Abstract
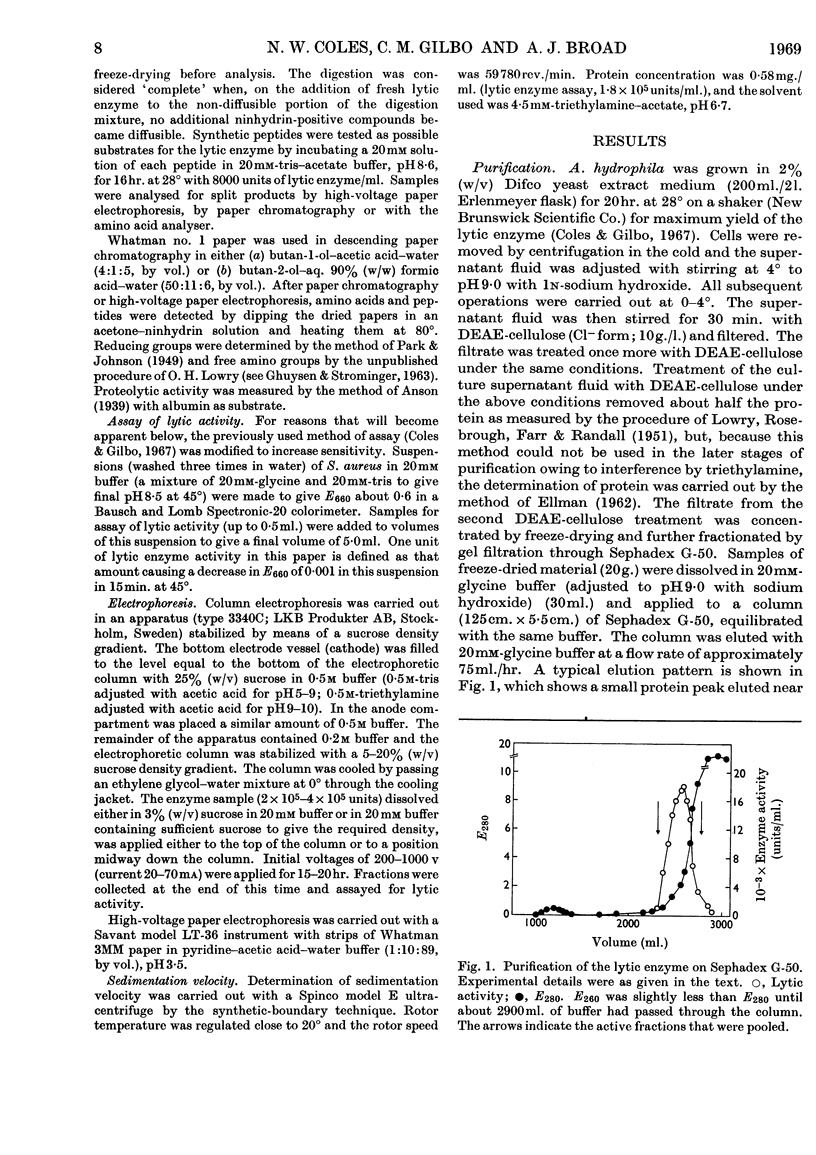
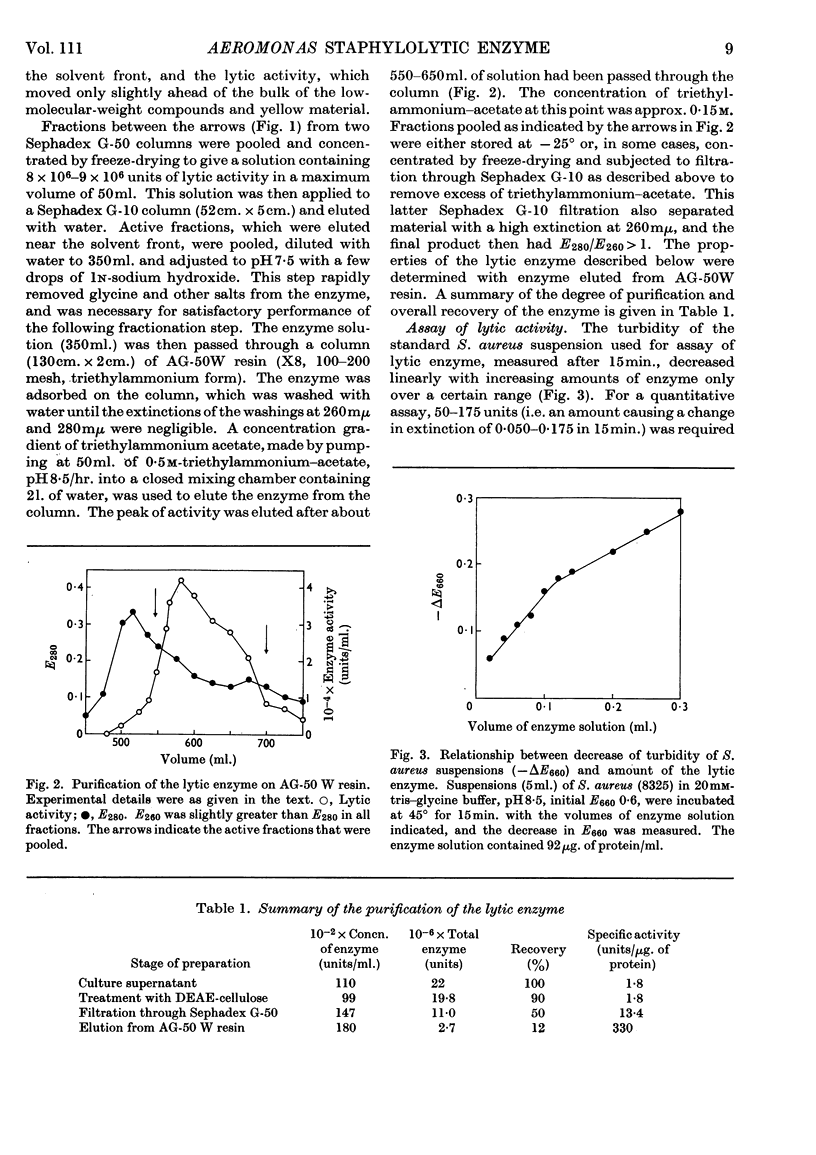
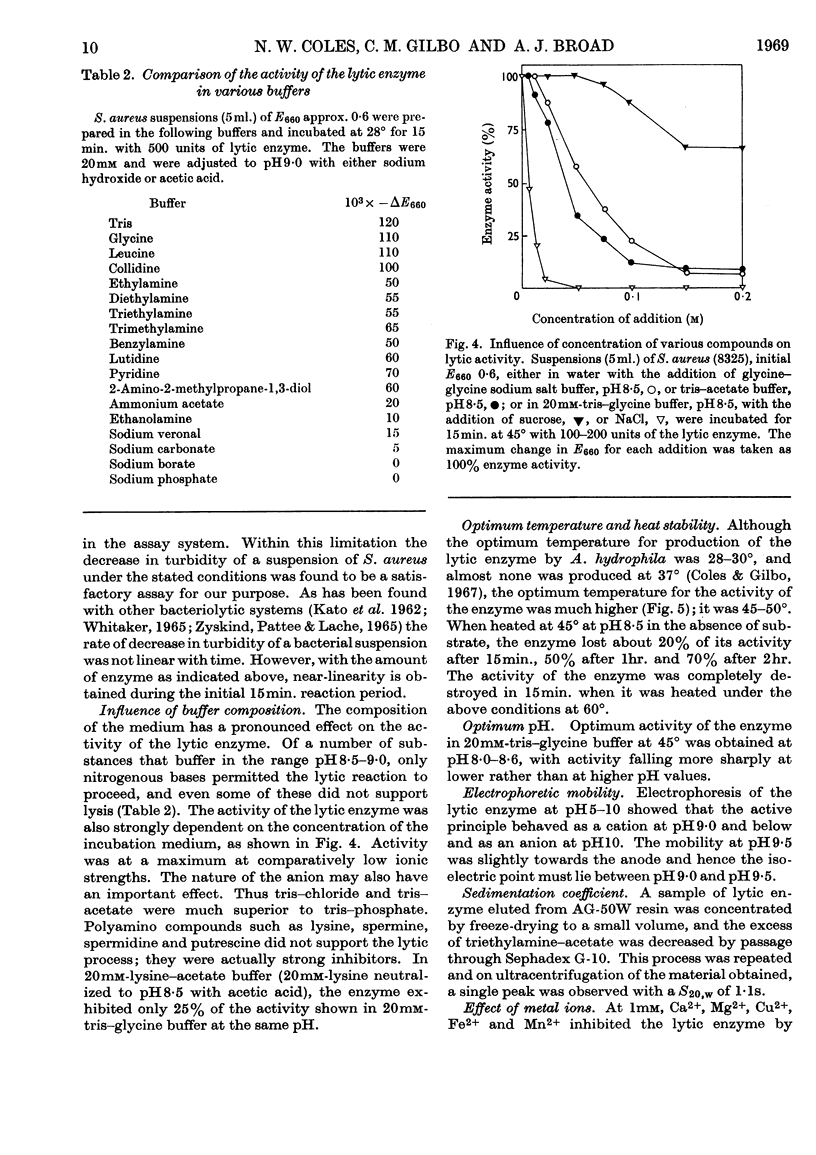
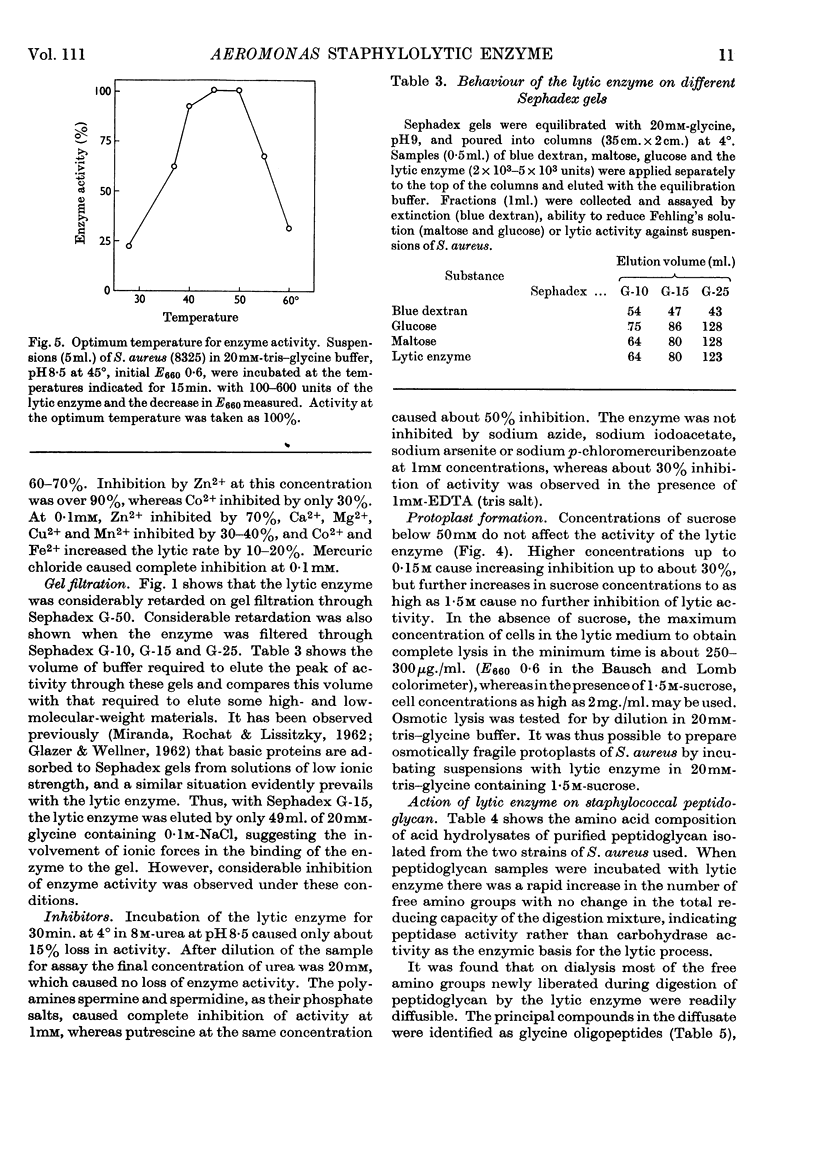
1. An enzyme produced by Aeromonas hydrophila and capable of lysing Staphylococcus aureus cells was purified 180-fold by gel filtration and chromatography on columns of AG-50 W resin. 2. Physical measurements on the purified enzyme suggest that it is a small basic protein with an isoelectric point between pH9·0 and pH9·5. 3. Maximum lytic activity was obtained in 20mm-tris–glycine buffer, pH8·5, at 45°, with no detectable activity in the absence of a nitrogenous base. 4. The enzyme is active in the above buffer containing 1·5m-sucrose, and is useful for the preparation of protoplasts of Staphylococcus aureus. 5. Purified cell wall peptidoglycans of two strains of Staphylococcus aureus, differing in amino acid composition, were hydrolysed by the enzyme with the liberation of glycine oligopeptides, principally diglycine and triglycine. 6. Synthetic glycine oligopeptides larger than triglycine, but not polyglycine, were hydrolysed, as were a number of leucine-containing dipeptides and tripeptides, but no proteolytic activity could be demonstrated. 7. It is concluded that the enzyme is lytic towards Staphylococcus aureus because it splits the pentaglycine cross-links of the cell-wall peptidoglycan.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWDER H. P., ZYGMUNT W. A., YOUNG J. R., TAVORMINA P. A. LYSOSTAPHIN: ENZYMATIC MODE OF ACTION. Biochem Biophys Res Commun. 1965 Apr 23;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- Coles N. W., Gilbo C. M. Lysis of Staphylococcus aureus by culture supernatant fluids of a species of Aeromonas. J Bacteriol. 1967 Mar;93(3):1193–1194. doi: 10.1128/jb.93.3.1193-1194.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles N. W., Gross R. The induced formation of penicillinase in Staphylococcus aureus. Aust J Exp Biol Med Sci. 1965 Dec;43(6):725–736. doi: 10.1038/icb.1965.57. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. The biuret reaction: changes in the ultraviolet absorption spectra and its application to the determination of peptide bonds. Anal Biochem. 1962 Jan;3:40–48. doi: 10.1016/0003-2697(62)90042-8. [DOI] [PubMed] [Google Scholar]
- ENSIGN J. C., WOLFE R. S. LYSIS OF BACTERIAL CELL WALLS BY AN ENZYME ISOLATED FROM A MYXOBACTER. J Bacteriol. 1965 Aug;90:395–402. doi: 10.1128/jb.90.2.395-402.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign J. C., Wolfe R. S. Characterization of a small proteolytic enzyme which lyses bacterial cell walls. J Bacteriol. 1966 Feb;91(2):524–534. doi: 10.1128/jb.91.2.524-534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHUYSEN J. M., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. I. PREPARATION OF FRAGMENTS BY ENZYMATIC HYDROLYSIS. Biochemistry. 1963 Sep-Oct;2:1110–1119. doi: 10.1021/bi00905a035. [DOI] [PubMed] [Google Scholar]
- GILLESPIE D. C., COOK F. D. EXTRACELLULAR ENZYMES FROM STRAINS OF SORANGIUM. Can J Microbiol. 1965 Feb;11:109–118. doi: 10.1139/m65-014. [DOI] [PubMed] [Google Scholar]
- GLAZER A. N., WELLNER D. Adsorption of proteins on 'Sephadex'. Nature. 1962 Jun 2;194:862–863. doi: 10.1038/194862a0. [DOI] [PubMed] [Google Scholar]
- Gilbo C. M., Beaton C. D., Coles N. W. Electron microscopy of the lysis of Staphylococcus aureus cell walls by Aeromonas lytic factor. J Bacteriol. 1967 Jun;93(6):1972–1975. doi: 10.1128/jb.93.6.1972-1975.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Wolfe R. S. Composition, properties, and substrate specificities of Myxobacter AL-1 protease. J Biol Chem. 1968 Mar 10;243(5):879–888. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Izaki K., Matsuhashi M., Tipper D. J. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Fed Proc. 1967 Jan-Feb;26(1):9–22. [PubMed] [Google Scholar]
- Tsai C. S., Whitaker D. R., Jurásek L., Gillespie D. C. Lytic enzymes of Sorangium sp. Action of the alpha- and beta-lytic proteases on two bacterial mucopeptides. Can J Biochem. 1965 Dec;43(12):1971–1983. doi: 10.1139/o65-220. [DOI] [PubMed] [Google Scholar]
- Whitaker D. R. Lytic enzymes of Sorangium sp. Isolation and enzymatic properties of the alpha- and beta-lytic proteases. Can J Biochem. 1965 Dec;43(12):1935–1954. doi: 10.1139/o65-217. [DOI] [PubMed] [Google Scholar]
- ZYSKIND J. W., PATTEE P. A., LACHE M. STAPHYLOLYTIC SUBSTANCE FROM A SPECIES OF PSEUDOMONAS. Science. 1965 Mar 19;147(3664):1458–1459. doi: 10.1126/science.147.3664.1458. [DOI] [PubMed] [Google Scholar]


