Abstract
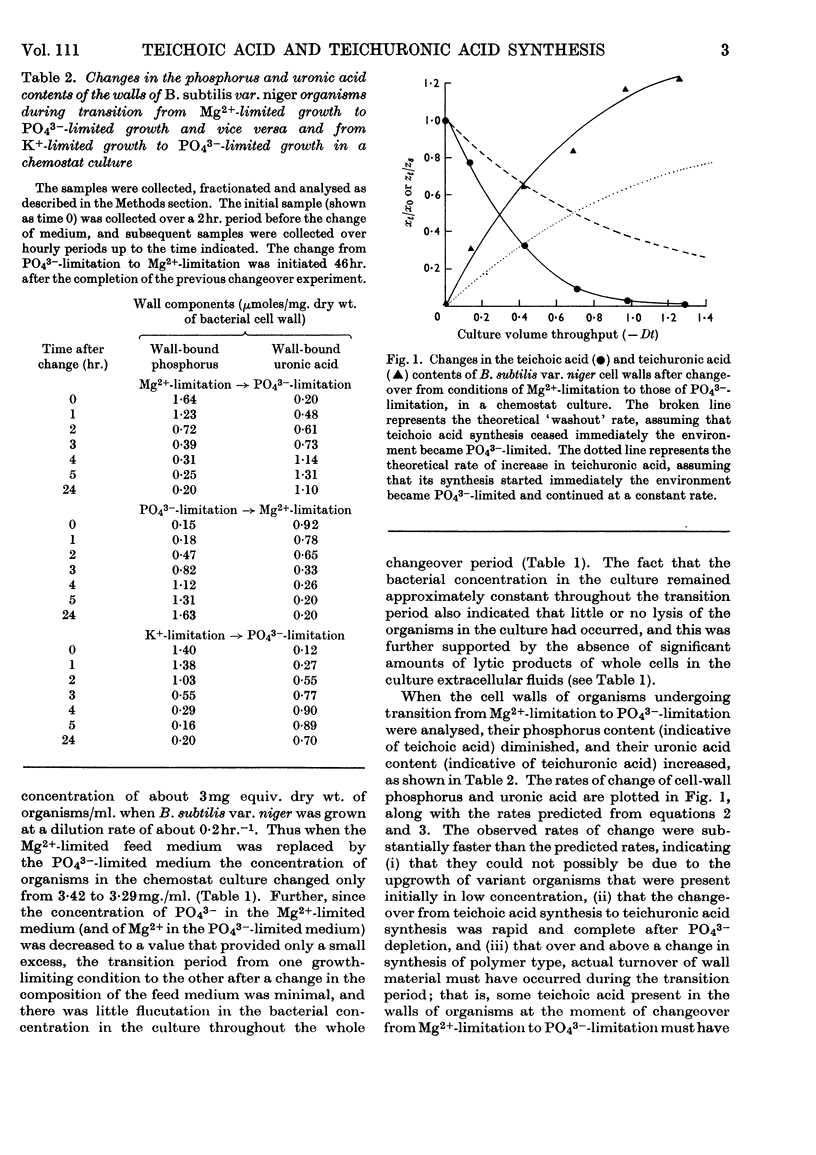
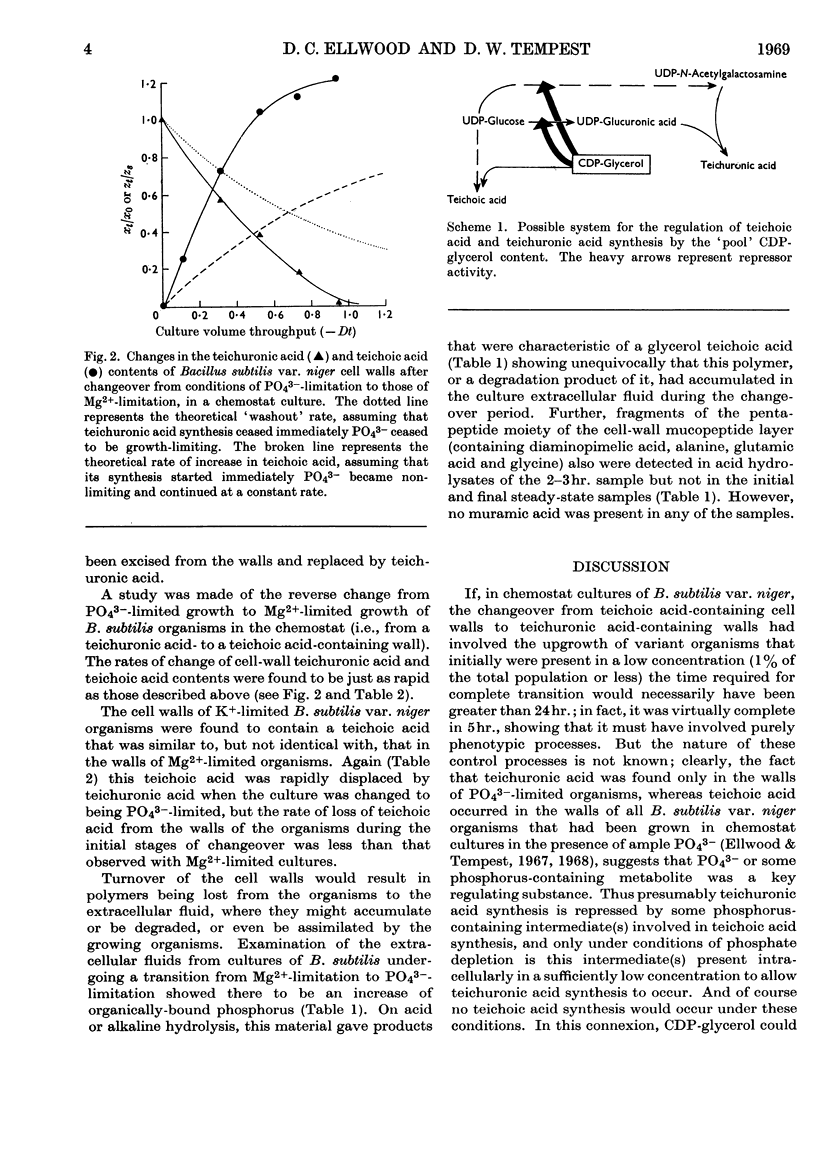
1. Quantitative determination of the anionic polymers present in the walls of Bacillus subtilis var. niger organisms undergoing transition, in a chemostat culture, from either Mg2+-limitation to PO43−-limitation or K+-limitation to PO43−-limitation showed that teichuronic acid synthesis started immediately the culture became PO43−-limited and proceeded at a rate substantially faster than the rate of biomass synthesis. 2. Simultaneously, the cell-wall teichoic acid content diminished at a rate greater than that due to dilution by newly synthesized wall material, and fragments of teichoic acid and mucopeptide accumulated in the culture extracellular fluid. 3. Equally rapid reverse changes occurred when a PO43−-limited B. subtilis var. niger culture was returned to being Mg2+-limited. 4. It is concluded that in this organism both teichoic acid and teichuronic acid syntheses are expressions of a single genotype, and a mechanism for the control of synthesis of both polymers is suggested. 5. These results are discussed with reference to the constantly changing environmental conditions that obtain in a batch culture and the variation in bacterial cell-wall composition that is reported to occur throughout the growth cycle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellwood D. C., Kelemen M. V., Baddiley J. The glycerol teichoic acid from the walls of Staphylococcus albus N.T.C.C. 7944. Biochem J. 1963 Feb;86(2):213–225. doi: 10.1042/bj0860213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Tempest D. W. Teichoic acid or teichuronic acid in the walls of Bacillus subtilis var. niger, grown in a chemostat. Biochem J. 1967 Sep;104(3):69P–69P. [PMC free article] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Herbert D., Phipps P. J., Tempest D. W. The chemostat: design and instrumentation. Lab Pract. 1965 Oct;14(10):1150–1161. [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346: Biosynthesis of the teichuronic acid. Biochem J. 1966 Dec;101(3):692–697. doi: 10.1042/bj1010692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL E. O. Criteria for the growth of contaminants and mutants in continuous culture. J Gen Microbiol. 1958 Feb;18(1):259–268. doi: 10.1099/00221287-18-1-259. [DOI] [PubMed] [Google Scholar]
- Powell E. O. Theory of the chemostat. Lab Pract. 1965 Oct;14(10):1145–passim. [PubMed] [Google Scholar]
- Tempest D. W., Dicks J. W., Ellwood D. C. Influence of growth condition on the concentration of potassium in Bacillus subtilis var. niger and its possible relationship to cellular ribonucleic acid, teichoic acid and teichuronic acid. Biochem J. 1968 Jan;106(1):237–243. doi: 10.1042/bj1060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Dicks J. W., Meers J. L. Magnesium-limited growth of Bacillus subtilis, in pure and mixed cultures, in a chemostat. J Gen Microbiol. 1967 Oct;49(1):139–147. doi: 10.1099/00221287-49-1-139. [DOI] [PubMed] [Google Scholar]


