Abstract
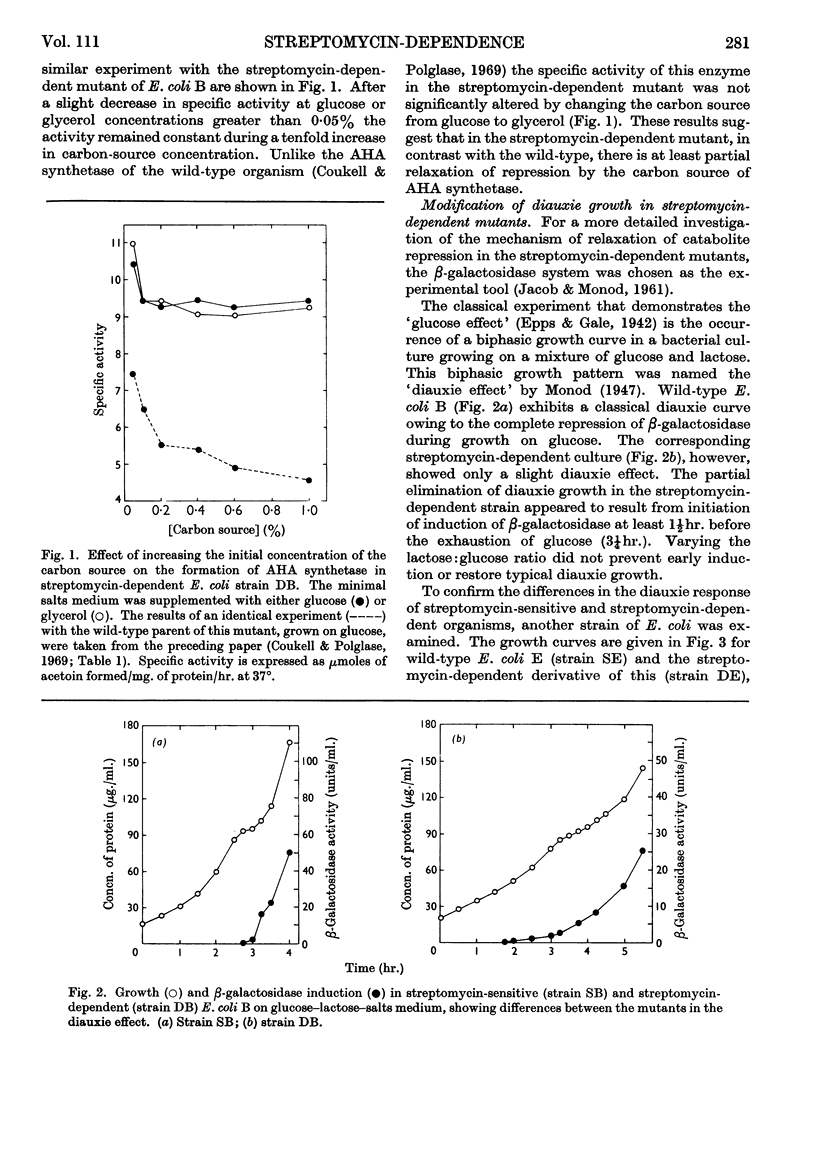
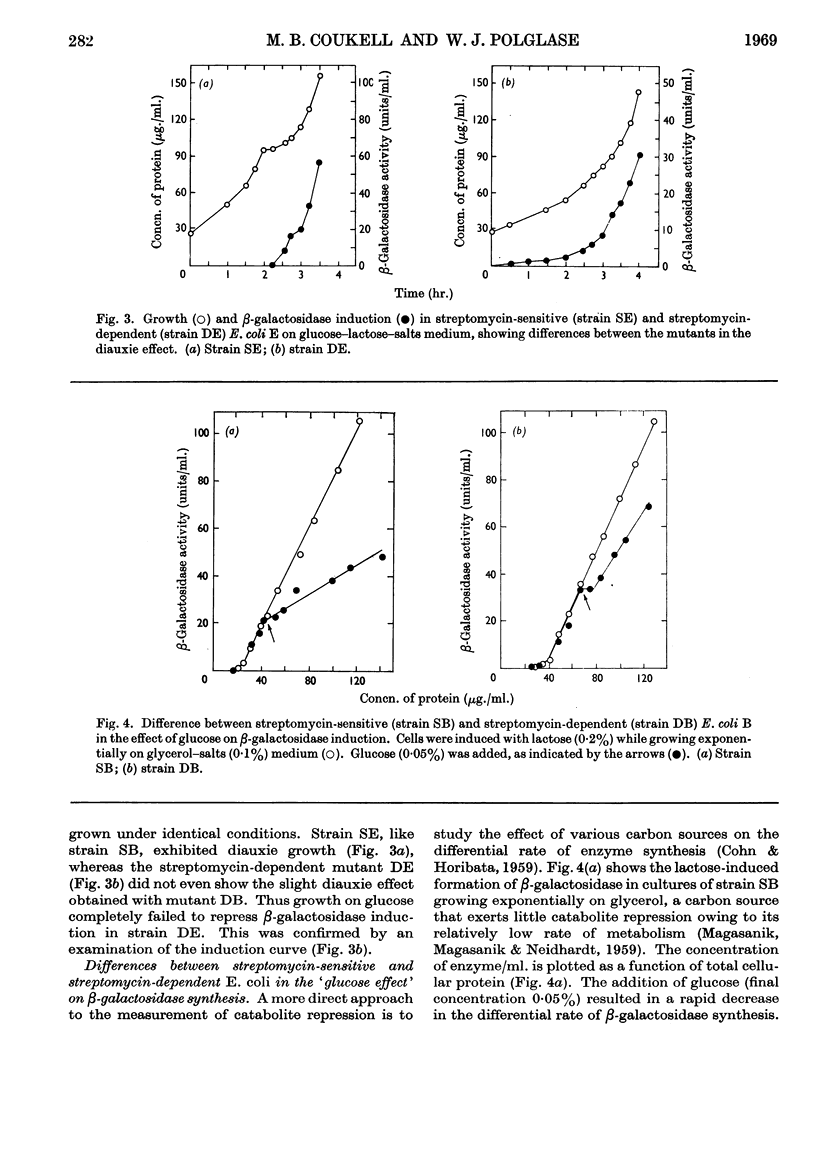
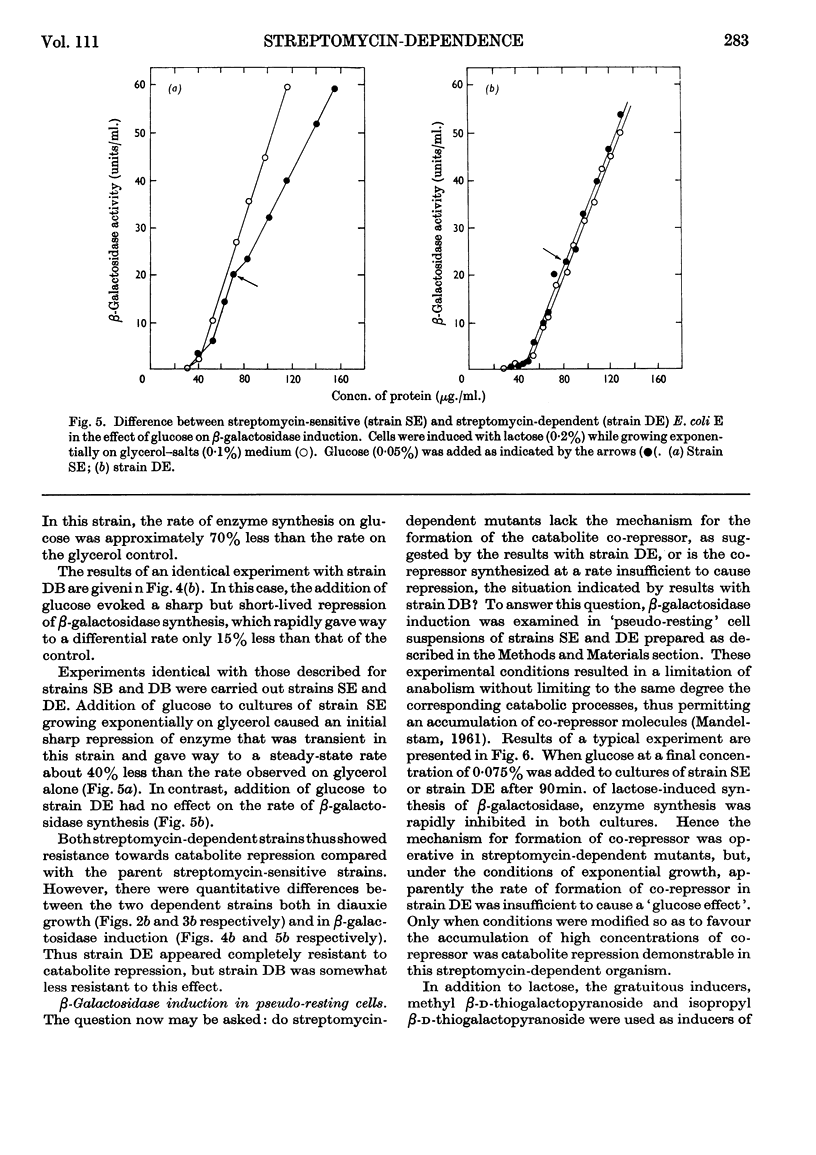
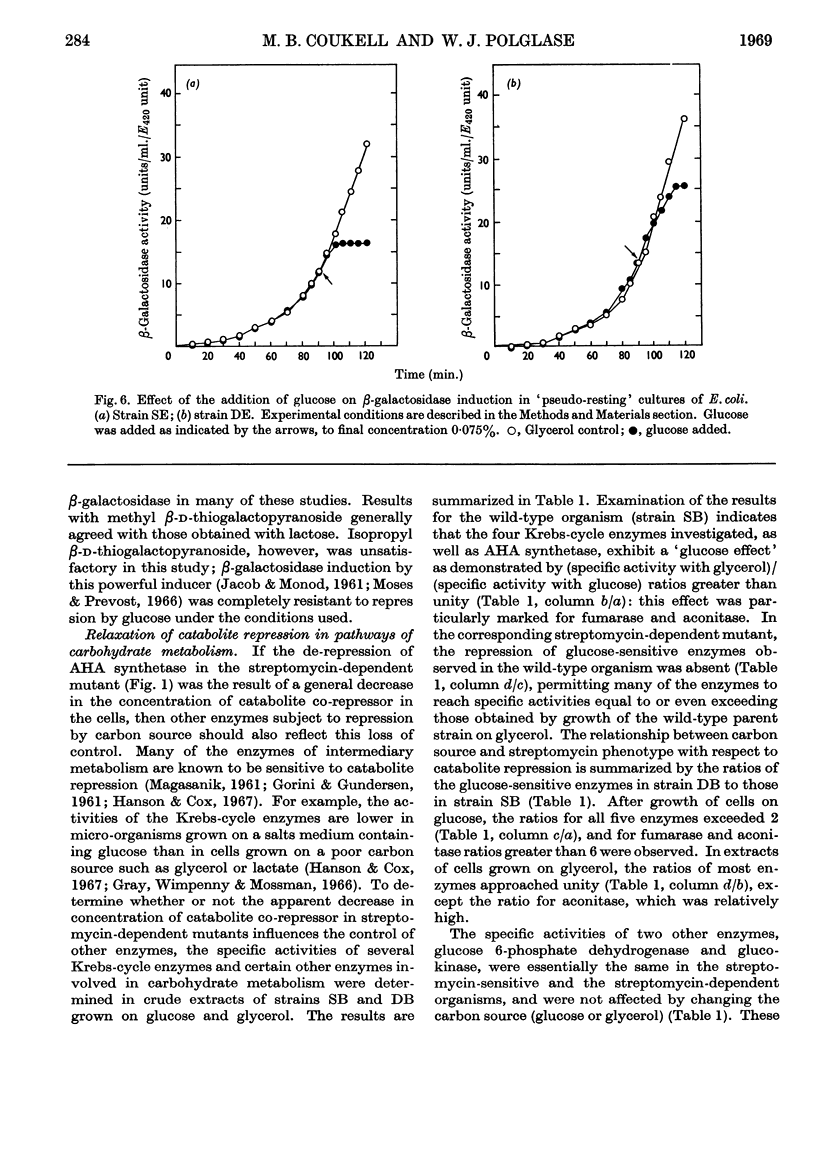
Acetohydroxy acid synthetase, which is sensitive to catabolite repression in wild-type Escherichia coli B, was relatively resistant to this control in a streptomycin-dependent mutant. The streptomycin-dependent mutant was found to be inducible for β-galactosidase in the presence of glucose, although repression of β-galactosidase by glucose occurred under experimental conditions where growth of the streptomycin-dependent mutant was limited. Additional glucose-sensitive enzymes of wild-type E. coli B (citrate synthase, fumarase, aconitase and isocitrate dehydrogenase) were found to be insensitive to the carbon source in streptomycin-dependent mutants: these enzymes were formed by streptomycin-dependent E. coli B in equivalent quantities when either glucose or glycerol was the carbon source. Two enzymes, glucokinase and glucose 6-phosphate dehydrogenase, that are glucose-insensitive in wild-type E. coli B were formed in equivalent quantity on glucose or glycerol in both streptomycin-sensitive and streptomycin-dependent E. coli B. The results indicate a general decrease or relaxation of catabolite repression in the streptomycin-dependent mutant. The yield of streptomycin-dependent cells from glucose was one-third less than that of the streptomycin-sensitive strain. We conclude that the decreased efficiency of glucose utilization in streptomycin-dependent E. coli B is responsible for the relaxation of catabolite repression in this mutant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAGG P. D., POLGLASE W. J. Extracellular metabolites of streptomycin mutants of Escherichia coli. J Bacteriol. 1962 Aug;84:370–374. doi: 10.1128/jb.84.2.370-374.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Physiology of the inhibition by glucose of the induced synthesis of the beta-galactosideenzyme system of Escherichia coli. J Bacteriol. 1959 Nov;78:624–635. doi: 10.1128/jb.78.5.624-635.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coukell M. B., Polglase W. J. Acetolactate formation by streptomycin mutants of Escherichia coli. Can J Microbiol. 1965 Dec;11(6):905–911. doi: 10.1139/m65-120. [DOI] [PubMed] [Google Scholar]
- Coukell M. B., Polglase W. J. Repression by glucose of acetohydroxy acid synthetase in Escherichia coli B. Biochem J. 1969 Feb;111(3):273–278. doi: 10.1042/bj1110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai I. D., Polglase W. J. Characterization and properties of acetohydroxy acid synthetase of streptomycin-dependent Escherichia coli. Biochim Biophys Acta. 1965 Oct 25;110(1):181–188. doi: 10.1016/s0926-6593(65)80107-2. [DOI] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., GUNDERSEN W. Induction by arginine of enzymes of arginine biosynthesis in Escherichia coli B. Proc Natl Acad Sci U S A. 1961 Jul 15;47:961–971. doi: 10.1073/pnas.47.7.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. E., Spotts C. R. Effects of streptomycin deprivation on enzyme synthesis in streptomycin-dependent Escherichia coli. J Bacteriol. 1967 Oct;94(4):1154–1161. doi: 10.1128/jb.94.4.1154-1161.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Mossman M. R. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):33–41. doi: 10.1016/0304-4165(66)90149-8. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Induction and repression of beta-galactosidase in non-growing Escherichia coli. Biochem J. 1961 Jun;79:489–496. doi: 10.1042/bj0790489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses V., Prevost C. Catabolite repression of beta-galactosidase synthesis in Escherichia coli. Biochem J. 1966 Aug;100(2):336–353. doi: 10.1042/bj1000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C. Mutant of Aerobacter aerogenes lacking glucose repression. J Bacteriol. 1960 Oct;80:536–543. doi: 10.1128/jb.80.4.536-543.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polglase W. J. Regulation of acetohydroxy and synthetase in streptomycin-dependent Escherichia coli. Can J Biochem. 1966 May;44(5):599–606. doi: 10.1139/o66-072. [DOI] [PubMed] [Google Scholar]
- SRERE P. A., KOSICKI G. W. The purification of citrate-condensing enzyme. J Biol Chem. 1961 Oct;236:2557–2559. [PubMed] [Google Scholar]
- TIRUNARAYANAN M. O., VISCHER W. A., RENNER U. Streptomycin and amino acid metabolism of bacteria. Antibiot Chemother (Northfield) 1962 Feb;12:117–122. [PubMed] [Google Scholar]


