 0(0)a | 0(0)a | 0(0)a | 0(0)a | 0(0)a | 1(2.9)a | *** | *** |
| Catheter tip | 3(15.0)a | 0(0)a | 0(0)a | 0(0)a | 0(0)a | 0(0)a | 0(0)a | *** | *** |
| Others | 0(0)a | 0(0)a | 0(0)a | 1(2.0)a | 0(0)a | 0(0)a | 0(0)a | *** | *** |
| Urinary tract | 0(0)a | 0(0)a | 1(4.5)a | 1(2.0)a | 0(0)a | 1(1.8)a | 1(2.9)a | *** | *** |
a,bThe same letters between any 2 years indicate P > 0.05, whereas different letters between any 2 years indicate P < 0.05
***The specific values were not calculated separately; please refer to Note 1 for specific statistical significance
−−−The memory is insufficient to calculate
MRSA Methicillin-resistant Staphylococcus aureus
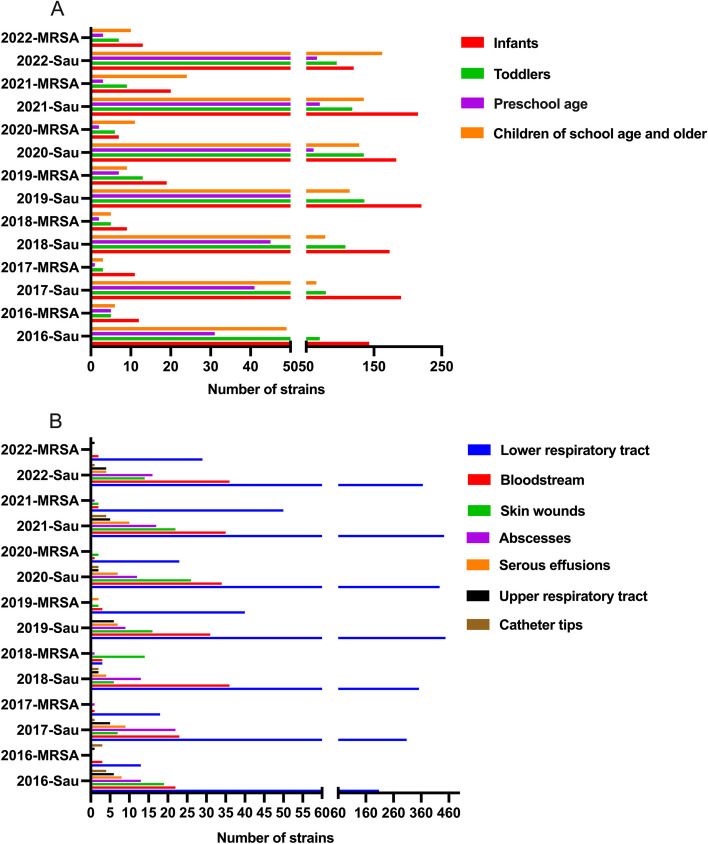
The detection rate of S. aureus and MRSA in children with different ages are shown in Tables 2 and 3 and Fig. 3A. Among the 3147 strains of S. aureus in 7 years, the top 3 highest detection rate of S. aureus in age groups were infants (1244, 39.7%), toddlers (741, 23.7%), and children at school age and older (731, 23.4%) in order (Table 2). For MRSA, The top 3 in age groups were the same as that of S. aureus, but in a different order, infants (91, 38.9%), children at school age and older (87, 29.1%), and toddlers (48, 20.5%). It can be seen that infants were most likely to be infected with S. aureus, and showed a higher proportion of MRSA. In the distribution of annual detection rate of S. aureus, only in 2022, children at school age and older ranked first. In the other 6 years, infants ranked first. For MRSA, children at school age and older and infants randomly ranked first. The detection rate of S. aureus was statistically different in the distribution of age stratification (P < 0.05). And the statistical difference of detection rate of S. aureus in the same age group between any two years is shown in Table 2. There was no statistically significant difference in these two aspects of MRSA (P > 0.05).
Fig. 3.
The distribution of S. aureus and MRSA in age and infected sites. A shows the distribution of S. aureus and MRSA in age (B) shows the distribution of S. aureus and MRSA. Notes: Sau: S. aureus; MRSA: methicillin-resistant S. aureus
The infected sites of S. aureus were shown in Tables 2 and 3 and Fig. 3B. Among all the S. aureus isolates from 2016 to 2022, the top 3 highest detection rate of S. aureus in infected sites were the lower respiratory tract (2,552, 81.7%), bloodstream (217, 6.5%), and skin wounds (110, 3.9%). For MRSA, The top 3 in infected sites were the lower respiratory tract (156, 77.9%), skin wounds (47, 8.8%), and bloodstream (15, 6.6%). Thus, the lower respiratory tract is the most common infected site of S. aureus and MRSA.
In the 7 years, the top 2 highest annual detection rate of S. aureus in infected sites is the lower respiratory tract and bloodstream. S. aureus isolated from abscesses ranked third in 2017 and 2018, and skin wounds ranked third in the other 5 years. For MRSA, the distribution of infected sites is similar to S. aureus, except the skin wound ranked first in 2018 (63.6%). The detection rate of S. aureus and MRSA was statistically different in the distribution of infected sites (P < 0.05). And the statistical difference of detection rate of S. aureus and MRSA in infected sites between any two years is shown in Tables 2 and 3.
AMR in S. aureus
The AMR profiles of 3,147 strains of S. aureus from 2016 to 2022 were shown in Table 4. All the strains of S. aureus were sensitive to tigecycline, nitrofurantoin, vancomycin, and linezolid. The annual changes in AMR of S. aureus from 2016 to 2022 were shown in Table 4 and Fig. 4. The resistant rate of S. aureus, to penicillin G was as high as 87.5% at least, to erythromycin was as high as 51.8% at least, to benzocillin was as high as 38.0% at least, to cefoxitin was as high as 35.5% at least, and to clindamycin was as high as 32.7% at least. The resistant rates of S. aureus to erythromycin, clindamycin, cefoxitin TMP-SMX and gentamicin were declining annually, to penicillin G and benzoxiline fluctuated within 10%. The resistance trends of S. aureus to tetracycline, quinupristin/dalfopristin, and fosfomycin showed a similar trend, increasing initially and then decreasing. S. aureus showed similar resistance trend to levofloxacin, ciprofloxacin, and moxifloxacin, with the resistant rate slowly increasing to being less than 10%. It’s resistant rate to fosfomycin, rifampicin, and minocycline was less than 5.0%. The significant differences in the AMR of S. aureus to commonly used antimicrobials between any 2 years from 2016 to 2022 were shown in Table 4.
Table 4.
Resistance of S. aureus to commonly used antimicrobial drugs [Strain (%)]
| Antimicrobial drugs | The year | ||||||
|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| Penicillin G | 251(93.7)a,b | 328(90.9)b | 388(92.1)a,b | 477(91.2)a,b | 471(87.5)a,b,c | 551(89.8)a,c | 462(93.3)c |
| Benzocillin | 126(47.0)a | 155(43.3)a | 162(44.1)a | 228(43.8)a | 197(38.0)a | 229(42.6)a | 188(42.0)a |
| Erythromycin | 167(60.8)a | 227(61.4)a | 248(59.3)a | 309(59.1)a | 292(56.3)a | 300(55.6)a | 232(51.8)a |
| Clindamycin | 126(42.4)a | 153(41.4)a | 182(43.4)a | 213(41.0)a | 207(40.0)a | 209(38.8)a | 145(32.7)a |
| Cefoxitin | 47(39.5)a,b,c | 99(54.7)c | 94(44.5)b,c | 83(38.0)a,b | 60(38.0)a | 88(35.7)b | 48(35.5)a |
| Tetracycline | 46(16.5)a,b | 56(15.1)a,b | 52(14.9)a,b | 83(19.8)b | 79(20.4)b | 48(12.8)a | 35(12.2)a |
| Levofloxacin | 15(5.7)a,b | 19(5.8)a,b | 28(7.4)a,b | 34(8.1)a,b | 29(5.7)a,b | 46(9.5)a,b | 31(8.3)a,b |
| Ciprofloxacin | 18(6.4)a | 19(5.1)a | 25(6.2)a | 29(6.9)a | 24(6.2)a | 26(7.0)a | 29(10.1)a |
| Moxifloxacin | 11(4.5)a | 15(4.8)a | 19(6.1)a | 26(6.5)a | 24(5.2)a | 40(8.8)a | 25(6.8)a |
| TMP-SMZ | 42(13.9)a | 52(14.2)a | 44(10.5)a,b | 46(8.8)a,b | 36(6.9)a,b | 37(6.9)b | 26(6.8)b |
| Fosfomycin | 2(2.1)a | 7(7.1)a | 1(0.8)a | 4(3.2)a | 3(2.2)a | 6(3.5)a | 3(3.7)a |
| Rifampicin | 9(3.2)a | 6(1.6)a,b | 5(1.2)a,b | 7(1.4)a,b | 8(1.8)b | 5(1.0)a,b | 5(1.2)a,b |
| Gentamicin | 28(9.3)a | 29(8.0)a,b | 22(5.3)a,b,c | 8(1.7)d | 19(3.7)b,c,d | 21(3.9)b,c,d | 10(2.2)c,d |
| Vancomycin | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| Minocycline | 0a | 4(3.7)a | 1(1.4)a | 3(4.5)a | 1(1.2)a | 6(3.6)a | 0a |
| Nitrofurantoin | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| Linezolid | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| Quinopridin/Dafeptin | 22(10.0)a | 33(12.5)a | 51(17.4)a | 46(13.3)a | 54(16.2)a | 48(15.1)a | 26(10.2)a |
| Tigecycline | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
a, b, c The same letters between any 2 years indicate P > 0.05, whereas different letters between any 2 years indicate P < 0.05
Fig. 4.
Resistance of S. aureus to commonly used antimicrobial drugs
AMR in MRSA
The AMR profiles of the 234 strains of MRSA from 2016 to 2022 were shown in Table 5. All the strains of MRSA were sensitive to vancomycin, linezolid, quinupristin/dalfopristin, and tigecycline.The annual changes in AMR of MRSA from 2016 to 2022 were shown in Table 5 and Fig. 5. Of these 234 strains of MRSA, 179 (76.5%) were resistant to erythromycin, 116 (49.6%) to clindamycin, 39 (16.7%) to tetracycline, 29 (12.4%) to levofloxacin, 27 (11.5%) to ciprofloxacin, 27 (11.5%) to moxifloxacin, 14 (6.0%) to TMP-SMX, eight (3.4%) to rifampicin, and six (2.6%) to gentamicin. The resistant rates of MRSA to erythromycin, clindamycin, and tetracycline varied considerably, but showed a similar increasing trend. The resistance trends of MRSA to levofloxacin, ciprofloxacin, and moxifloxacin were similar. The resistant rates of MRSA to TMP-SMX, rifampicin, and gentamicin did not exceed 10% after 2017. The significant differences in the AMR of MRSA to commonly used antimicrobials, except tetracyclin, between any 2 years from 2016 to 2022 were shown in Table 5.
Table 5.
Resistance of MRSA to commonly used antimicrobial drugs [Strain (%)]
| Antimicrobial drugs | The year | ||||||
|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| Erythromycin | 21(75.0)a | 18(90.0)a | 18(81.8)a | 42(85.7)a | 19(73.1)a | 44(78.6)a | 17(51.5)a |
| Clindamycin | 9(34.6)a | 9(45.0)a | 9(40.9)a | 37(75.5)a | 14(53.8)a | 27(48.2)a | 11(34.4)a |
| Tetracycline | 6(22.2)a,b | 8(40.0)b | 3(13.6)a,b | 13(31.0)a,b | 2(28.6)a,b | 4(8.5)a | 3(12.0)a,b |
| Levofloxacin | 3(10.7)a | 1(5.0)a | 2(9.1)a | 5(12.2)a | 3(11.5)a | 13(28.9)a | 2(9.1)a |
| Ciprofloxacin | 3(10.7)a | 1(5.0)a | 2(9.1)a | 5(11.9)a | 2(28.6)a | 9(19.1)a | 5(20.0)a |
| Moxifloxacin | 3(10.7)a | 0a | 2(9.1)a | 5(12.2)a | 3(11.5)a | 13(28.9)a | 1(4.5)a |
| TMP-SMZ | 4(14.3)a | 2(10.0)a | 1(4.5)a | 2(4.1)a | 1(3.8)a | 2(3.6)a | 2(6.1)a |
| Rifampicin | 4(14.2)a | 0a | 1(4.6)a | 1(2.1)a | 1(3.8)a | 0a | 1(3.0)a |
| Gentamicin | 3(10.7)a | 0a | 0a | 1(2.0)a | 0a | 0a | 2(6.1)a |
| Vancomycin | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| Linezolid | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| Quinopridin/Dafeptin | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| Tigecycline | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
a, bThe same letters between any 2 years indicate P > 0.05, whereas different letters between any 2 years indicate P < 0.05
Fig. 5.
Resistance of MRSA to commonly used antimicrobial drugs
Discussion
This is the first multi-center retrospective study, in which we retrospectively analyzed the profile and evolution of S. aureus in 17 PICUs in China from 2016 to 2022.
S. aureus is a very common human pathogenic microorganism that was first discovered in 1880 by Alexander Ogston, a Scottish surgeon. With the evolution of bacteria and the use of antimicrobial drugs, the AMR of S. aureushas gradually changed, especially after the emergence of MRSA, which has made the diagnosis and treatment more difficult [13, 14]. Children in ICUs are critically ill and vulnerable to be infected, so that S. aureusmay cause significant morbidity and mortality in neonatal ICUs and PICUs [15]. Therefore, S. aureus in ICUs needs to be continuously monitored. A study in Germany found that the incidence of S. aureusand MRSA decreased in hospitals over a 10-year period from 2007 to 2016, but gradually increased in ICUs, with a decrease incidence of MRSA (35.2% to 20.7%) [16]. The China Antimicrobial Surveillance Network (CHINET) reported that the incidence of MRSA in S. aureusdecreased from 38.4% in 2016 to 30.0% in 2021 [17, 18]. In this study, during the 7 years, from 2016 to 2022, although the top 10 bacterial strains from the 17 PICUs were mainly gram-negative bacilli, S. aureus occupied the second place with an absolute advantage, with the incidence of MRSA ranged from 4.99% to 10.35%. The incidence of S. aureus in PICUs increased from 24.75% to 36.19%, whereas the incidence of MRSA decreased from 9.27% to 7.30%. Thus, the results of our study are similar to the findings of researches above.Few epidemiological studies have focused on S. aureus in PICUs alone, so our study may provide some reference value. The decreased incidence of MRSA in PICUs may be affected by pathogen surveillance and feedback systems and multidisciplinary management of antimicrobial drugs.
The incidence of S. aureusis very high [19, 20]. Many previous studies have reported gender differences in S. aureusbacteremia (SAB), with some studies showing a higher incidence of SAB in females than in males [21–25], Whereas some others studies have reported that there was no difference in the incidence of MRSA between the sexes [26–28]. In the study, there was no gender difference in the incidence of S. aureus and MRSA, which was consistent with the findings of See et al. and Campbell et al. [15, 29] It may be related to the origin of the subjects, the inherent biological differences between men and women, and social factors. Studies undertaken in pediatric wards have shown that the group infected by S. aureusis most common in infants [15], and the group infected by MRSA is most common in children younger than 5 year sold [30, 31]. Few studies have investigated the age distribution infected by S. aureus and MRSA in PICUs. In our study, both the group infected by S. aureus and MRSA were most common in infants. Multiple sites or organs in human can be infected by S. aureus. Comprehensive data from home and abroad showed that the most common infected site of MRSA is the respiratory tract (36.3%−48.0%), followed by the bloodstream (15%−35.7%), wounds or catheter exit sites (25.2%−44%), and the urinary tract (2.8%−28%), with the least common source of specimen being puncture fluid [31–33]. Wu et al. [34] reported that the top three most common infected sites of MRSA in children were the lower respiratory tract (58.28%), abscess (16.78%), and secretions (7.61%), and the least common specimen sources were cerebrospinal fluid and urine. Zhang et al. [35] found that MRSA was detected ranging from 40 to 50% in bloodstream infections with S. aureus. In the study, the most common infected site of S. aureus and MRSA was the lower respiratory tract, followed by the bloodstream and skin wounds. The least common infected sites of S. aureus and MRSA were “others” (including peritoneal dialysis fluid, drainage fluid and etc.) and the gastrointestinal tract, respectively. In summary, it can be seen that S. aureus is the main pathogen of respiratory tract whether at home or abroad, adults or children, general wards or intensive care units, and the same to MRSA. In this study, we also found that the incidence of S. aureus and MRSA in skin wounds was higher in children in PICU, which was mostly related to the colonization of Staphylococcus aureus on the skin surface.
AMR of S. aureus is a major concern and plays a decisive role in the clinical efficacy. There are many researches have reported the resistance of commonly used antimicrobial drugs of S. aureus. According to the CHINET report on trends in MRSA resistance from 2016 to 2021, all strains of MRSA were found to be sensitive to vancomycin, teicoplanin, and linezolid, with a decreased trend in resistance to erythromycin, clindamycin, tetracycline, levofloxacin, ciprofloxacin, moxifloxacin, TMP-SMX, gentamicin, and rifampicin [17, 18]. Studies about MRSA in pediatric patients, all strains of MRSA were sensitive to vancomycin and linezolid, and the highest resistant rate to erythromycin was 62%, followed by clindamycin (14%−57%), TMP-SMX (3%−24%), gentamicin (24%), rifampicin (12%), and minocycline (10%) [36, 37]. In this study, we also found that all the all strains of MRSA were sensitive to vancomycin, teicoplanin, and linezolid, with high resistant rates to erythromycin, clindamycin, and tetracycline but low resistant rates to relevofloxacin, ciprofloxacin, moxifloxacin, TMP-SMX, gentamicin, and rifampicin. We also found that the resistance of MRSA to commonly used antimicrobials showed an overall downward trend with wide year-to-year variations, except clindamycin and quinolone antibiotics. The resistant rate of MRSA to clindamycin increased from 34.6% in 2016 to 75.5% in 2019, and then decreased back to 34.4% in 2022. We analysed that the fluctuations resistant rate of MRSA varied widely to these antimicrobials, possibly related to the usage of the drugs. Studies have shown that strains from different sources may exhibit different sensitivity to antimicrobial, and bloodstream-derived MRSAs are more susceptible to drug resistance than than skin-derived and soft tissue-derived MRSAs [38]. Liang et al. [39] reported that resistant rates to gentamicin and ciprofloxacin were significantly higher in MRSAs from the respiratory tract than those from skin and soft tissues or blood.
For staphylococci, resistance to drugs can either develop by horizontal transfer of resistance determinants encoded by mobile genetic elements viz plasmids, transposons and the staphylococcal cassette chromosome or by mutations in chromosomal genes. Horizontally acquired resistance can occur by one of them: enzymatic drug modification and inactivation, enzymatic modification of the drug binding site, drug efflux, bypass mechanisms involving acquisition of a novel drug-resistant target, displacement of the drug to protect the target. Acquisition of resistance by mutation can result from alteration of the drug target that prevents the inhibitor from binding, derepression of chromosomally encoded multi-drug resistance efflux pumps and multiple stepwise mutations that alter the structure and composition of the cell wall and/or membrane to reduce drug access to its target. The development of resistance to many antibiotics by S. aureushas involved acquisition of determinants by horizontal gene transfer of mobile genetic elements [40]. Vancomycin was the first choice for pneumonia patients with hospital-acquired MRSA. Recent evidence suggests that the minimum inhibitory concentration for vancomycin is increasing and linezolid is another option [41]. The standard treatment for MRSA bacteremia is vancomycin or daptomycin. But, the efficacy is limited and there are many disadvantages such as poor tissue permeability and slow killing time. Another strategy is to combine the standard regimen with other drugs, such as antistaphylococcal β-lactam (ASBL). However, whether the combination has obvious better outcomes is still inconclusive [5]. The drug resistance of S. aureus inevitably change. Therefore, it is necessary to understand its drug resistance changes in specific areas. Now, there are few studies on the mechanism of S. aureus resistance in PICUs. In this study, the resistance mechanism of S. aureus was not explored, which is a major regret. It is planned to be explored in the follow-up related research work.
This study has certain limitations, due to the lack of relevant information that has not been fully matched with clinical data, such as primary disease, seasonal correlation, geographical correlation, isolation of colonized bacteria and pathogenic bacteria, and the difference between community-acquired and hospital-acquired bacteria, etc. We will gradually optimize the CHIPS to incorporate the relevant information in the later data so that these questions will be answered in future studies.
Conclusions
In this study, the 7-year surveillance of bacterial strains in 17 PICUs in China revealed that although gram-negative bacilli were the predominant etiological agents of infections, S. aureus, a gram-positive bacterium, ranked second in frequency. Infants were most susceptible to S. aureus and MRSA, and the lower respiratory tract was the most common infected site. S. aureus showed the highest resistant rate to penicillin G, followed by erythromycin, benzathine, cefoxitin, and clindamycin. The resistant rates of S. aureus to levofloxacin, ciprofloxacin, and moxifloxacin were less than 10%, to fosfomycin, rifampicin, and minocycline were less than 5%. MRSA showed a highly resistance to erythromycin and clindamycin, but the resistant rates to tetracycline, levofloxacin, ciprofloxacin, moxifloxacin, TMP-SMX, rifampicin, and gentamicin were low. No strains resistant to vancomycin, linezolid and tigecycline were found in this study, but the supervision of these antimicrobial drugs should not be ignored, and they should be used rationally to maintain the last line of defense in the treatment of S. aureus. When considering to be infected by S. aureus clinically, it is necessary to select antimicrobial drugs reasonably based on the age, infected site, and local epidemiological characteristics.
Acknowledgements
We would like to thank the members of CHIPS for their contributions to this study. The CHIPS members are as follows:
1. Hong Renc, Shanghai Children’s Medical Center affiliated to Shanghai Jiao Tong University School of Medicine;
2. Juan-zhen Lic, Shanghai Children’s Medical Center affiliated to Shanghai Jiao Tong University School of Medicine;
3. Yu-cai Zhangd, Shanghai Children’s Hospital affiliated to Shanghai Jiaotong University;
4. Yi-ping Zhoud, Shanghai Children’s Hospital affiliated to Shanghai Jiaotong University;
5. Cheng-mei Zhange, Children’s Hospital Affiliated to Zhejiang University Medical School;
6. Zhen-jie Chene, Children’s Hospital Affiliated to Zhejiang University Medical School;
7. Ming-ming Zhoue, Children’s Hospital Affiliated to Zhejiang University Medical School;
8. Zheng-jiang Baif, Children’s Hospital of Soochow University;
9. Sai-hu Hangf, Children’s Hospital of Soochow University;
10. Li-li Hangf, Children’s Hospital of Soochow University;
11. He-he Cheng, Ningbo Women and Children’s Hospital;
12. Yao Zhengg, Ningbo Women and Children’s Hospital;
13. Qun-ying Cheng, Ningbo Women and Children’s Hospital;
14. Peng-wei Zhuh, Wuxi Children’s Hospital;
15. Yong Lih, Wuxi Children’s Hospital;
16. Yan Xuh, Wuxi Children’s Hospital;
17. Bi-zhen Zhui, the First Affiliated Hospital of Xiamen University;
18. Hui-xuan Shii, the First Affiliated Hospital of Xiamen University;
19. Shao-xian Hongj, Xiamen Children's Hospital;
20. Yu-kun Huangj, Xiamen Children's Hospital;
21. Mei-lian Huangj, Xiamen Children's Hospital;
22. Dan-qun Jingk, Anhui Children’s Hospital;
23. Wen-jia Tongk, Anhui Children’s Hospital;
24. Cheng-Yu Zhangk, Anhui Children’s Hospital;
25. Wei-guo Yangl, Shenzhen Children’s Hospital;
26. Wei-ke Mangl, Shenzhen Children’s Hospital;
27. Qing Mengl, Shenzhen Children’s Hospital;
28. Yi-bing Chengm, Henan Children’s Hospital;
29. Qun-qun Zhangm, Henan Children’s Hospital;
30. Kai-jie Gaom, Henan Children’s Hospital;
31. Hui-ming Xun, Kaifeng Children's Hospital;
32. Yu-xia Lin, Kaifeng Children's Hospital;
33. Hang-hai Dingn, Kaifeng Children's Hospital;
34. Xiu-lan Luo, Hunan Children's Hospital;
35. Jiao-tian Huango, Hunan Children's Hospital;
36. Jian-long Liuo, Hunan Children's Hospital;
37. You-rong Zhup, Jiangxi Provincial Children’s Hospital;
38. Yuan-yuan Chenp, Jiangxi Provincial Children’s Hospital;
39. Shu-fang Xiaoq, Kunming Children's Hospital;
40. Juan Heq, Kunming Children's Hospital;
41. Li Jiangq, Kunming Children's Hospital;
42. Jian-li Chenr, Guiyang Children’s Hospital;
43. Yi Linr, Guiyang Children’s Hospital;
44. Jia Leir, Guiyang Children’s Hospital.
Abbreviations
- PICU
Pediatric intensive care units
- CHINET
China antimicrobial surveillance network
- CLSL
Clinical and laboratory standards institute
- S. aureu
Staphylococcus aureus
- AMR
Antimicrobial resistance
- MSSA
Methicillin-sensitive staphylococcus aureus
- MRSA
Methicillin-resistant staphylococcus aureus
- CHIPS
China paediatric intensive care unit pathogen surveillance network
- DST
Drug susceptibility testing
- TMP-SMX
Sulfamethoxazole-trimethoprim
- GM +
Gram-positive
- SAB
Staphylococcus aureus Bacteremia
Authors' contributions
X.L. Zhang, J. Liu, and P. Fu contributed equally to this work as co-first authors. X.L. Zhang, J. Liu, and P. Fu wrote the first manuscript and revised verson. C.Q. Wang, G.P. Lu, and G.F. Yan conceptualized and designed the study, and took responsibility for the integrity of the whole work. The others performed the collection, analysis, and interpretation of data. All authors contributed to the article and approved the submitted version.
Funding
The study were supported by the National Key Research and Development Program of China (2021YFC2701800, 2021YFC2701805), the Scientific Research Project of Shanghai Municipal Health Commission (No. 202140442), and the Shanghai Municipal Health System major supports discipline projects (2023ZDFC0103).
Data availability
Data and materials used in this work are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The study was approved by the institutional review board at the Children’s Hospital of Fudan University in Shanghai, and the requirement for informed consent was waived (approval number [2021] 431). The study strictly adheres to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiao-Lei Zhang, Jing Liu and Pan Fu first authors contributed equally to this article.
Chuan-Qing Wang, Guo-Ping Lu and Gang-Feng Yan corresponding authors contributed equally to this article.
Contributor Information
Chuan-Qing Wang, Email: chuanqing523@163.com.
Guo-Ping Lu, Email: 13788904150@163.com.
Gang-Feng Yan, Email: gangfeng_yan@fudan.edu.cn.
the China paediatric Intensive care Unit Pathogen Surveillance Network (CHIPS) Study Group:
Hong Ren, Juan-zhen Li, Yu-cai Zhang, Yi-ping Zhou, Cheng-mei Zhang, Zhen-jie Chen, Ming-ming Zhou, Zheng-jiang Bai, Sai-hu Hang, Li-li Hang, He-he Chen, Yao Zheng, Qun-ying Chen, Peng-wei Zhu, Yong Li, Yan Xu, Bi-zhen Zhu, Hui-xuan Shi, Shao-xian Hong, Yu-kun Huang, Mei-lian Huang, Dan-qun Jing, Wen-jia Tong, Cheng-Yu Zhang, Wei-guo Yang, Wei-ke Mang, Qing Meng, Yi-bing Cheng, Qun-qun Zhang, Kai-jie Gao, Hui-ming Xu, Yu-xia Li, Hang-hai Ding, Xiu-lan Lu, Jiao-tian Huang, Jian-long Liu, You-rong Zhu, Yuan-yuan Chen, Shu-fang Xiao, Juan He, Li Jiang, Jian-li Chen, Yi Lin, and Jia Lei
References
- 1.Paonessa JR, Shah RD, Pickens CI, Lizza BD, Donnelly HK, Malczynski M, et al. Rapid Detection of Methicillin-Resistant Staphylococcus aureus in BAL: A Pilot Randomized Controlled Trial. Chest. 2019;155:999–1007. 10.1016/j.chest.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265–73. 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr Opin Microbiol. 2012;15(5):588–95. 10.1016/j.mib.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Lakhundi S, Zhang K. Methicillin-resistant staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31(4):e00020-18. 10.1128/CMR.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X, Lu N, Song P, Zhou M, Li Y, Wang Z, et al. Vancomycin, Daptomycin, Antistaphylococcal β-Lactam, and Trimethoprim-Sulfamethoxazole Monotherapy and Combination Therapy in the Management of Methicillin-Resistant Staphylococcus aureus: A Network Meta-Analysis. Front Pharmacol. 2022;13:805966. 10.3389/fphar.2022.805966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su W, Liu Y, Wang Q, Yuan L, Gao W, Yao KH, et al. Antibiotic susceptibility and clonal distribution of Staphylococcus aureus from pediatric skin and soft tissue infections: 10-year trends in multicenter investigation in China. Front Cell Infect Microbiol. 2023;13:1179509. 10.3389/fcimb.2023.1179509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Y, Glaser K, Speer CP. New Threats from an Old Foe: Methicillin-Resistant Staphylococcus aureus Infections in Neonates. Neonatology. 2018;114(2):127–34. 10.1159/000488582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blinova E, Lau E, Bitnun A, et al. Point prevalence survey of antimicrobial utilization in the cardiac and pediatric critical care unit[J]. Pediatr Crit Care Med. 2013;14(6):e280-288. 10.1097/PCC.0b013e31828a846d. [DOI] [PubMed] [Google Scholar]
- 9.Fischer JE, Ramser M, Fanconi S. Use of antibiotics in pediatric intensive care and potential savings[J]. Intensive Care Med. 2000;26(7):959–66. 10.1007/s00134005. [DOI] [PubMed] [Google Scholar]
- 10.Kathleen C, Jennifer B, Juri B, et al. 1134. Antibiotic Indications and Appropriateness in the Pediatric Intensive Care Unit: A 10 Center Point Prevalence Study[J]. Open Forum Infectious Diseases. 2019;(Supplement-2):S403-S404. 10.1093/ofid/ofz360.998.
- 11.Shang H, Wang YS, Shen ZY. National Guide to Clinical Laboratory Procedures (4th edition). Beijing: People’s Medical Publishing House; 2015. [Google Scholar]
- 12.Clinical Laboratory Standards Institute.Performance Standards for Antimicrobial Susceptibility Testing. CLSI. 2020:M100-S30.
- 13.Aires-de-Sousa M. Methicillin-resistant Staphylococcus aureus among animals: current overview. Clin Microbiol Infect. 2017;23:373–80. 10.1016/j.cmi.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Gatadi S, Madhavi YV, Chopra S, Nanduri S. Promising antibacterial agents against multidrug resistant Staphylococcus aureus. Bioorg Chem. 2019;92:103252. 10.1016/j.bioorg.2019.103252. [DOI] [PubMed] [Google Scholar]
- 15.See P, Bonacorsi S, Toumazi A, Doit C, Naudin J, Chomton M, et al. Factors linked to Staphylococcus aureus healthcare-associated infections among pediatric intensive care unit colonized patients. Arch Pediatr. 2023;30(3):153–7. 10.1016/j.arcped.2023.01.002. [DOI] [PubMed]
- 16.Kramer TS, Schroeder C, Behnke M, Aghdassi SJ, Geffers C, Gastmeier R, et al. Decrease of methicillin resistance in staphylococcus aureus in nosocomial infections in Germany-a prospective analysis over 10 years. J Infection. 2019;78(3):215–9. 10.1016/j.jinf.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Hu F, Guo Y, Zhu D, Wang F, Jiang XF, Xu YC, et al. CHINET surveillance of bacterial resistance in China: 2016 report. Chin J Infect Chemother. 2017;17(5):481–91. 10.16718/j.1009-7708.2017.05.001. [Google Scholar]
- 18.Hu F, Guo Y, Zhu D, Wang F, Jiang XF, Xu YC, et al. CHINET surveillance of bacterial resistance in China: 2021 report. Chin J Infect Chemother. 2022;22(5):521–30. 10.16718/j.1009-7708.2022.05.001. [Google Scholar]
- 19.Lam JC, Gregson DB, Robinson S, Somayaji R, Conly JM, Parkins MD. Epidemiology and outcome determinants of Staphylococcus aureus bacteremia revisited: a population-based study. Infection. 2019;47(6):961–71. 10.1007/s15010-019-01330-5. [DOI] [PubMed] [Google Scholar]
- 20.Souli M, Ruffin F, Choi SH, Park LP, Gao S, Lent NC, et al. Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, longitudinal study. Clin Infect Dis. 2019;69(11):1868–77. 10.1093/cid/ciz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smit J, Lopez-Cortes LE, Kaasch AJ, Søgaard M, Thomsen RW, Schønheyder HC, et al. Gender differences in the outcome of community-acquired Staphylococcus aureus bacteraemia: a historical population-based cohort study. Clin Microbiol Infect. 2017;23(1):27–32. 10.1016/j.cmi.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Austin ED, Sullivan SS, Macesic N, Mehta M, Miko BA, Nematollahi S, et al. Reduced mortality of Staphylococcus aureus bacteremia in a retrospective cohort study of 2139 patients: 2007–2015. Clin Infect Dis. 2020;70(8):1666–74. 10.1093/cid/ciz498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB, et al. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev. 2012;25(2):362–86. 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphreys H, Fitzpatick F, Harvey BJ. Gender differences in rates of carriage and bloodstream infection caused by methicillin-resistant Staphylococcus aureus: are they real, do they matter and why? Clin Infect Di. 2015;61(11):1708–814. 10.1093/cid/civ576. [DOI] [PubMed] [Google Scholar]
- 25.Mansur N, Hazzan R, Paul M, Bishara J, Leibovici L. Does sex affect 30-day mortality in Staphylococcus aureus bacteremia? Gend Med. 2012;9(6):463–70. 10.1016/j.genm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Kang CK, Kwak YG, Park Y, Song KH, Kim ES, Jung SI, et al. Gender affects prognosis of methicillin-resistant Staphylococcus aureus bacteremia differently depending on the severity of underlying disease. Eur J Clin Microbiol Infect Dis. 2018;37(6):1119–23. 10.1007/s10096-018-3226-6. [DOI] [PubMed] [Google Scholar]
- 27.Forsblom E, Kakriainen A, Ruotsalainen E, J€arvinen A. Comparison of patient characteristics, clinical management, infectious specialist consultation, and outcome in men and women with methicillin-sensitive Staphylococcus aureus bacteremia: a propensity-score adjusted retrospective study. Infection. 2018;46(6):837–45. 10.1007/s15010-018-1216-3. [DOI] [PubMed] [Google Scholar]
- 28.Jokinen E, Laine J, Huttunen R, Rahikka P, Huhtala H, Vuento R, et al. Comparison of outcome and clinical characteristics of bacteremia caused bymethicillin-resistant, penicillin-resistant and penicillin-susceptible Staphylococcus aureus strains. Infect Dis (Lond). 2017;49(7):493–500. 10.1080/23744235.2017.1292046. [DOI] [PubMed] [Google Scholar]
- 29.Campbell AJ, Al Yazidi LS, Phuong LK, Leung C, Best EJ, Webb RH, et al. Pediatric Staphylococcus aureus Bacteremia. Clin Infect Dis. 2022;74(4):604–13. 10.1093/cid/ciab510. [DOI] [PubMed] [Google Scholar]
- 30.Perovic O, Iyaloo S, Kularatne R, Lowman W, Bosman N, Wadula J, et al. Prevalence and trends of staphylococcus aureus bacteraemia in hospitalized patients in south Africa 2010 to 2012: Laboratory-based surveillance mapping of antimicrobial resistance and molecular epidemiology. PloS One. 2015;10(12):e0145429. 10.1371/journal.pone.0145429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahimi F, Shokoohizadeh L. Characterization of methicillin resistant Staphylococcus aureus strains among inpatients and outpatients in a referral hospital in Tehran, Iran. Microb Pathog. 2016;97:89–93. 10.1016/j.micpath.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Huang L, Zhang R, Hu Y, Zhou H, Cao J, Lv H, et al. Epidemiology and risk factors of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci infections in Zhejiang China from 2015 to 2017. Antimicrob Resist Infect Control. 2019;8:90. 10.1186/s13756-019-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samadi R, Ghalavand Z, Mirnejad R, Nikmanesh B, Eslami G. Antimicrobial resistance and molecular characteristics of methicillin-resistant staphylococcus aureus isolates from children patients in Iran. Infect Drug Resist. 2019;12:3849–57. 10.2147/IDR.S229394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X, Wang C, He L, Xu HM, Jing CM, Chen YH, et al. Antimicrobial resistance profile of methicillin-resistant Staphylococcus aureus isolates in children reported from the ISPED surveillance of bacterial resistance, 2016–2021. Front Cell Infect Microbiol. 2023;13:1102779. 10.3389/fcimb.2023.1102779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Sun Z, Tian L. Antimicrobial resistance among pathogens causing bloodstream infections: A multicenter surveillance report over 20 years, (1998–2017). Infect Drug Resist. 2022;15:249–60. 10.2147/IDR.S344875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acree ME, Morgan E, David MZS. aureus infections in Chicago 2006–2014: increase in CA MSSA and decrease in MRSA incidence. Infect Control Hosp Epidemiol. 2017;38(10):1226–34. 10.1017/ice.2017.177. [DOI] [PubMed] [Google Scholar]
- 37.Liang Y, Tu C, Tan C, Ahmed M, Dai M, Xia Y, et al. Antimicrobial resistance, virulence genes profiling and molecular relatedness of methicillin-resistant staphylococcus aureus strains isolated from hospitalized patients in guangdong province. China Infect Drug Resist. 2019;12:447–59. 10.2147/IDR.S192611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milstone AM, Carroll KC, Ross T, Shangraw KA, Perl TM. Community-associated methicillin-resistant staphylococcus aureus strains in pediatric intensive care unit. Emerg Infect Dis. 2010;16(4):647–55. 10.3201/eid1604.090107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miguel CPV, Mejias A, Leber A, Sanchez P. A decade of antimicrobial resistance in staphylococcus aureus: A single center experience. PloS One. 2019;14(2):e0212029. 10.1371/journal.pone.0212029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen SO, Lyon BR. Genetics of antimicrobial resistance in Staphylococcus aureus[J]. Future Microbiology. 2009;4(5):565–82. 10.2217/fmb.09.30. [DOI] [PubMed] [Google Scholar]
- 41.Lesher B, Gao X, Chen Y, Liu Z. Methicillin-resistant Staphylococcus aureus nosocomial pneumonia: role of linezolid in the People’s Republic of China. Clinicoecon Outcomes Res. 2016;24(8):63–72. 10.2147/CEOR.S91985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials used in this work are available from the corresponding author upon request.