Abstract
Objective:
Women with gestational diabetes have higher future risk of type 2 diabetes; however, whether other adverse pregnancy outcomes are independently associated with type 2 diabetes is unclear. We examined long-term diabetes risk after preterm delivery or hypertensive disorders of pregnancy in a large population-based cohort.
Methods:
A national cohort study was conducted of all 2,184,417 women with a singleton delivery in Sweden during 1973–2015 and no pre-existing diabetes, followed up for type 2 diabetes identified from nationwide outpatient and inpatient diagnoses through 2018. Cox regression was used to compute hazard ratios (HRs) for type 2 diabetes associated with preterm delivery or hypertensive disorders of pregnancy, adjusting for gestational diabetes and other maternal factors. Co-sibling analyses assessed for confounding by shared familial (genetic or environmental) factors.
Results:
Both preterm delivery and hypertensive disorders of pregnancy were independently associated with higher type 2 diabetes risk. Within 10 years after delivery, adjusted HRs for type 2 diabetes associated with specific pregnancy outcomes were: any preterm (<37 weeks) delivery, 1.96 (95% CI, 1.83–2.09); extremely preterm (22–27 weeks) delivery, 2.53 (2.03–3.16); and hypertensive disorders of pregnancy, 1.52 (1.43–1.63). All HRs remained significantly elevated (1.1- to 1.7-fold) even 30–46 years after delivery. These findings were largely unexplained by shared familial factors.
Conclusions:
In this large national cohort, women with preterm delivery or hypertensive disorders of pregnancy had subsequent increased risk for type 2 diabetes up to 46 years later. Women with these pregnancy complications need early preventive actions and long-term monitoring for type 2 diabetes.
INTRODUCTION
Type 2 diabetes affects over 500 million people globally and is a leading cause of morbidity and mortality (1). Gestational diabetes and delivery of a high birth weight infant are well-established risk factors for future development of type 2 diabetes in women (2–4). However, less is known about whether other adverse pregnancy outcomes, such as preterm delivery and hypertensive disorders of pregnancy, are independently associated with type 2 diabetes. Approximately 10% of all deliveries worldwide occur preterm (gestational age <37 weeks) (5), and 5% to 10% of all pregnant women develop hypertensive disorders of pregnancy (6). A better understanding of the long-term impacts on type 2 diabetes may help further identify high-risk women early in life, thus enabling earlier preventive actions to reduce the risk of diabetes and its complications across the life course.
Prior studies have shown that women with preterm delivery or hypertensive disorders of pregnancy have higher future risks of chronic hypertension and cardiovascular disorders (7–10). Both preterm delivery and hypertensive disorders also have been linked with endothelial dysfunction and chronic vascular inflammation (11), which have been shown to precede the development of hyperglycemia (12). However, evidence linking these pregnancy outcomes with type 2 diabetes has been inconsistent. Some (13–19) but not all (20–24) studies have suggested that preterm delivery or hypertensive disorders of pregnancy are associated with higher future risks of type 2 diabetes. These discrepancies may be related to study design differences, including ascertainment of pregnancy complications and diabetes by self report in many studies, and differences in follow-up time or control for confounding. Large population-based cohort studies with prospective, clinically diagnosed pregnancy outcomes and type 2 diabetes are needed. Moreover, it is unclear whether previously reported associations might be explained by the unmasking of preexistent risk as opposed to eliciting of new risk for type 2 diabetes. Family-based designs that control for unmeasured shared familial (genetic or environmental) factors may help further elucidate potential causality.
To address these knowledge gaps, we conducted a national cohort study of >2 million women in Sweden. Our goals were to: (1) examine long-term risks for type 2 diabetes associated with preterm delivery and hypertensive disorders of pregnancy in a large population-based cohort, adjusting for gestational diabetes and other maternal factors; (2) assess changes in such risks across the life course with up to 46 years of follow-up; and (3) assess for potential confounding by unmeasured shared genetic or environmental factors in families using co-sibling analyses. We hypothesized that women who experienced either preterm delivery or hypertensive disorders of pregnancy would have long-term increased risks of type 2 diabetes, and that such risks would only partially be explained by shared familial factors.
METHODS
Study Population
The Swedish Medical Birth Register contains prenatal and birth information for nearly all deliveries in Sweden since 1973. Using this register, we identified 2,194,939 women who had a singleton delivery during 1973–2015. Singleton deliveries were chosen to improve internal comparability, given the higher prevalence of adverse pregnancy outcomes and their different underlying causes in multiple gestation pregnancies. We excluded 10,522 (0.5%) women who had a diagnosis of diabetes before pregnancy in the Swedish Hospital or Outpatient Registers or primary care records (identified using International Classification of Diseases [ICD] codes 250 in ICD-8/9 and E10-E14 in ICD-10), leaving 2,184,417 women for inclusion in the study. The Swedish Hospital Register contains all primary and secondary hospital discharge diagnoses from six populous counties in southern Sweden starting in 1964 and with nationwide coverage starting in 1987 (25). The Swedish Outpatient Register contains all diagnoses from specialty clinics with approximately 87% nationwide coverage starting in 2001. In addition, primary care diagnoses were available for 20% of the Swedish population starting in 1998, 45% starting in 2001, and 90% starting in 2008.
This study was approved by the Regional Ethical Review Board in Lund, Sweden (No. 2008/471 and later amendments). Participant consent was not required because this study used only pseudonymized registry-based secondary data.
Ascertainment of Preterm Delivery and Hypertensive Disorders of Pregnancy
Preterm delivery and hypertensive disorders of pregnancy were identified from prenatal and birth records in the Swedish Medical Birth Register (8). Gestational age at delivery was based on maternal report of last menstrual period in the 1970s and ultrasound estimation starting in the 1980s and onward (>70% of the cohort), and was classified as any preterm (<37 weeks), extremely preterm (22–27 weeks), moderately preterm (28–33 weeks), late preterm (34–36 weeks), early term (37–38 weeks), full-term (39–41 weeks, used as the reference group), or post-term (≥42 weeks). Hypertensive disorders of pregnancy included gestational hypertension (new-onset hypertension at ≥20 weeks of gestation) or preeclampsia as identified from diagnostic codes in the Medical Birth Register (Supplemental Table 1 in the Appendix). Gestational age in the Medical Birth Register has been found to be highly reliable (26). Hypertension and other chronic disorders also have high validity in Swedish registries (25), although to our knowledge have not been formally validated in the Medical Birth Register.
Type 2 Diabetes Ascertainment
The study cohort was followed up for the earliest diagnosis of type 2 diabetes from first delivery through December 31, 2018 (maximum follow-up time, 46 years; median, 25). To exclude gestational diabetes from type 2 diabetes, the outcome was defined as any diagnosis of type 2 diabetes occurring >2 weeks after delivery. Consistent with prior epidemiologic criteria (27, 28), type 2 diabetes was identified based on ICD-8/9 code 250 or ICD-10 codes E11-E14 in the Swedish Hospital and Outpatient Registers or primary care records without meeting other specific criteria for type 1 diabetes (i.e., excluding ICD-9 codes 250.X1 and 250.X3 or insulin prescription before age 30 years). Insulin prescriptions were identified based on code A10 in the Swedish Pharmacy Register, which includes all medication prescriptions nationwide since July 1, 2005. Type 2 diabetes diagnoses in the Swedish Hospital Register have been reported to have a positive predictive value >99% (29).
Covariates
Other maternal characteristics that may be associated with preterm delivery or hypertensive disorders of pregnancy and type 2 diabetes were identified using the Swedish Medical Birth Register and national census data, which were linked using a pseudonymous serial number. Maternal age was adjusted for in all analyses as the Cox model time scale (as described below). Covariates included the following maternal factors, with all time-varying factors updated for each pregnancy: calendar year of delivery (modeled simultaneously as continuous and categorical variables in 5-year groups to allow for potential non-linearity), parity (1, 2, 3, 4, ≥5), education level (≤9, 10–12, >12 years), employment status (yes/no) and income (quartiles) in the year prior to delivery, country of origin (Sweden, other European, non-European), and other diabetes risk factors including body mass index (BMI; continuous and categorical [<18.5, 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2]), smoking (0, 1–9, ≥10 cigarettes/day), pre-existing hypertension or hyperlipidemia (Supplemental Table 1 in the Appendix), gestational diabetes (yes/no), and infant size for gestational age (within-cohort birth weight percentile standardized for gestational age).
Maternal BMI and smoking were assessed at the beginning of prenatal care starting in 1982 and were available for 56% and 67% of women, respectively. Data were >99% complete for all other variables. Missing data were multiply imputed with 20 imputations using all other covariates and type 2 diabetes as predictors. As alternatives to multiple imputation, sensitivity analyses were performed that (1) restricted to women with complete data (N=1,196,041), or (2) coded missing data for each variable as a separate category.
Statistical Analysis
Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for subsequent risk of type 2 diabetes associated with preterm delivery or hypertensive disorders of pregnancy. These associations were examined across the maximum possible follow-up (up to 46 years after delivery) and within narrower intervals of follow-up (<10, 10–19, 20–29, 30–46 years) among women still living in Sweden without a prior diagnosis of type 2 diabetes at the beginning of the respective interval. Preterm delivery and hypertensive disorders of pregnancy were modeled as time-dependent variables based on “ever” experiencing the respective outcome. For example, if a woman’s first delivery had neither of these outcomes and her second was preterm with gestational hypertension, she entered the exposure categories for preterm delivery and hypertensive disorders of pregnancy at the date of her second delivery. If a woman’s first and second deliveries were both preterm, she entered the preterm exposure category at the date of her first delivery (7, 8).
Maternal age was used as the Cox model time axis with age at each delivery as “time zero” (i.e., each singleton delivery was included as a separate observation, with exposures updated for each delivery). Women were censored at death as identified in the Swedish Death Register (n=85,366; 4%) or at emigration as determined by absence of a Swedish residential address in census data (n=92,673; 4%). All models were adjusted for covariates (as described above) and included both preterm delivery and hypertensive disorders of pregnancy so that they were mutually adjusted for each other. The proportional hazards assumption was assessed by examining log-log survival plots and was met in each model. Incidence rate differences with 95% CIs, attributable fraction in the exposed (AFe), and population attributable fraction (PAF) were also computed.
Co-sibling analyses were performed to assess for potential confounding by unmeasured familial (genetic or early-life environmental) factors that may be shared determinants of preterm delivery or hypertensive disorders of pregnancy and type 2 diabetes. Shared environmental factors within families may include lifestyle factors such as diet and physical activity, or ambient exposures such as passive smoking. These analyses included all 1,191,237 (54%) women with at least one full sister who had a singleton delivery (regardless of pregnancy outcome status). Stratified Cox models were performed using a separate stratum for each unique set of sisters identified by their mother’s and father’s pseudonymous serial numbers. Each stratum has its own baseline hazard function reflecting shared genetic and environmental factors, thus controlling for shared exposures within each family (7, 8). In addition, these analyses were further adjusted for the same covariates as in the main analyses.
A secondary analysis also examined risk of type 2 diabetes associated with spontaneous or medically indicated preterm delivery, which was systematically recorded starting in 1990 (N=2,658,024 births among 1,452,305 women; maximum 29 years of follow-up). In addition, sensitivity analyses were performed that repeated the main analyses after (1) excluding women with gestational diabetes, instead of adjusting for gestational diabetes as a covariate; and (2) restricting to deliveries that occurred in 1990 or later to explore the potential influence of temporal changes in diagnostic practices or gestational age measurement. All statistical tests were two-sided and used a significance level of 0.05. All analyses were conducted using Stata version 16.1.
RESULTS
Table 1 summarizes characteristics of all women and those with preterm delivery or hypertensive disorders of pregnancy. Women with preterm delivery were more likely to be younger at first delivery and/or have low education level or income. Women with hypertensive disorders were more likely to be older and have low education level and/or high BMI.
Table 1.
Baseline maternal characteristics by ever occurrence of preterm delivery or hypertensive disorders of pregnancy, Sweden, 1973–2015.
| Characteristic | All women | Preterm deliverya | Hypertensive disorders of pregnancya |
|---|---|---|---|
| N=2,184,417 | N=199,888 | N=138,927 | |
| n (%) | n (%) | n (%) | |
| Age at first delivery (years) | |||
| <20 | 119,710 (5.5) | 17,020 (8.5) | 7,241 (5.2) |
| 20–24 | 601,766 (27.5) | 60,146 (30.1) | 37,105 (26.7) |
| 25–29 | 786,853 (36.0) | 66,337 (33.2) | 47,240 (34.0) |
| 30–34 | 479,158 (21.9) | 39,226 (19.6) | 30,752 (22.1) |
| 35–39 | 163,858 (7.5) | 14,085 (7.1) | 13,099 (9.4) |
| ≥40 | 33,072 (1.5) | 3,074 (1.5) | 3,490 (2.5) |
| Year of first delivery | |||
| 1973–1979 | 528,377 (24.2) | 43,883 (22.0) | 46,226 (33.3) |
| 1980–1989 | 442,789 (20.3) | 48,069 (24.0) | 28,329 (20.4) |
| 1990–1999 | 443,023 (20.3) | 44,036 (22.0) | 22,802 (16.4) |
| 2000–2009 | 457,198 (20.9) | 41,729 (20.9) | 23,479 (16.9) |
| 2010–2015 | 313,030 (14.3) | 22,171 (11.1) | 18,091 (13.0) |
| Education (years) | |||
| ≤9 | 304,146 (13.9) | 31,017 (15.5) | 21,428 (15.4) |
| 10–12 | 966,928 (44.3) | 93,528 (46.8) | 64,892 (46.7) |
| >12 | 913,343 (41.8) | 75,343 (37.7) | 52,607 (37.9) |
| Employed | 1,885,274 (86.3) | 170,335 (85.2) | 125,272 (90.2) |
| Income (quartile) | |||
| 1st (highest) | 545,763 (25.0) | 44,716 (22.4) | 32,128 (23.1) |
| 2nd | 546,304 (25.0) | 47,629 (23.8) | 34,959 (25.2) |
| 3rd | 547,162 (25.0) | 50,435 (25.2) | 38,039 (27.4) |
| 4th (lowest) | 545,188 (25.0) | 57,118 (28.6) | 33,801 (24.3) |
| Country of origin | |||
| Sweden | 1,792,242 (82.0) | 164,900 (82.5) | 119,741 (86.2) |
| Other European | 205,457 (9.4) | 17,542 (8.8) | 12,211 (8.8) |
| Non-European | 186,718 (8.5) | 17,446 (8.7) | 6,975 (5.0) |
| Body mass index (kg/m 2 ) | |||
| <18.5 | 52,751 (2.4) | 5,928 (3.0) | 1,670 (1.2) |
| 18.5–24.9 | 839,921 (38.5) | 71,149 (35.6) | 36,714 (26.4) |
| 25.0–29.9 | 241,515 (11.1) | 21,112 (10.6) | 17,790 (12.8) |
| ≥30.0 | 90,965 (4.2) | 9,205 (4.6) | 11,064 (8.0) |
| Unknown | 959,265 (43.9) | 92,494 (46.2) | 71,689 (51.6) |
| Smoking (cigarettes/day) | |||
| 0 | 1,244,022 (56.9) | 108,621 (54.3) | 70,227 (50.6) |
| 1–9 | 152,835 (7.0) | 16,539 (8.3) | 6,990 (5.0) |
| ≥10 | 68,358 (3.1) | 8,672 (4.3) | 2,961 (2.1) |
| Unknown | 719,202 (32.9) | 66,056 (33.0) | 58,749 (42.3) |
| Prior diagnoses | |||
| Hypertension | 1,739 (0.1) | 365 (0.2) | 927 (0.7) |
| Hyperlipidemia | 1,332 (0.1) | 155 (0.1) | 144 (0.1) |
| Gestational diabetes | 9,412 (0.4) | 1,394 (0.7) | 1,201 (0.9) |
| Infant birth weight for gestational age (percentile) | |||
| <10th | 218,442 (10.0) | 25,279 (12.6) | 22,709 (16.3) |
| 10th to 90th | 1,747,534 (90.0) | 162,714 (81.4) | 104,520 (75.2) |
| ≥90th | 218,441 (10.0) | 11,895 (6.0) | 11,698 (8.4) |
All characteristics differed significantly compared to women without the respective adverse pregnancy outcome (P<0.05) using the Kruskal-Wallis test for equality of populations.
In 55 million person-years of follow-up, 122,332 (5.6%) women were diagnosed with type 2 diabetes. The median age at first delivery was 27 (interquartile range [IQR], 23 to 30), at type 2 diabetes diagnosis was 54 (IQR, 45 to 61), and at end of follow-up was 53 (IQR, 43 to 64) years. The median follow-up time in women who did not die was 27 (IQR, 14 to 39) years after first delivery. Incidence rates for type 2 diabetes by pregnancy outcome and follow-up time are shown in Supplemental Table 2 in the Appendix.
Preterm Delivery, Hypertensive Disorders of Pregnancy, and Risk of Type 2 Diabetes
Across the entire follow-up period (up to 46 years after delivery), both preterm delivery and hypertensive disorders of pregnancy were independently associated with increased risk of type 2 diabetes. After mutually adjusting for each other and all covariates, the HRs for type 2 diabetes associated with preterm delivery or hypertensive disorders were 1.35 (95% CI, 1.33–1.38) and 1.72 (1.70–1.75), respectively (Supplemental Tables 2–3 in the Appendix). Risk of type 2 diabetes was higher by earlier gestational age at delivery, with adjusted HRs ranging from 1.55 (95% CI, 1.44–1.67) for women who delivered extremely preterm (22–27 weeks) to 1.18 (1.16–1.19) for those who delivered at early term (37–38 weeks), relative to full-term (39–41 weeks).
Adjusted HRs for type 2 diabetes associated with preterm delivery were highest in the first 10 years after delivery and then subsequently declined, but remained significantly elevated even 30–46 years after delivery (1.17; 95% CI, 1.14–1.21) (Figure 1 and Supplemental Table 2). In contrast, adjusted HRs associated with hypertensive disorders of pregnancy were relatively stable across time and remained similarly elevated even 30–46 years after delivery (1.65; 95% CI, 1.61–1.69) (Figure 1 and Supplemental Table 3).
Figure 1.

Adjusted HRs and 95% CIs for type 2 diabetes associated with preterm delivery (PTD) or hypertensive disorders of pregnancy, by time since delivery.
Incidence rate differences (i.e., excess type 2 diabetes cases) associated with preterm delivery peaked at 20–29 years after delivery, whereas those associated with hypertensive disorders continued to increase with longer follow-up to older ages. Across the entire follow-up period, an estimated 35.5% of all type 2 diabetes cases in women who delivered preterm and 6.1% of all type 2 diabetes cases in this entire population of women could be attributed directly to preterm delivery (Table 2). The corresponding estimates for hypertensive disorders of pregnancy were 54.1% and 7.5%, respectively (Table 2). Figure 2 shows the cumulative incidence of type 2 diabetes associated with preterm delivery or hypertensive disorders of pregnancy by time since delivery.
Table 2.
Attributable fractions for type 2 diabetes associated with preterm delivery or hypertensive disorders of pregnancy.
| Attributable fraction among the exposed (AFe) | Population attributable fraction (PAF) | |
|---|---|---|
| Up to 46 years after delivery | ||
| Preterm delivery | 35.5% | 6.1% |
| Hypertensive disorders of pregnancy | 54.1% | 7.5% |
| <10 years after delivery | ||
| Preterm delivery | 66.3% | 17.1% |
| Hypertensive disorders of pregnancy | 59.1% | 8.8% |
| 10–19 years after delivery | ||
| Preterm delivery | 51.9% | 12.3% |
| Hypertensive disorders of pregnancy | 58.0% | 8.3% |
| 20–29 years after delivery | ||
| Preterm delivery | 34.9% | 6.5% |
| Hypertensive disorders of pregnancy | 55.2% | 8.0% |
| 30–46 years after delivery | ||
| Preterm delivery | 14.9% | 1.5% |
| Hypertensive disorders of pregnancy | 44.3% | 5.9% |
Figure 2.
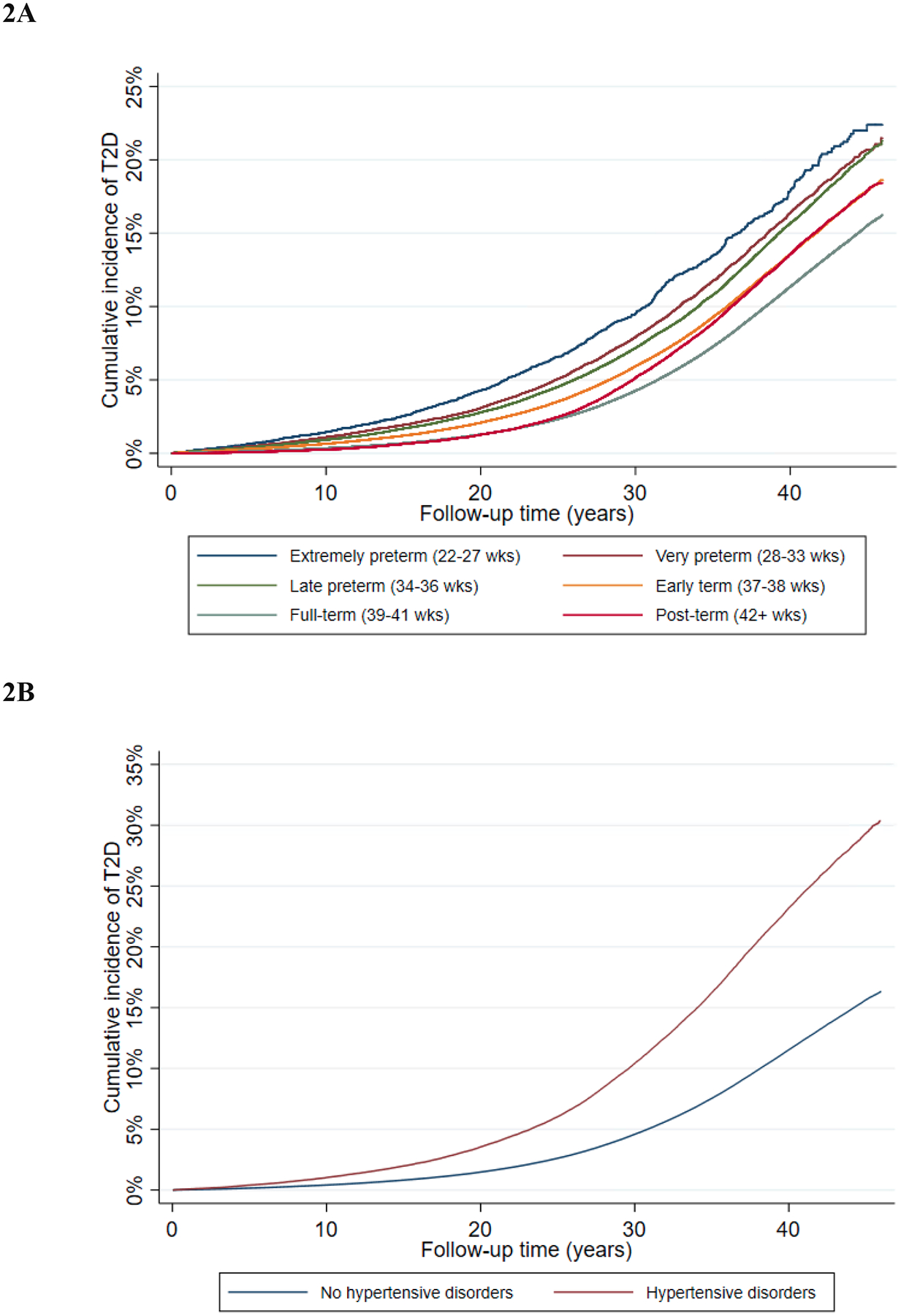
Cumulative incidence of type 2 diabetes associated with preterm delivery (2A) or hypertensive disorders of pregnancy (2B), by time since delivery.
Women who experienced both preterm delivery and hypertensive disorders of pregnancy (n=25,198) had even higher risks of type 2 diabetes. Compared to women with neither of these outcomes, the adjusted HR for type 2 diabetes across the entire follow-up period (up to 46 years after delivery) in women with both outcomes was 2.17 (95% CI, 2.09–2.25). In narrower follow-up intervals, the corresponding HR for type 2 diabetes ranged from 2.66 (95% CI, 2.38–2.97) at <10 years to 1.90 (1.78–2.03) at 30–46 years after delivery.
Co-Sibling Analyses
Co-sibling analyses to control for unmeasured shared familial (genetic and early-life environmental) factors resulted in partial attenuation of certain risk estimates and increases in others (Table 3). Across the entire follow-up (up to 46 years after delivery), the adjusted HR for type 2 diabetes associated with preterm delivery was 1.35 (95% CI, 1.33–1.38) in the primary analysis vs. 1.31 (1.24–1.38) in the co-sibling analysis. The corresponding HRs associated with hypertensive disorders of pregnancy were 1.72 (95% CI, 1.70–1.75) vs. 1.65 (1.56–1.73). These findings suggest that the main results were mostly unexplained by shared familial factors.
Table 3.
Co-sibling analyses of preterm delivery or hypertensive disorders of pregnancy and subsequent risk of type 2 diabetes.
| Type 2 diabetes cases | Adjusted HR (95% CI)a | |
|---|---|---|
| Up to 46 years after delivery | ||
| Preterm delivery (<37 wks) | 9,066 | 1.31 (1.24, 1.38) |
| Early term (37–38 wks) | 19,437 | 1.14 (1.10, 1.19) |
| Full-term (39–41 wks) | 37,752 | Reference |
| Hypertensive disorders | 9,307 | 1.65 (1.56, 1.73) |
| No hypertensive disorders | 59,147 | Reference |
| <10 years after delivery | ||
| Preterm delivery (<37 wks) | 2,445 | 2.25 (1.59, 3.18) |
| Early term (37–38 wks) | 4,318 | 1.24 (0.97, 1.59) |
| Full-term (39–41 wks) | 4,933 | Reference |
| Hypertensive disorders | 1,889 | 1.47 (1.01, 2.13) |
| No hypertensive disorders | 9,993 | Reference |
| 10–19 years after delivery | ||
| Preterm delivery (<37 wks) | 2,379 | 1.64 (1.34, 2.01) |
| Early term (37–38 wks) | 5,011 | 1.24 (1.07, 1.43) |
| Full-term (39–41 wks) | 7,417 | Reference |
| Hypertensive disorders | 2,014 | 2.10 (1.69, 2.61) |
| No hypertensive disorders | 13,218 | Reference |
| 20–29 years after delivery | ||
| Preterm delivery (<37 wks) | 2,650 | 1.46 (1.30, 1.64) |
| Early term (37–38 wks) | 6,142 | 1.21 (1.12, 1.32) |
| Full-term (39–41 wks) | 12,306 | Reference |
| Hypertensive disorders | 2,880 | 1.50 (1.34, 1.68) |
| No hypertensive disorders | 19,283 | Reference |
| 30–46 years after delivery | ||
| Preterm delivery (<37 wks) | 1,592 | 1.10 (1.00, 1.21) |
| Early term (37–38 wks) | 3,966 | 1.03 (0.96, 1.10) |
| Full-term (39–41 wks) | 13,096 | Reference |
| Hypertensive disorders | 2,524 | 1.65 (1.51, 1.81) |
| No hypertensive disorders | 17,812 | Reference |
Adjusted for shared familial (genetic and/or environmental) factors, and additionally for maternal age, year of delivery, parity, education, employment, income, country of origin, BMI, smoking, pre-existing hypertension or hyperlipidemia, gestational diabetes, and other adverse pregnancy outcomes.
Secondary Analyses
Both spontaneous and medically indicated preterm delivery were associated with similarly increased relative rates of type 2 diabetes compared with full-term delivery (adjusted HR, spontaneous preterm: 1.69; 95% CI, 1.61–1.78; medically indicated preterm: 1.72; 1.63–1.81; P=0.71 for difference in HRs) (Supplemental Table 4 in the Appendix).
Sensitivity analyses that alternately excluded women with gestational diabetes, restricted to deliveries in 1990 or later, or assessed alternative approaches for missing data yielded similar results as the main analyses and the conclusions were unchanged (Supplemental Results in the Appendix).
DISCUSSION
In this large national cohort, women with preterm delivery or hypertensive disorders of pregnancy had substantially increased long-term risks for type 2 diabetes, after adjusting for gestational diabetes and other maternal factors. In the 10 years following delivery, the relative rate of type 2 diabetes was elevated nearly 2-fold in women with preterm delivery (2.5-fold for extremely preterm) and 1.5-fold in those with hypertensive disorders of pregnancy. These relative rates remained significantly elevated (1.1- to 1.7-fold) even 30–46 years after delivery. Co-sibling analyses suggested that these findings were largely unexplained by genetic or early-life environmental factors that may be shared determinants of these pregnancy outcomes and type 2 diabetes within families.
To our knowledge, this is the largest study to date to examine these major pregnancy outcomes in relation to future risk of type 2 diabetes, and the first to assess for potential unmeasured familial confounding. Prior studies of preterm delivery include a US study of 57,904 women that reported a nearly 1.2-fold subsequent risk of type 2 diabetes in those who delivered preterm, with higher risks in the first 10 years after delivery (13). Another US study of 31,101 black women reported that those who delivered preterm, with or without gestational diabetes, had a 1.2-fold higher risk of type 2 diabetes (16). A Danish study of 782,287 women found that those who delivered at 32–36 weeks of gestation had a 1.9-fold higher subsequent risk of type 2 diabetes, compared with ≥37 weeks (14). Another Danish study of 100,669 women reported that those who delivered at 32–37 weeks had a 2.2-fold subsequent risk of type 2 diabetes (15). In contrast, a US study of 49,717 women (20) and a UK study of 15,969 women (21) found no association between preterm delivery and type 2 diabetes. These prior studies had substantial heterogeneity, possibly related to differences in ascertainment of pregnancy outcomes and diabetes, follow-up times, and control for confounding.
Prior studies of hypertensive disorders in pregnancy also have varied widely, but most have reported positive associations with type 2 diabetes. A US study of 49,717 women reported a 1.2-fold odds of type 2 diabetes among women who experienced any hypertensive disorder in pregnancy (20). A study of 6,587 women in northern Sweden found that those with hypertensive disorders of pregnancy had a nearly 2-fold odds of type 2 diabetes between ages 50 and 60 years (17). A meta-analysis of 17 studies (mean number of diabetes cases <3000) reported that women with hypertensive disorders in pregnancy had a 1.5-fold subsequent risk of type 2 diabetes (pooled relative risk, 1.56; 95% CI, 1.21–2.01) (30).
The present study extends prior evidence by examining two adverse pregnancy outcomes in a large national cohort with >120,000 type 2 diabetes cases, thus enabling well-powered assessment of their independent associations. The findings suggest that both preterm delivery and hypertensive disorders of pregnancy are independently associated with elevated type 2 diabetes risks up to 46 years later. Both spontaneous and medically indicated preterm delivery were associated with similarly increased (1.7-fold) risks. The relative rate of type 2 diabetes associated with preterm delivery was highest within the first 10 years after delivery and then declined, whereas those associated with hypertensive disorders of pregnancy were relatively stable across time and remained similarly elevated even 30–46 years after delivery. However, the excess cases of type 2 diabetes associated with either of these pregnancy outcomes, and thus the public health impact, increased with additional follow-up to older ages. These associations also persisted after controlling for shared familial (genetic and environmental) factors in co-sibling analyses, consistent with potential causal relationships.
These findings have important clinical implications. Preterm delivery and hypertensive disorders of pregnancy should be considered long-term independent risk factors for type 2 diabetes, in addition to previously established risk factors such as gestational diabetes (2) and delivery of a high birth weight infant (3, 4). These pregnancy outcomes should be routinely tracked in electronic health records to facilitate transition of women from obstetric to primary care clinics, where they need early preventive evaluation and long-term monitoring for type 2 diabetes. In women with these pregnancy complications, lifestyle interventions to promote metabolic health soon after delivery may play a vital role in long-term diabetes prevention. Such actions should include counseling to reduce other known risk factors, including obesity, physical inactivity, unhealthy diet, and smoking (1), followed by long-term clinical monitoring for timely detection and treatment of type 2 diabetes.
Strengths and Limitations
A key strength of the present study is its large national cohort design with up to 46 years of follow-up. The availability of highly complete nationwide birth and medical registry data, both inpatient and outpatient, helped minimize potential selection and ascertainment biases. The large sample size enabled high statistical power for assessment of both preterm delivery and hypertensive disorders while adjusting for multiple potential confounders, as well as controlling for unmeasured shared familial factors using co-sibling analyses.
This study also had certain limitations. Detailed clinical records were unavailable to validate type 2 diabetes diagnoses, although high positive predictive values have been reported in the Swedish registries (25, 29). Type 2 diabetes diagnoses were available from primary care records starting in 1998 and specialty outpatient clinics in 2001, resulting in underreporting in earlier years; consequently, the absolute risks associated with preterm delivery or hypertensive disorders of pregnancy may potentially be higher than those reported. Changes in diagnostic criteria for type 2 diabetes and gestational diabetes occurred during the decades spanned by this study; however, calendar year of delivery and elapsed time were adjusted for in all analyses, and a sensitivity analysis that excluded the earliest decades further corroborated the main findings. Despite controlling for multiple maternal factors and shared familial exposures in co-sibling analyses, residual confounding by maternal BMI, smoking, gestational diabetes, or other factors during pregnancy is still possible. Country of origin was adjusted for in all analyses, but information on race/ethnicity was unavailable. This study will need replication in other countries when feasible, including diverse populations to explore for potential racial/ethnic differences.
Conclusions
In this large national cohort, women with preterm delivery or hypertensive disorders of pregnancy had long-term increased risks for type 2 diabetes, after adjusting for gestational diabetes and other maternal factors. These risks remained significantly elevated up to 46 years after delivery. Preterm delivery and hypertensive disorders of pregnancy should now be recognized as long-term independent risk factors for type 2 diabetes. Women with these pregnancy outcomes need early preventive evaluation and long-term monitoring for type 2 diabetes.
Supplementary Material
Funding:
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL139536); the Swedish Research Council; and the Swedish Heart-Lung Foundation. The funding agencies had no role in the design and conduct of the study; collection, analysis, and interpretation of data; or the writing of the manuscript or the decision to submit it for publication.
Footnotes
Conflicts of interest: The authors report no conflict of interest.
Contributor Information
Casey Crump, Departments of Family and Community Medicine and of Epidemiology, The University of Texas Health Science Center, Houston, Texas, USA
Jan Sundquist, Department of Clinical Sciences, Lund University, Malmö, Sweden
Kristina Sundquist, Department of Clinical Sciences, Lund University, Malmö, Sweden
REFERENCES
- 1.Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet 2022. Nov 19;400(10365):1803–20. [DOI] [PubMed] [Google Scholar]
- 2.Dennison RA, Chen ES, Green ME, Legard C, Kotecha D, Farmer G, et al. The absolute and relative risk of type 2 diabetes after gestational diabetes: A systematic review and meta-analysis of 129 studies. Diabetes Res Clin Pract 2021. Jan;171:108625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller C, Lim E. The risk of diabetes after giving birth to a macrosomic infant: data from the NHANES cohort. Matern Health Neonatol Perinatol 2021. May 12;7(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabeya Y, Goto A, Kato M, Takahashi Y, Matsushita Y, Inoue M, et al. History of having a macrosomic infant and the risk of diabetes: the Japan public health center-based prospective diabetes study. PLoS One 2013;8(12):e84542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohuma EO, Moller AB, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet 2023. Oct 7;402(10409):1261–71. [DOI] [PubMed] [Google Scholar]
- 6.Fu R, Li Y, Li X, Jiang W. Hypertensive Disorders in Pregnancy: Global Burden From 1990 to 2019, Current Research Hotspots and Emerging Trends. Curr Probl Cardiol 2023. Jul 20;48(12):101982. [DOI] [PubMed] [Google Scholar]
- 7.Crump C, Sundquist J, Sundquist K. Preterm Delivery and Long-term Risk of Hypertension in Women. JAMA Cardiol 2022. Jan 1;7(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crump C, Sundquist J, McLaughlin MA, Dolan SM, Govindarajulu U, Sieh W, Sundquist K. Adverse pregnancy outcomes and long term risk of ischemic heart disease in mothers: national cohort and co-sibling study. BMJ 2023. Feb 1;380:e072112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K, Adderley NJ. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ 2020. Oct 7;371:m3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garovic VD, White WM, Vaughan L, Saiki M, Parashuram S, Garcia-Valencia O, et al. Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J Am Coll Cardiol 2020. May 12;75(18):2323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane-Cordova AD, Gunderson EP, Carnethon MR, Catov JM, Reiner AP, Lewis CE, et al. Pre-pregnancy endothelial dysfunction and birth outcomes: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Hypertens Res 2018. Apr;41(4):282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caballero AE. Endothelial dysfunction, inflammation, and insulin resistance: a focus on subjects at risk for type 2 diabetes. Curr Diab Rep 2004. Aug;4(4):237–46. [DOI] [PubMed] [Google Scholar]
- 13.Tanz LJ, Stuart JJ, Williams PL, Missmer SA, Rimm EB, James-Todd TM, Rich-Edwards JW. Preterm Delivery and Maternal Cardiovascular Disease Risk Factors: The Nurses’ Health Study II. J Womens Health (Larchmt) 2019. May;28(5):677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lykke JA, Paidas MJ, Damm P, Triche EW, Kuczynski E, Langhoff-Roos J. Preterm delivery and risk of subsequent cardiovascular morbidity and type-II diabetes in the mother. BJOG 2010. Feb;117(3):274–81. [DOI] [PubMed] [Google Scholar]
- 15.Naver KV, Secher NJ, Ovesen PG, Gorst-Rasmussen A, Lundbye-Christensen S, Nilas L. Offspring preterm birth and birth size are related to long-term risk of maternal diabetes. Eur J Epidemiol 2013. May;28(5):427–32. [DOI] [PubMed] [Google Scholar]
- 16.James-Todd T, Wise L, Boggs D, Rich-Edwards J, Rosenberg L, Palmer J. Preterm birth and subsequent risk of type 2 diabetes in black women. Epidemiology 2014. Nov;25(6):805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timpka S, Markovitz A, Schyman T, Mogren I, Fraser A, Franks PW, Rich-Edwards JW. Midlife development of type 2 diabetes and hypertension in women by history of hypertensive disorders of pregnancy. Cardiovasc Diabetol 2018. Sep 10;17(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang IK, Tsai IJ, Chen PC, Liang CC, Chou CY, Chang CT, et al. Hypertensive disorders in pregnancy and subsequent diabetes mellitus: a retrospective cohort study. Am J Med 2012. Mar;125(3):251–7. [DOI] [PubMed] [Google Scholar]
- 19.Feig DS, Shah BR, Lipscombe LL, Wu CF, Ray JG, Lowe J, et al. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PLoS Med 2013;10(4):e1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu K, Wactawski-Wende J, Mendola P, Parikh NI, LaMonte MJ, Barnabei VM, et al. Adverse pregnancy outcomes and risk of type 2 diabetes in postmenopausal women. Am J Obstet Gynecol 2024. Jul 23;230(1):93.e1–.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song A, Okoth K, Adderley NJ. Association between preterm delivery and subsequent maternal risk of hypertension and type 2 diabetes mellitus in a UK population-based retrospective cohort study. BMJ Open 2023. Nov 24;13(11):e078167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marin R, Gorostidi M, Portal CG, Sanchez M, Sanchez E, Alvarez J. Long-term prognosis of hypertension in pregnancy. Hypertens Pregnancy 2000;19(2):199–209. [DOI] [PubMed] [Google Scholar]
- 23.Kurabayashi T, Mizunuma H, Kubota T, Kiyohara Y, Nagai K, Hayashi K. Pregnancy-induced hypertension is associated with maternal history and a risk of cardiovascular disease in later life: Japanese cross-sectional study. Maturitas 2013. Jul;75(3):227–31. [DOI] [PubMed] [Google Scholar]
- 24.Heida KY, Franx A, van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, et al. Earlier Age of Onset of Chronic Hypertension and Type 2 Diabetes Mellitus After a Hypertensive Disorder of Pregnancy or Gestational Diabetes Mellitus. Hypertension 2015. Dec;66(6):1116–22. [DOI] [PubMed] [Google Scholar]
- 25.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cnattingius S, Kallen K, Sandstrom A, Rydberg H, Mansson H, Stephansson O, et al. The Swedish medical birth register during five decades: documentation of the content and quality of the register. Eur J Epidemiol 2023. Jan 3;38(1):109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rawshani A, Rawshani A, Franzen S, Eliasson B, Svensson AM, Miftaraj M, et al. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N Engl J Med 2017. Apr 13;376(15):1407–18. [DOI] [PubMed] [Google Scholar]
- 28.Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia 2020. Mar;63(3):508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragnarson Tennvall G, Apelqvist J, Eneroth M. The inpatient care of patients with diabetes mellitus and foot ulcers. A validation study of the correspondence between medical records and the Swedish Inpatient Registry with the consequences for cost estimations. J Intern Med 2000. Nov;248(5):397–405. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Wang Z, Wang L, Qiu M, Wang Y, Hou X, et al. Hypertensive disorders during pregnancy and risk of type 2 diabetes in later life: a systematic review and meta-analysis. Endocrine 2017. Mar;55(3):809–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


