Abstract
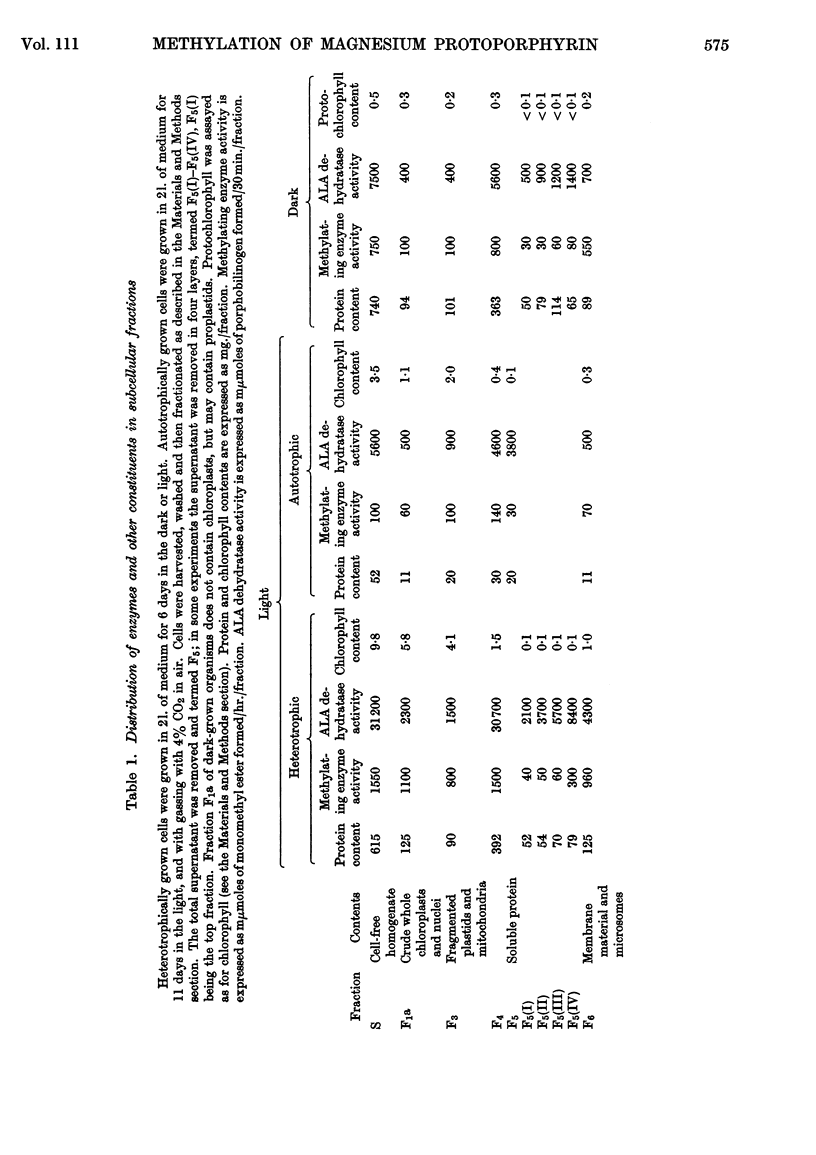
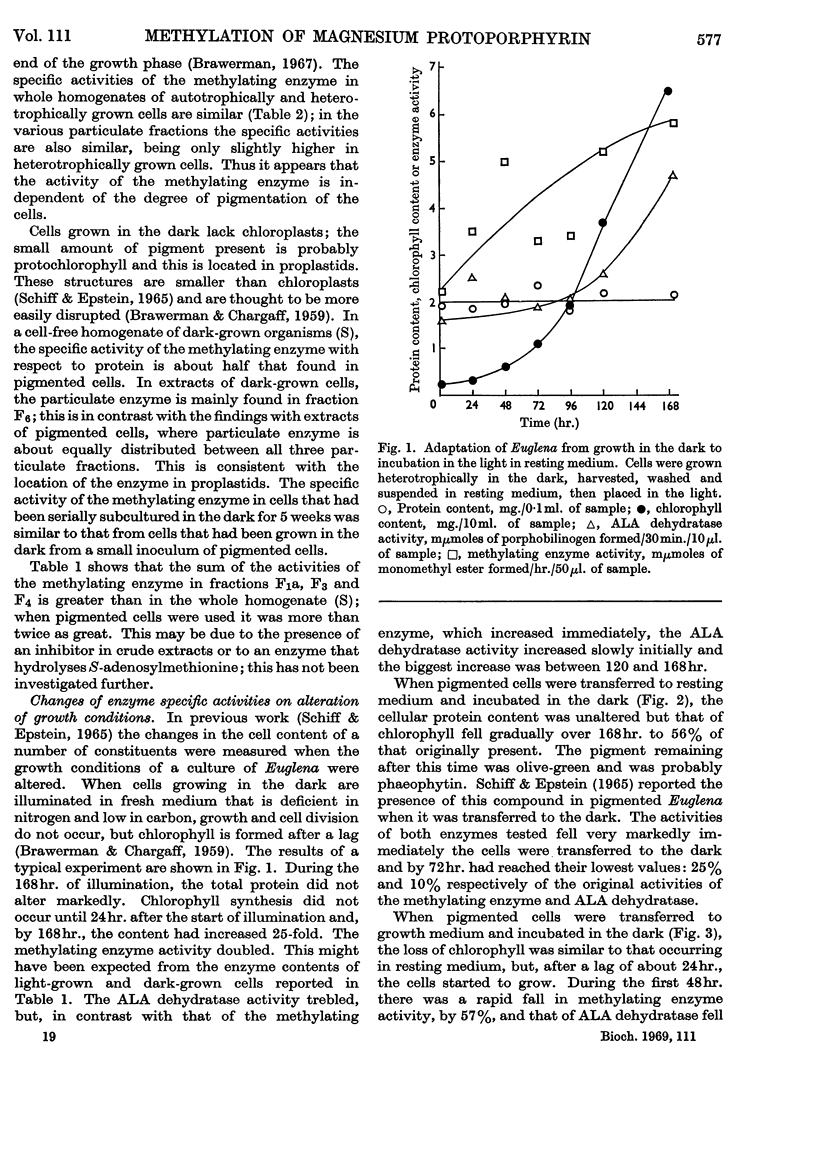
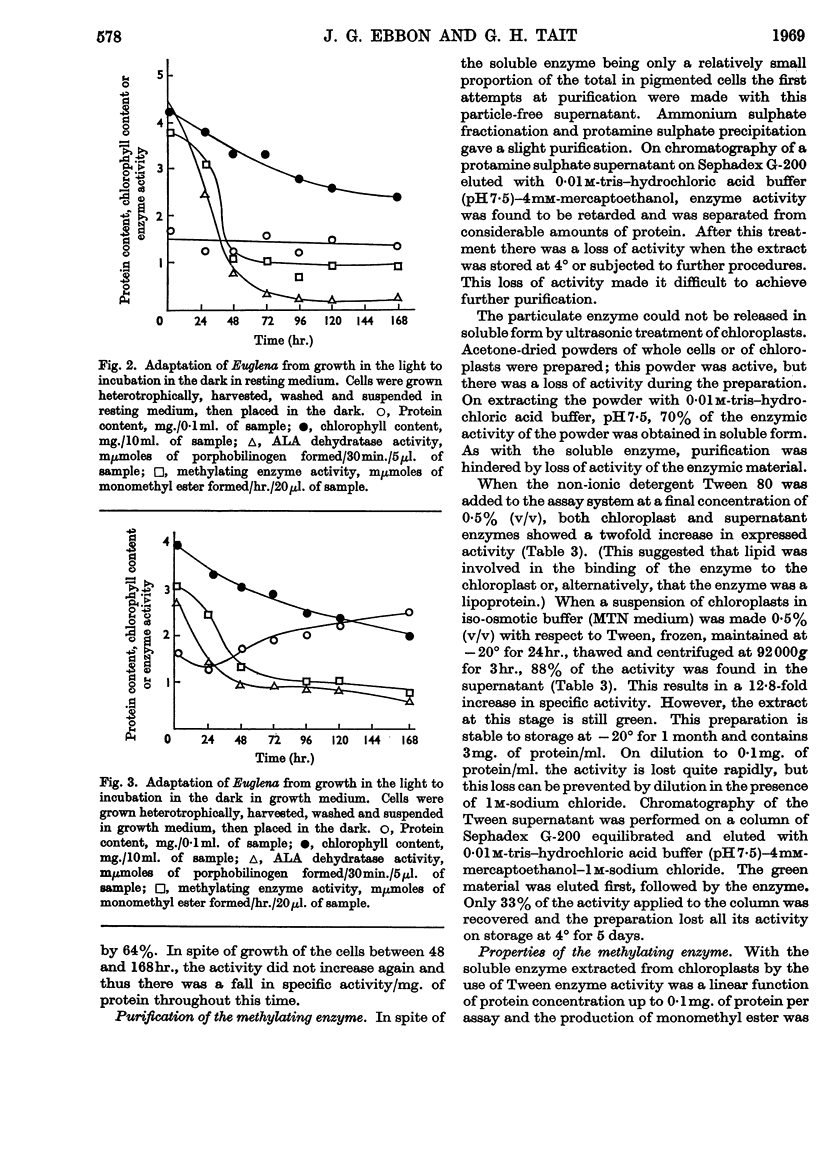
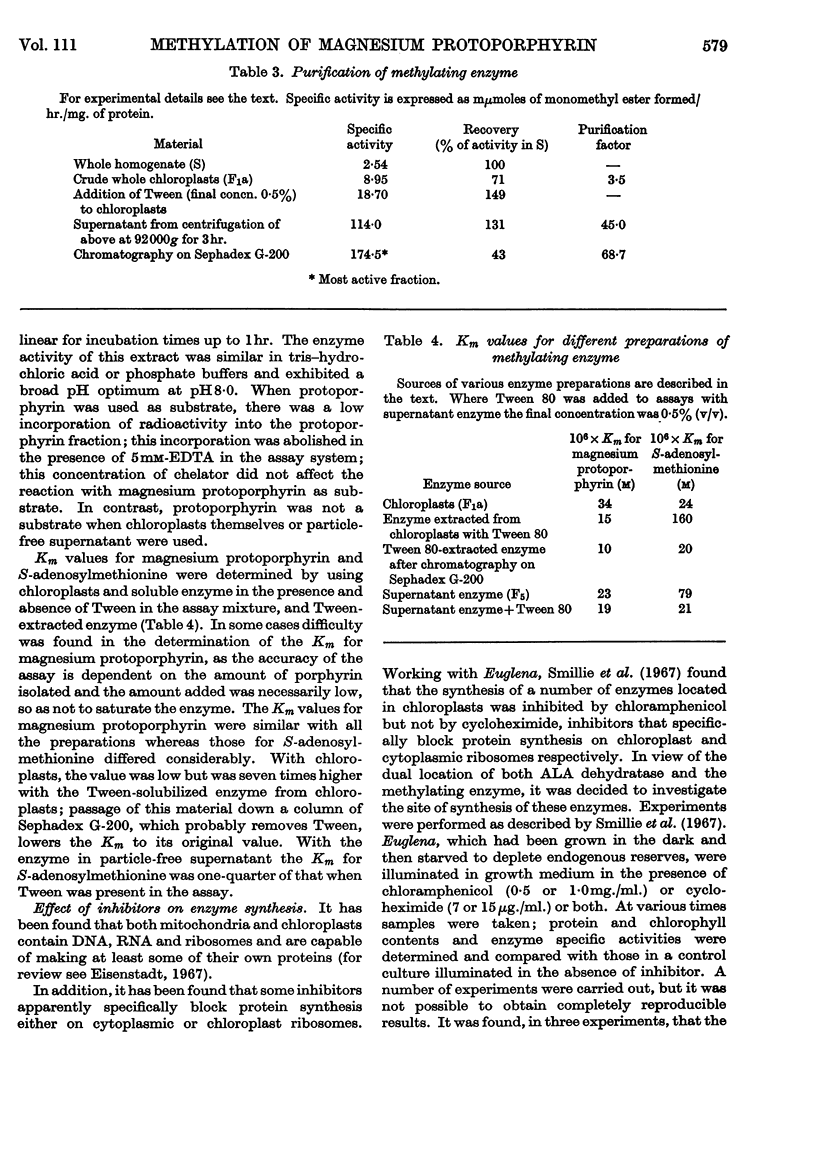
1. An enzyme that methylates magnesium protoporphyrin was detected in extracts of light-grown and dark-grown cells of Euglena gracilis. The activity in light-grown cells is two to three times that in cells grown in the dark. 2. The activity is mainly located in the chloroplast fraction from light-grown cells and in proplastids in dark-grown cells. However, in cells grown either in the light or dark, about 15–20% is found in particle-free supernatant. 3. The chloroplast methylating enzyme was solubilized by the action of Tween 80 and partially purified. The properties were investigated. 4. From experiments in which etiolated cells were illuminated in the presence of inhibitors of chloroplast or cytoplasmic protein synthesis, it appears that the methylating enzyme is made on cytoplasmic ribosomes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUM S. J., BURNHAM B. F., PLANE R. A. STUDIES ON THE BIOSYNTHESIS OF CHLOROPHYLL: CHEMICAL INCORPORATION OF MAGNESIUM INTO PORPHYRINS. Proc Natl Acad Sci U S A. 1964 Dec;52:1439–1442. doi: 10.1073/pnas.52.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAWERMAN G., CHARGAFF E. Changes in protein and ribonucleic acid during the formation of chlorplasts in Euglena gracilis. Biochim Biophys Acta. 1959 Jan;31(1):164–171. doi: 10.1016/0006-3002(59)90452-4. [DOI] [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARELL E. F., KAHN J. S. SYNTHESIS OF PORPHYRINS BY ISOLATED CHLOROPLASTS OF EUGLENA. Arch Biochem Biophys. 1964 Oct;108:1–6. doi: 10.1016/0003-9861(64)90347-9. [DOI] [PubMed] [Google Scholar]
- EISENSTADT J. M., BRAWERMAN G. THE PROTEIN-SYNTHESIZING SYSTEMS FROM THE CYTOPLASM AND THE CHLOROPLASTS OF EUGLENA GRACILIS. J Mol Biol. 1964 Dec;10:392–402. doi: 10.1016/s0022-2836(64)80060-7. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. STUDIES ON THE BIOSYNTHESIS OF PORPHYRIN AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS SPHEROIDES. 4. S-ADENOSYLMETHIONINEMAGNESIUM PROTOPORPHYRIN METHYLTRANSFERASE. Biochem J. 1963 Aug;88:325–334. doi: 10.1042/bj0880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Krishnan S. M., Padmanaban G., Sarma P. S. Role of iron in the regulation of heme biosynthesis in Neurospora crassa. Biochem Biophys Res Commun. 1968 May 10;31(3):333–339. doi: 10.1016/0006-291x(68)90480-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Menon I. A., Shemin D. Concurrent decrease of enzymic activities concerned with the synthesis of coenzyme B 12 and of propionic acid in propionibacteria. Arch Biochem Biophys. 1967 Aug;121(2):304–310. doi: 10.1016/0003-9861(67)90080-x. [DOI] [PubMed] [Google Scholar]
- RICHARDSON S. H., HULTIN H. O., GREEN D. E. STRUCTURAL PROTEINS OF MEMBRANE SYSTEMS. Proc Natl Acad Sci U S A. 1963 Nov;50:821–827. doi: 10.1073/pnas.50.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., SUTTIE J. W., WORK T. S. Protein synthesis in mitochondria. 2. Rate of incorporation in vitro of radioactive amino acids into soluble proteins in the mitochondrial fraction, including catalase, malic dehydrogenase and cytochrome c. Biochem J. 1962 Apr;83:29–40. doi: 10.1042/bj0830029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Graham D., Dwyer M. R., Grieve A., Tobin N. F. Evidence for the synthesis in vivo of proteins of the Calvin cycle and of the photosynthetic electron-transfer pathway on chloroplast ribosomes. Biochem Biophys Res Commun. 1967 Aug 23;28(4):604–610. doi: 10.1016/0006-291x(67)90356-7. [DOI] [PubMed] [Google Scholar]
- Spencer D. Protein synthesis by isolated spinach chloroplasts. Arch Biochem Biophys. 1965 Aug;111(2):381–390. doi: 10.1016/0003-9861(65)90200-6. [DOI] [PubMed] [Google Scholar]
- Stocking C. R. Chloroplast Isolation in Nonaqueous Media. Plant Physiol. 1959 Jan;34(1):56–61. doi: 10.1104/pp.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAIT G. H., GIBSON K. D. The enzymic formation of magnesium protoporphyrin monomethyl ester. Biochim Biophys Acta. 1961 Sep 30;52:614–616. doi: 10.1016/0006-3002(61)90432-2. [DOI] [PubMed] [Google Scholar]


