Abstract
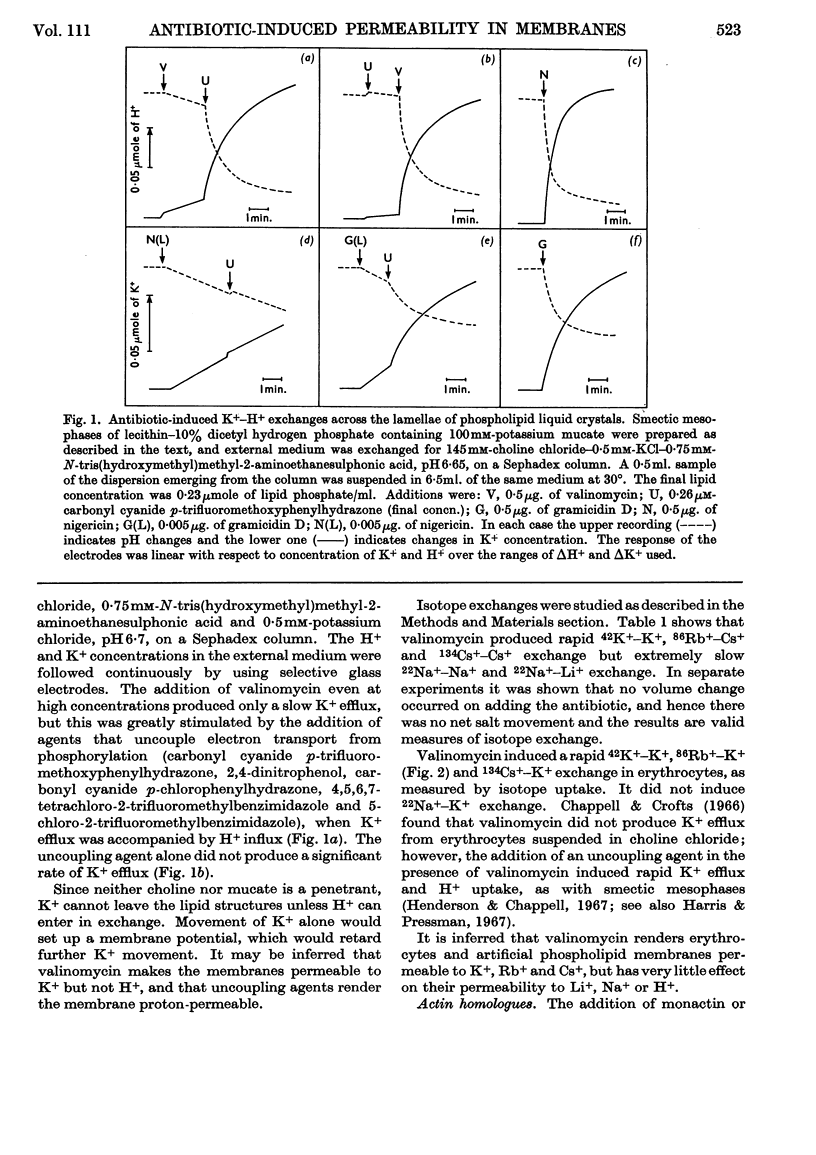
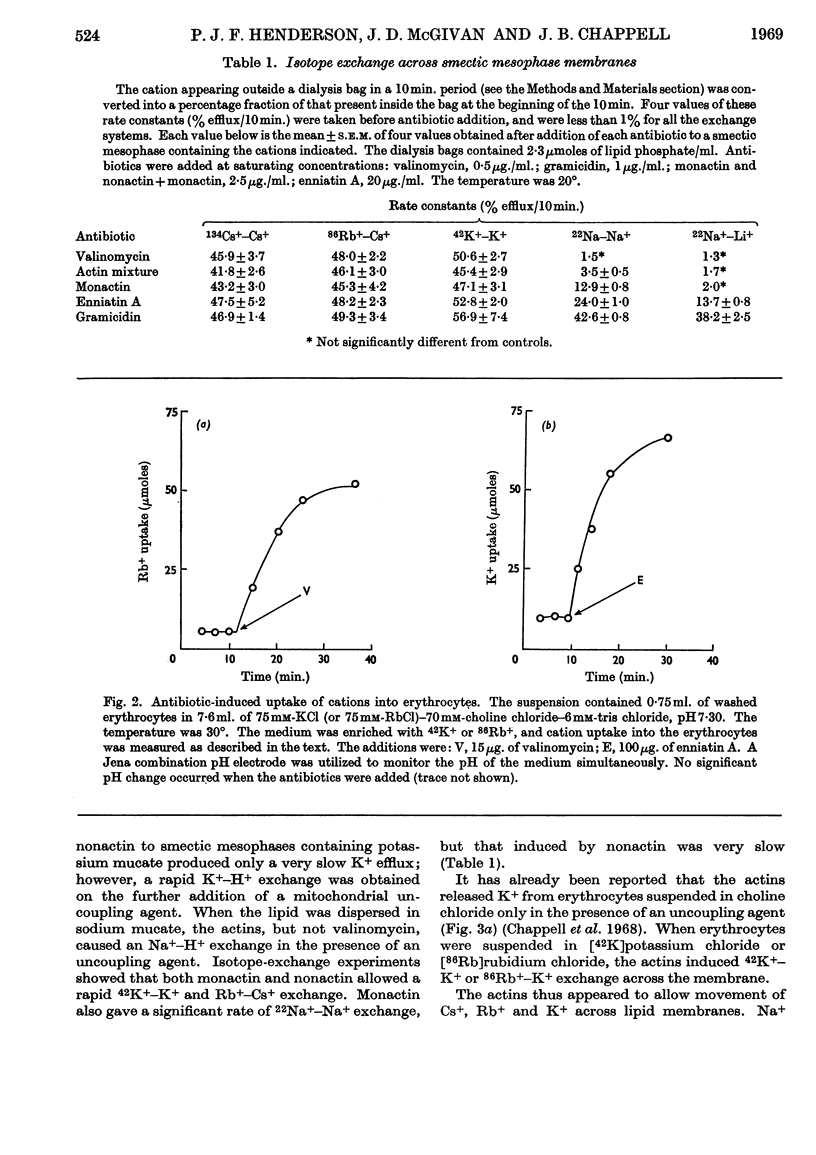
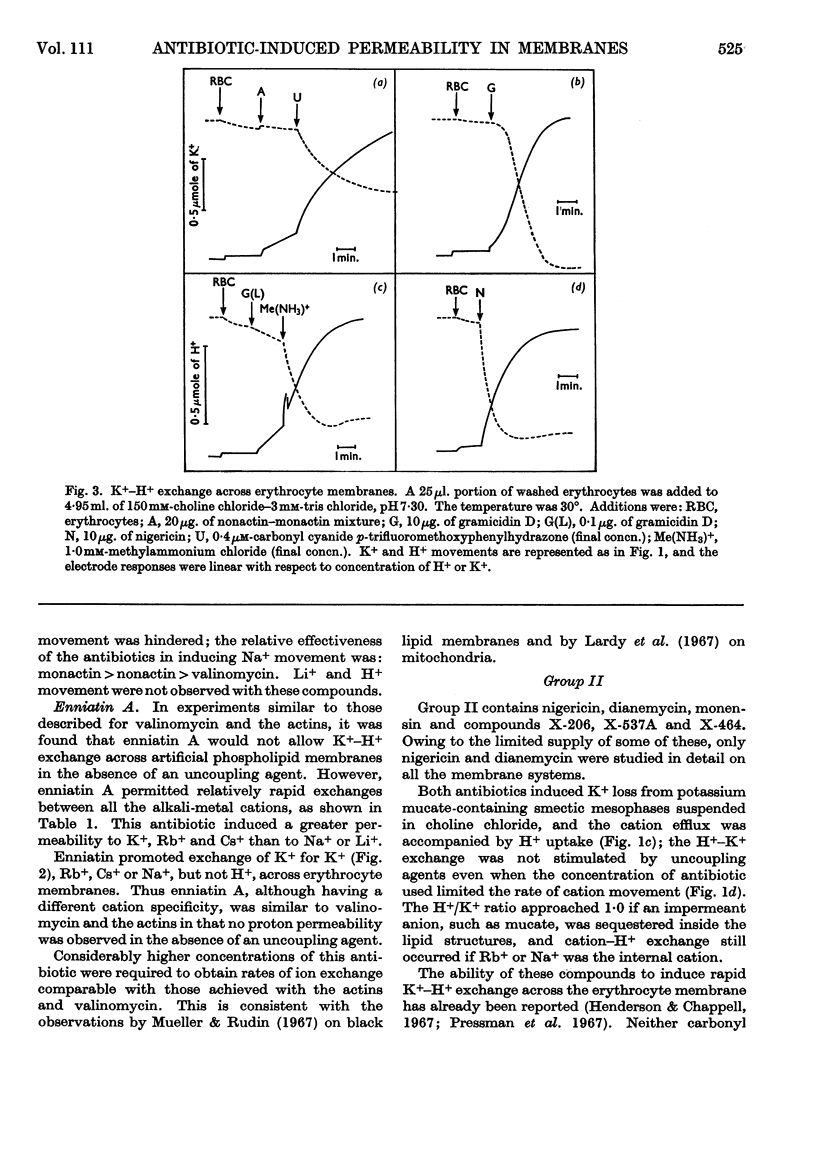
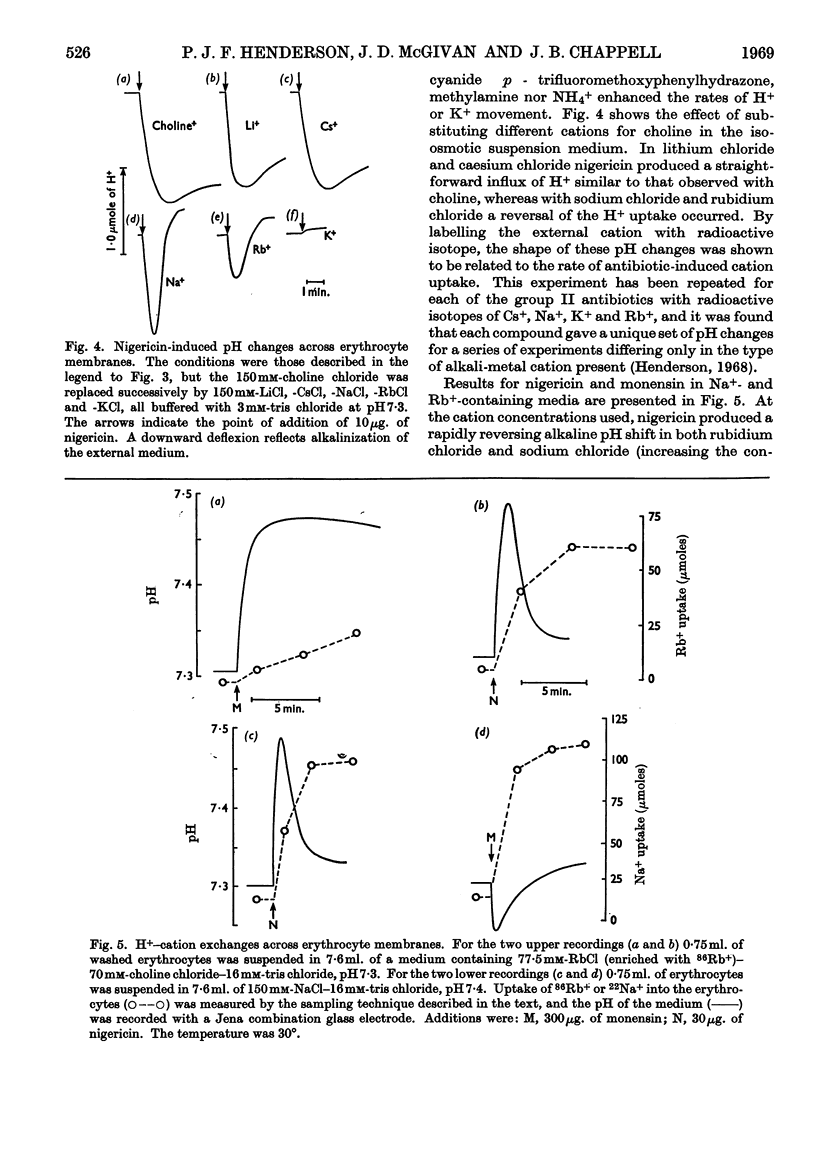
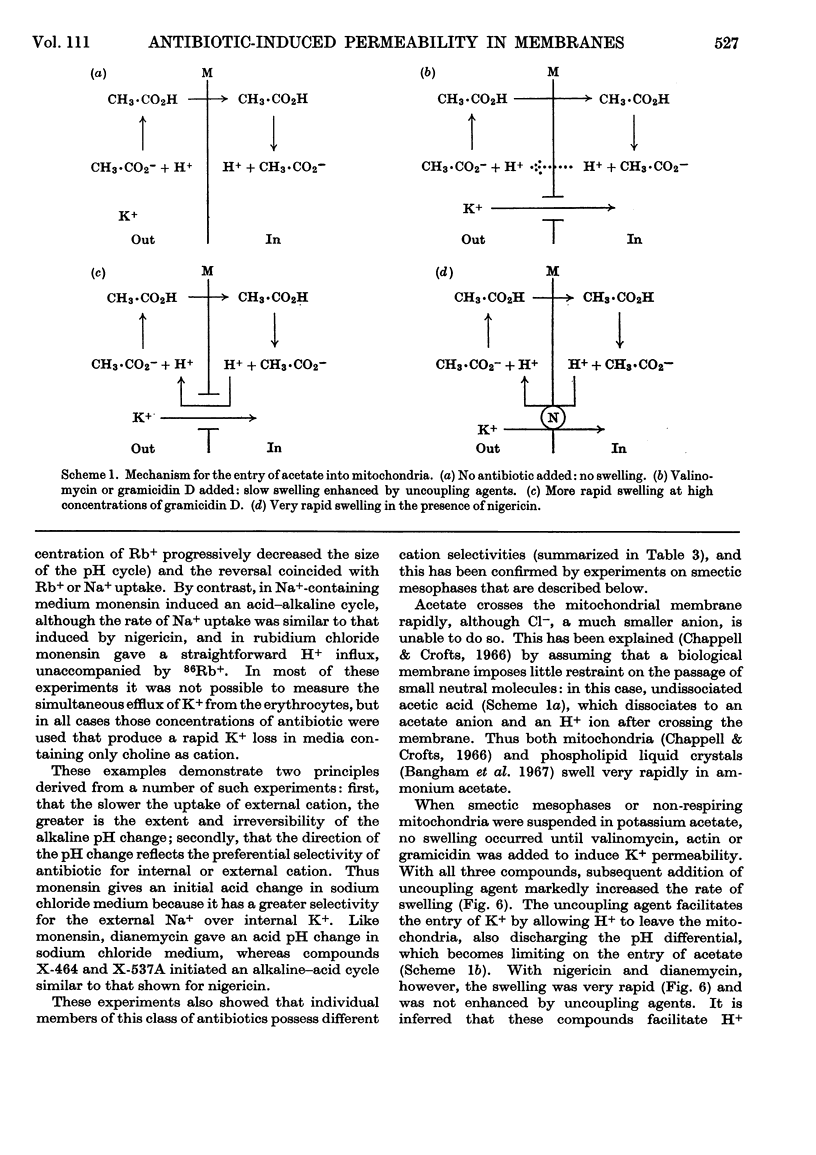
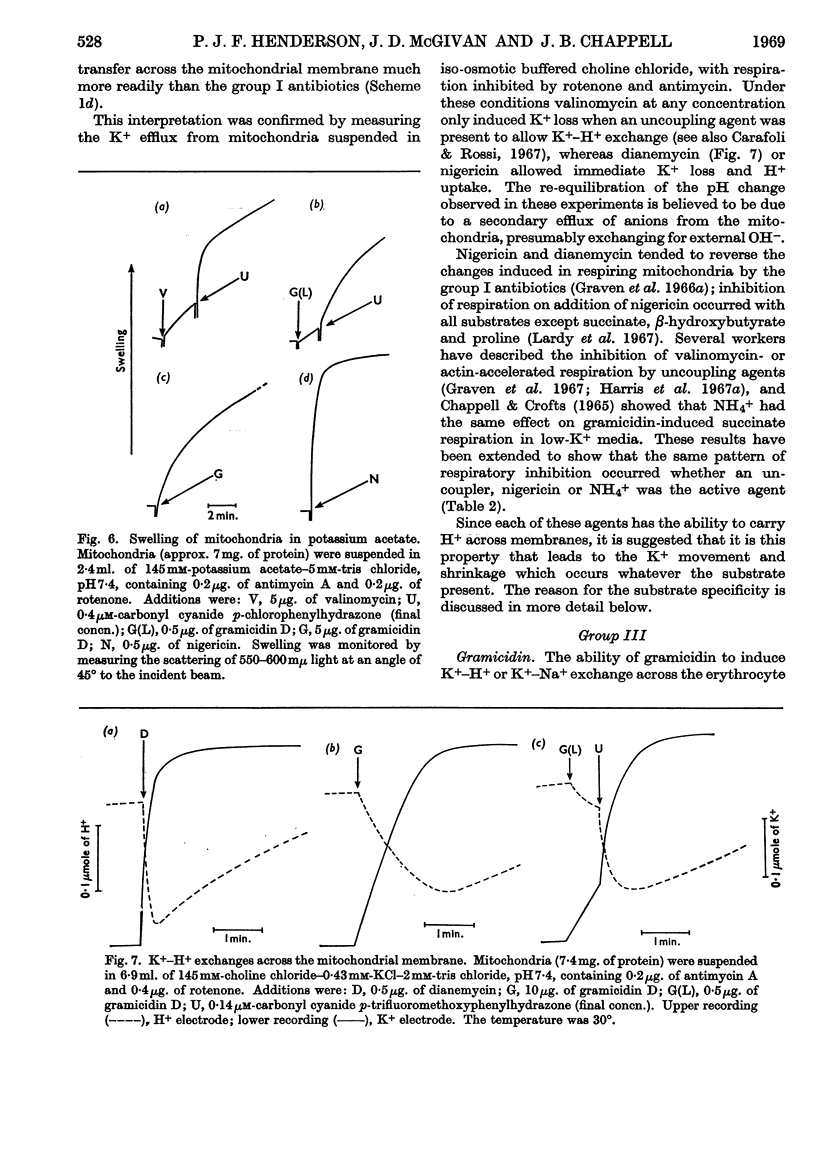
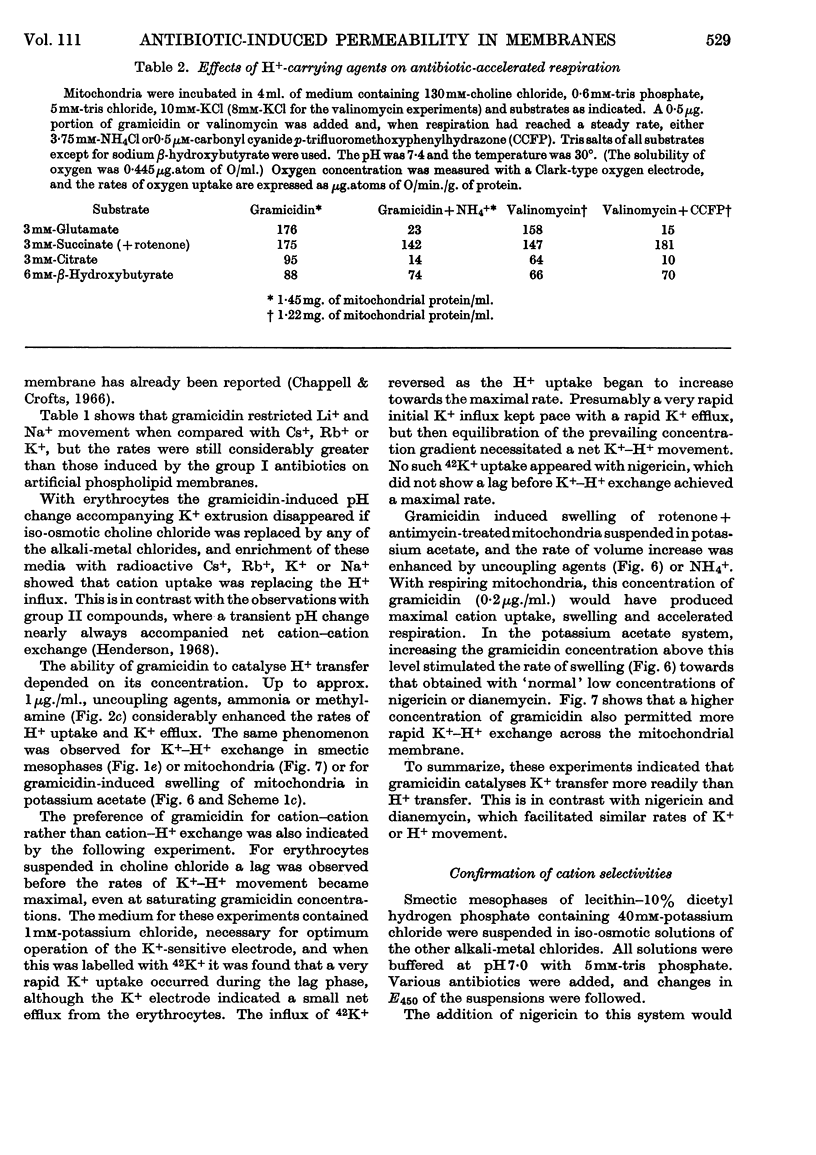
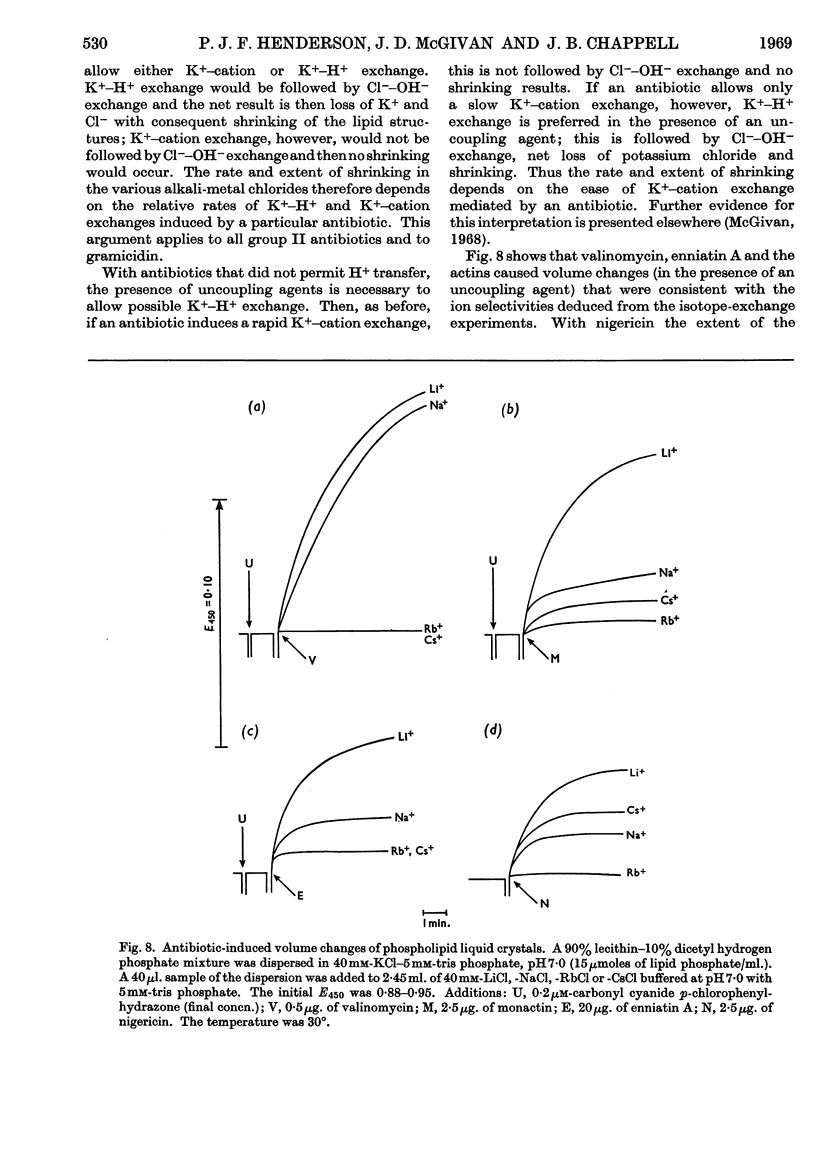
1. The action of the antibiotics enniatin A, valinomycin, the actin homologues, gramicidin, nigericin and dianemycin on mitochondria, erythrocytes and smectic mesophases of lecithin–dicetyl hydrogen phosphate was studied. 2. These antibiotics induced permeability to alkali-metal cations on all three membrane systems. 3. The ion specificity on each membrane system was the same. 4. Enniatin A, valinomycin and the actins did not induce permeability to protons, whereas nigericin and dianemycin rendered all three membrane systems freely permeable to protons. 5. Several differences were noted between permeability induced by nigericin and that induced by gramicidin. 6. The action of all these antibiotics on mitochondrial respiration could be accounted for by changes in passive ion permeability of the mitochondrial membrane similar to those induced in erythrocytes and phospholipid membranes, if it is assumed that a membrane potential is present in respiring mitochondria.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agtarap A., Chamberlin J. W., Pinkerton M., Steinrauf L. The structure of monensic acid, a new biologically active compound. J Am Chem Soc. 1967 Oct 25;89(22):5737–5739. doi: 10.1021/ja00998a062. [DOI] [PubMed] [Google Scholar]
- Andreoli T. E., Tieffenberg M., Tosteson D. C. The effect of valinomycin on the ionic permeability of thin lipid membranes. J Gen Physiol. 1967 Dec;50(11):2527–2545. doi: 10.1085/jgp.50.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham A. D., Standish M. M., Watkins J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965 Aug;13(1):238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. GRAMICIDIN AND ION TRANSPORT IN ISOLATED LIVER MITOCHONDRIA. Biochem J. 1965 May;95:393–402. doi: 10.1042/bj0950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E., Rossi C. S. The effect of dinitrophenol on the permeability of the mitochondrial membrane. Biochem Biophys Res Commun. 1967 Oct 26;29(2):153–157. doi: 10.1016/0006-291x(67)90579-7. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. ON THE MECHANISM OF ACTION OF PHOSPHOLIPASE A. Biochem J. 1963 Sep;88:414–423. doi: 10.1042/bj0880414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-O S., Graven S. N., Lardy H. A. Potassium Ion-dependent hydrolysis of adenosine triphosphate induced by nigericin in mitochondria. J Biol Chem. 1967 Jun 25;242(12):2925–2932. [PubMed] [Google Scholar]
- Glynn I. M. Involvement of a membrane potential in the synthesis of ATP by mitochondria. Nature. 1967 Dec 30;216(5122):1318–1319. doi: 10.1038/2161318a0. [DOI] [PubMed] [Google Scholar]
- Graven S. N., Estrada-O S., Lardy H. A. Alkali metal cation release and respiratory inhibition induced by nigericin in rat liver mitochondria. Proc Natl Acad Sci U S A. 1966 Aug;56(2):654–658. doi: 10.1073/pnas.56.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graven S. N., Lardy H. A., Estrada-O S. Antibiotics as tools for metabolic studies. 8. Effect of nonactin homologs on alkali metal cation transport and rate of respiration in mitochondria. Biochemistry. 1967 Feb;6(2):365–371. doi: 10.1021/bi00854a001. [DOI] [PubMed] [Google Scholar]
- Graven S. N., Lardy H. A., Johnson D., Rutter A. Antibiotics as tools for metabolic studies. V. Effect of nonactin, monactin, dinactin, and trinactin on oxidative phosphorylation and adenosine triphosphatase induction. Biochemistry. 1966 May;5(5):1729–1735. doi: 10.1021/bi00869a040. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Gramicidin, valinomycin, and cation permeability of Streptococcus faecalis. J Bacteriol. 1967 Jul;94(1):53–60. doi: 10.1128/jb.94.1.53-60.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Catlin G., Pressman B. C. Effect of transport-inducing antibiotics and other agents on potassium flux in mitochondria. Biochemistry. 1967 May;6(5):1360–1369. doi: 10.1021/bi00857a019. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Höfer M. P., Pressman B. C. Stimulation of mitochondrial respiration and phosphorylation by transport-inducing antibiotics. Biochemistry. 1967 May;6(5):1348–1360. doi: 10.1021/bi00857a018. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Pressman B. C. Obligate cation exchanges in red cells. Nature. 1967 Dec 2;216(5118):918–920. doi: 10.1038/216918a0. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R., von Stedingk L. V. Ion transport induced by light and antibiotics IN CHROMATOPHORES FROM Rhodospirillum rubrum. Eur J Biochem. 1968 Oct 17;6(1):41–54. doi: 10.1111/j.1432-1033.1968.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Kilbourn B. T., Dunitz J. D., Pioda L. A., Simon W. Structure of the K+ complex with nonactin, a macrotetrolide antibiotic possessing highly specific K+ transport properties. J Mol Biol. 1967 Dec 28;30(3):559–563. doi: 10.1016/0022-2836(67)90370-1. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- Lardy H. A., Graven S. N., Estrada S. Specific induction and inhibition of cation and anion transport in mitochondria. Fed Proc. 1967 Sep;26(5):1355–1360. [PubMed] [Google Scholar]
- Levinson C. Effect of valinomycin on net sodium and potassium transport in Ehrlich ascites tumour cells. Nature. 1967 Oct 7;216(5110):74–75. doi: 10.1038/216074a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Development of K+-Na+ discrimination in experimental bimolecular lipid membranes by macrocyclic antibiotics. Biochem Biophys Res Commun. 1967 Feb 21;26(4):398–404. doi: 10.1016/0006-291x(67)90559-1. [DOI] [PubMed] [Google Scholar]
- Pressman B. C., Harris E. J., Jagger W. S., Johnson J. H. Antibiotic-mediated transport of alkali ions across lipid barriers. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1949–1956. doi: 10.1073/pnas.58.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARGES R., WITKOP B. GRAMICIDIN A. V. THE STRUCTURE OF VALINE- AND ISOLEUCINE-GRAMICIDIN A. J Am Chem Soc. 1965 May 5;87:2011–2020. doi: 10.1021/ja01087a027. [DOI] [PubMed] [Google Scholar]
- Shavit N., San Pietro A. K+ -dependent uncoupling of photophosphorylation by nigericin. Biochem Biophys Res Commun. 1967 Jul 21;28(2):277–283. doi: 10.1016/0006-291x(67)90441-x. [DOI] [PubMed] [Google Scholar]
- von Stedingk L. V., Baltscheffsky H. The light-induced, reversible pH change in chromatophores from Rhodospirillum rubrum. Arch Biochem Biophys. 1966 Nov;117(2):400–404. doi: 10.1016/0003-9861(66)90428-0. [DOI] [PubMed] [Google Scholar]


