Abstract
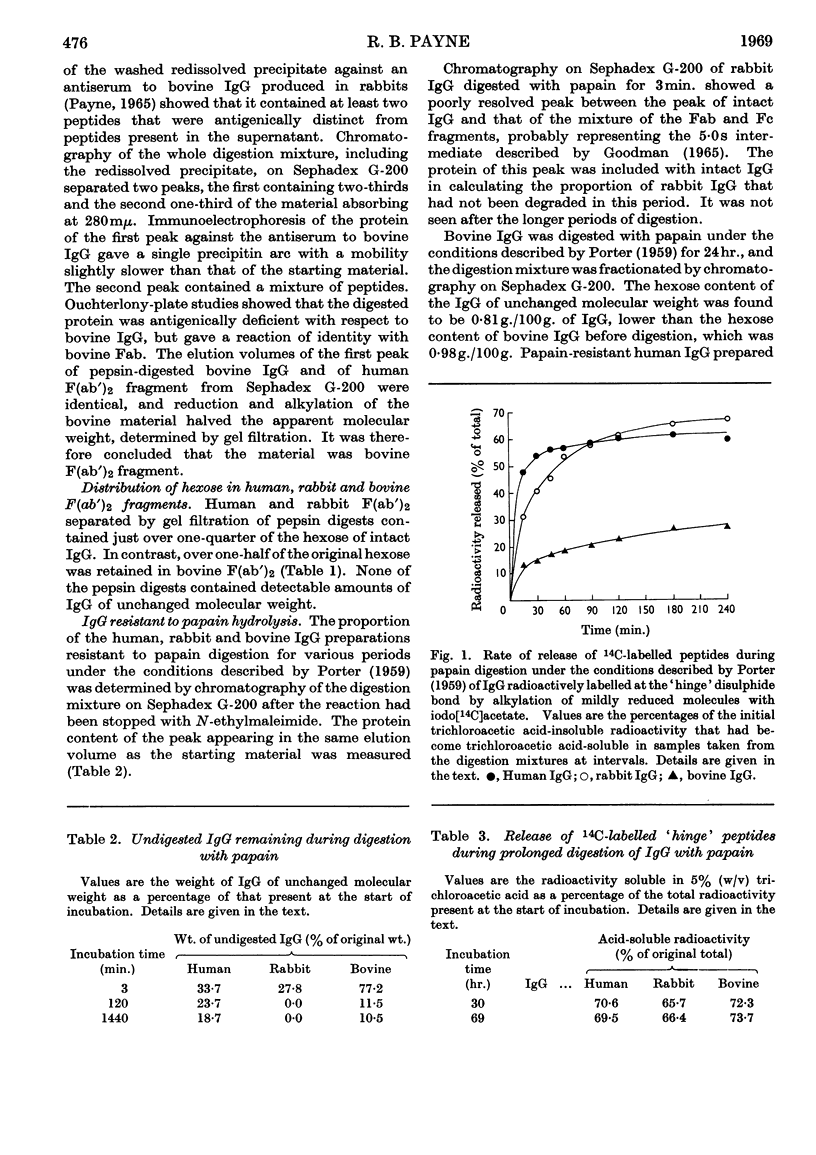
About two-thirds of the hexose of human and rabbit immunoglobulin G (IgG) was located in the Fc fragment and one-third in the `hinge' region of the γ (heavy) polypeptide chain at the junction of the Fab and Fc fragments. In contrast, bovine IgG contained more hexose in the `hinge' region than in the Fc fragment. The initial cleavage of susceptible IgG molecules into Fab and Fc fragments by papain under the conditions given by Porter (1959) had reached completion after digestion for 2hr., though bovine IgG was digested somewhat more slowly than human or rabbit IgG. The release of `hinge' peptides from human and rabbit IgG had also reached completion by 2hr., but was slower from bovine IgG and continued for several hours longer. Since bovine IgG molecules contained on the average a greater amount of hexose in the `hinge' region, carbohydrate on this part of the γ-chain may influence not only the initial rate of enzymic hydrolysis into Fab and Fc fragments, but also, and to a greater extent, the rate of further limited hydrolysis of the N-terminal regions of the Fc fragment. The presence of carbohydrate in the `hinge' region does not appear to account for the resistance of some IgG molecules to papain digestion and of some Fc fragments to N-terminal degradation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel C. A., Spiegelberg H. L., Grey H. M. The carbohydrate contents of fragments and polypeptide chains of human gamma-G-myeloma proteins of different heavy-chain subclasses. Biochemistry. 1968 Apr;7(4):1271–1278. doi: 10.1021/bi00844a004. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUMPTON M. J., WILKINSON J. M. AMINO ACID COMPOSITIONS OF HUMAN AND RABBIT GAMMA-GLOBULINS AND OF THE FRAGMENTS PRODUCED BY REDUCTION. Biochem J. 1963 Aug;88:228–234. doi: 10.1042/bj0880228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCOIS C., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 4. The determination of mannose in hen's-egg albumin by radioisotope dilution. Biochem J. 1962 May;83:335–341. doi: 10.1042/bj0830335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C. Structural units of human 7S gamma globulin. J Clin Invest. 1960 Dec;39:1933–1941. doi: 10.1172/JCI104218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIAO S., PUTNAM F. W. The cleavage of human gamma-globulin by papain. J Biol Chem. 1961 Jan;236:122–135. [PubMed] [Google Scholar]
- Hill R. L., Delaney R., Lebovitz H. E., Fellows R. E., Jr Studies on the amino acid sequence of heavy chains from rabbit immunoglobulin G. Proc R Soc Lond B Biol Sci. 1966 Nov 22;166(1003):159–175. doi: 10.1098/rspb.1966.0091. [DOI] [PubMed] [Google Scholar]
- Hong R., Nisonoff A. Relative labilities of the two types of interchain disulfide bond of rabbit gamma G-immunoglobulin. J Biol Chem. 1965 Oct;240(10):3883–3891. [PubMed] [Google Scholar]
- Howell J. W., Hood L., Sanders B. G. Comparative analysis of the IgG heavy chain carbohydrate peptide. J Mol Biol. 1967 Dec 28;30(3):555–558. doi: 10.1016/0022-2836(67)90369-5. [DOI] [PubMed] [Google Scholar]
- Inman F. P., Nisonoff A. Reversible dissociation of fragment Fc of rabbit gamma-G-immunoglobulin. J Biol Chem. 1966 Jan 25;241(2):322–329. [PubMed] [Google Scholar]
- MANDY W. J., RIVERS M. M., NISONOFF A. Recombination of univalent subunits derived from rabbit antibody. J Biol Chem. 1961 Dec;236:3221–3226. [PubMed] [Google Scholar]
- Melchers F., Lennox E. S., Facon M. A carbohydrate-containing mouse light chain-protein. Biochem Biophys Res Commun. 1966 Jul 20;24(2):244–251. doi: 10.1016/0006-291x(66)90727-3. [DOI] [PubMed] [Google Scholar]
- Milstein C. P., Feinstein A. Comparative studies of two types of bovine immunoglobulin G heavy chains. Biochem J. 1968 Apr;107(4):559–564. doi: 10.1042/bj1070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Osebold J. W., Aalund O. Physical heterogeneity of bovine gamma-globulins: characterization of gamma-M and gamma-G globulins. Arch Biochem Biophys. 1965 Oct;112(1):126–136. doi: 10.1016/0003-9861(65)90020-2. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- NOLAN C., SMITH E. L. Glycopeptides. II. Isolation and properties of glycopeptides from rabbit gamma-globulin. J Biol Chem. 1962 Feb;237:446–452. [PubMed] [Google Scholar]
- NOLAN C., SMITH E. L. Glycopeptides. III. Isolation and properties of glycopeptides from a bovine globulin of colostrum and from fraction II-3 of human globulin. J Biol Chem. 1962 Feb;237:453–458. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R. B. The reaction of rheumatoid factor with bovine IgG fragments obtained by papain digestion and by reduction. Immunology. 1965 Nov;9(5):449–456. [PMC free article] [PubMed] [Google Scholar]
- ROSEVEAR J. W., SMITH E. L. Glycopeptides. I. Isolation and properties of glycopeptides from a fraction of human gamma-globulin. J Biol Chem. 1961 Feb;236:425–435. [PubMed] [Google Scholar]
- Smyth D. S., Utsumi S. Structure at the hinge region in rabbit immunoglobulin-G. Nature. 1967 Oct 28;216(5113):332–335. doi: 10.1038/216332a0. [DOI] [PubMed] [Google Scholar]
- Steiner L. A., Porter R. R. The interchain disulfide bonds of a human pathological immunoglobulin. Biochemistry. 1967 Dec;6(12):3957–3970. doi: 10.1021/bi00864a043. [DOI] [PubMed] [Google Scholar]
- Utsumi S., Karush F. Chemical characterization of the peptic fragment of rabbit gamma-G-immunoglobulin. Biochemistry. 1967 Aug;6(8):2313–2315. doi: 10.1021/bi00860a006. [DOI] [PubMed] [Google Scholar]


