Highlights
-
•
Osteosarcoma cells can induce MSCs differentiate into CAFs.
-
•
TGF-β1 promotes osteosarcoma cells-induced differentiation from MSCs into CAFs.
-
•
METTL3 promotes osteosarcoma cells-induced MSCs self-differentiation to CAFs by mediating m6A modification of TGF-β1 mRNA.
-
•
METTL3/TGF-β1 signaling axis facilitates MSCs-mediated osteosarcoma progression.
Keywords: Osteosarcoma, Cancer-associated fibroblasts, Mesenchymal stem cells, Transforming growth factor-β1, Methyltransferase-like 3
Abstract
Osteosarcoma, a prevalent and aggressive skeletal malignancy, significantly impacts the prognosis of individuals, particularly young patients. Current treatments, including surgery and chemotherapy, often prove inadequate for advanced osteosarcoma with metastasis. This study investigates the role of the METTL3/TGF-β1 signaling axis in promoting osteosarcoma progression by inducing mesenchymal stem cells (MSCs) to differentiate into cancer-associated fibroblasts (CAFs). Utilizing co-culture technology, we demonstrated that osteosarcoma cells secrete TGF-β1, which is crucial for MSC differentiation into CAFs, as evidenced by the increased expression of CAF markers α-SMA, FSP-1, and FAP. Additionally, METTL3 was found to enhance the stability and expression of TGF-β1 mRNA through m6A modification, thereby facilitating the differentiation process of MSCs. In vivo xenograft experiments further confirmed that the METTL3/TGF-β1 axis significantly promotes tumor growth in osteosarcoma by mediating the differentiation of MSCs into CAFs. These findings provide new insights into the molecular mechanisms underlying osteosarcoma progression and highlight potential therapeutic targets for treating advanced stages of this malignancy.
1. Introduction
Osteosarcoma is one of the most common and serious primary malignant tumors of the skeletal system, accounting for over half of all bone sarcomas and exhibiting a bimodal age distribution. The majority of patient with osteosarcoma are adolescents and children, while a smaller group consists of elderly individuals over the age of 60 [1]. Osteosarcoma exhibits a significant propensity for both local invasion and distant metastasis, with nearly half of the patients experiencing recurrent disease [2]. Although the overall mortality rate for osteosarcoma is less than 40 % over a five-year period, the survival rate for patients with distant metastasis is only 20–30 % [3], [4], [5]. Currently, standard care programs for osteosarcoma include surgical interventions and chemotherapy; however, the outcomes for advanced osteosarcoma with metastasis remain significantly poor [6], [7], [8]. Therefore, there is an urgent need to understand the progression mechanisms of osteosarcoma and to identify effective therapeutic strategies.
Previous evidence has demonstrated that osteosarcoma cells originate from mesenchymal stem cells (MSCs) [9]. Consequently, neo-adjuvant and post-surgical adjuvant chemotherapies have been implemented for osteosarcoma since the 1970s. However, the survival rate of metastatic patients has not significantly improved over recent decades. Therefore, understanding the mechanisms involving MSCs and osteosarcoma cells to enhance the efficacy of osteosarcoma treatments remains a primary objective for researchers. The activation and functional properties of tumor cells are significantly influenced by the tumor microenvironment (TME) [10], [11], [12]. Activated cancer-associated fibroblasts (CAFs) are regarded as the most critical non-tumor cellular components within the TME. Research on various types of neoplasms indicates that CAFs overexpress a range of specific proteins, including fibroblast activation protein (FAP), alpha-smooth muscle actin (α-SMA), and fibroblast-specific protein 1 (FSP-1) [13]. CAFs play a crucial role in tumor pathogenesis, promoting tumor progression and remodeling the extracellular matrix by secreting cytokines that facilitate immune evasion [14], [15]. Previous studies have reported that MSCs may be closely associated with the invasion and development of osteosarcoma [16]. Additionally, Wang et al. demonstrated that osteosarcoma cells can directly induce bone marrow MSCs to differentiate into CAFs in co-culture conditions [17]. While it has established that bone marrow MSCs can promote tumor progression through their differentiation into fibroblasts, the mechanisms by which osteosarcoma cells induce MSCs to differentiate into CAFs remain uncertain [18], [19].
Transforming growth factor-β (TGF-β) acts as a tumor promoter by facilitating tumor growth, stimulating epithelial-mesenchymal transition (EMT), and inducing invasion. It is one of the most prevalent tumor-promoting growth factors in the microenvironment of osteosarcoma within the bone matrix [20], [21]. TGF-β1 has been shown to mediate the differentiation of MSCs into CAFs in colorectal carcinoma. Furthermore, osteosarcoma cells can induce MSCs to differentiate into CAFs by secreting TGF-β1 [3], [22], [23]. Methyltransferase-like 3 (METTL3) has been shown to be overexpressed in osteosarcoma cells [24], [25]. Multiple studies have demonstrated the presence of an N6-methyladenosine (m6A) methylation modification site on TGF-β1 mRNA that interacts with the METTL3 complex [25], [26], [27]. However, further research is needed to determine whether METTL3 regulates the differentiation of MSCs by promoting TGF-β1 in osteosarcoma cells.
In this study, we investigated the interaction between osteosarcoma and MSCs using co-culture technology to evaluate the tumor--promoting capabilities within this environment. Our findings demonstrated the existence of a vicious cycle in which osteosarcoma induces MSCs to differentiate into CAFs, while CAFs enhance the expression and stability of TGF-β1. Additionally, we examined the significant role of the METTL3/TGF-β1 axis in the differentiation of MSCs into CAFs induced by osteosarcoma cells, both in vitro and in vivo, to confirm these critical transfer and induction effects.
2. Materials and methods
2.1. Cell culture
Human bone marrow MSCs were obtained from Procell (Hubei, China), while two osteosarcoma cell lines, MG-63 and ROS, were acquired from ATCC (United States). Osteosarcoma cells were cultured in RPMI-1640 medium (Thermo Fisher, United States) supplemented with 10 percent of fetal bovine serum (v/v) and one percent of penicillin–streptomycin (w/v) at a constant temperature of 37 °C in a humidified atmosphere containing air and 5 percent of carbon dioxide. Meanswell, MSCs were cultured in completed human bone marrow MSC medium (Procell) under the same temperature and atmospheric conditions.
The interaction mechanism between osteosarcoma cells and MSCs was investigated using Transwell cell co-culture technology. Briefly, MSCs (2 × 104) were seeded in the lower chambers, while osteosarcoma cells (2 × 104) were placed in the upper chambers (0.45 μm pores, Corning, NY, USA). These cells were co-cultured for 48 h. For the blank control, only medium was added to the upper chamber in the control group.
2.2. Cell transfection
The tumor cell lines (2 × 105 cells per well) were cultured in 6-well plates until they adhered to the surface. For the inhibition of TGF-β1 or METTL3, short hairpin RNA (shRNA) against TGF-β1 (sh-TGF-β1: 5′-CAA GCA GAG TAC ACA CAG CAT-3′) or METTL3 (sh-METTL3: 5′-GCC TTA ACA TTG CCC ACT GAT-3′) and a negative control shRNA (sh-NC) were obtained from GeneCopoeia (Guangzhou, China). For TGF-β1 overexpression, the TGF-β1 sequence was inserted into the pcDNA3.1 vector (termed oe-TGF-β1), and the empty pcDNA3.1 vector (oe-NC) served as the control (RiboBio, Guangzhou, China). Lipofectamine 3000 (Invitrogen, USA) was used to transfect these plasmids into HOS or MG-63. The transfected cells were used for further experiments after a 48-hour incubation period. Furthermore, a second shRNA targeting TGF-β1 (sh-TGF-β1 #2: 5′-CCG GCC TTT CCT GCT TCT CAT-3′) or METTL3 (sh-METTL3 #2: 5′-GCA AGT ATG TTC ACT ATG AAA-3′) was used to validate a series of key experiments (Supplementary Fig. 1).
2.3. Quantitative real–time PCR (qPCR)
In this study, total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) from cultured osteosarcoma cells and neoplasm tissue samples. A reverse transcription kit (Takara, Japan) was then applied to synthesize the corresponding cDNA. qPCR assay was performed using the ABI PRISM 7900 System (Applied Biosystems, USA) with a SYBR Green PCR Kit (Takara, Japan). The mRNA level was relatively defined using the 2−ΔΔCT method and normalized to GAPDH, which served as an internal control. The primer sequences were given as follows: α-SMA (F: 5′-CTA TGC CTC TGG ACG CAC AAC T-3′, R: 5′-CAG ATC CAG ACG CAT GAT GGC A-3′), FSP-1 (F: 5′-CAG AAC TAA AGG AGC TGC TGA CC-3′, R: 5′-CTT GGA AGT CCA CCT CGT TGT C-3′), FAP (F: 5′-GGA AGT GCC TGT TCC AGC AAT G-3′, R: 5′-TGT CTG CCA GTC TTC CCT GAA G-3′), TGF-β1 (F: 5′-TAC CTG AAC CCG TGT TGC TCT C-3′, R: 5′-GTT GCT GAG GTA TCG CCA GGA A-3′), METTL3 (F: 5′-CTA TCT CCT GGC ACT CGC AAG A-3′, R: 5′-GCT TGA ACC GTG CAA CCA CAT C-3′), Ki-67 (F: 5′-GAA AGA GTG GCA ACC TGC CTT C-3′, R: 5′-GCA CCA AGT TTT ACT ACA TCT GCC-3′), and GAPDH (F: 5′- GTC TCC TCT GAC TTC AAC AGC G-3′, R: 5′-ACC ACC CTG TTG CTG TAG CCA A-3′).
2.4. Methylated RNA immunoprecipitation (MeRIP)-qPCR
Total RNA was extracted in this study from cells subjected to the indicated treatments using TRIzol reagent. Then, the mRNA was fragmented and immunoprecipitated with a mixture of anti-m6A (1:500, ab151230, Abcam) and Protein A beads (Thermo Fisher Scientific) after extraction and isolation. The complex with mRNA and bead-antibody was incubated in IP buffer overnight at 4 °C, followed by the quantification of target mRNA using qPCR.
2.5. Western blot
In the present study, total protein was isolated using RIPA lysis buffer (Thermo Fisher Scientific) from collected cells, and the concentration was measured with a BCA Protein Kit (Beyotime, Shanghai, China). Then, 150 μL of the lysate protein mixture was boiled for 5 min with 50 μL of 4 × loading buffer. Equal amounts of protein were separated by electrophoresis using 10 % SDS–PAGE. The separated proteins were then transferred onto PVDF membranes using the wet transfer method. After blocking with skimmed milk, the following primary antibodies were applied to the prepared membranes for immunoblotting: FSP-1 (1:1000, ab197896, Abcam), α-SMA (1:1000, ab5694, Abcam), METTL3 (1:1000, ab195352, Abcam), TGF-β1 (1:1000, ab215715, Abcam), FAP (1:1000, ab314456, Abcam), Ki-67 (1:5000, ab92742, Abcam) and β-actin (1:2500, ab9485, Abcam) at 4 °C overnight. After this process, the secondary antibodies conjugated with horseradish peroxidase were incubated with the membranes for 60 min at 25 °C following washing. Protein bands were visualized using a chemiluminescence system (Bio-Rad, USA) after incubation with ECL reagent from Thermo Fisher Scientific. The relative levels of protein expression were evaluated based on the gray densities of the bands and normalized to GAPDH.
2.6. Immunofluorescence
After treatment, cells were fixed for 20 min using paraformaldehyde (4 %). Then, cells were washed with Phosphate-Buffered Saline (PBS) and permeabilized with Triton X-100 (0.3 %) for 10 min. Following a 30-minute blocking, the cells were incubated overnight at 4 °C with α-SMA (1:250, ab124964, Abcam). After washing, the cells were incubated with a secondary antibody conjugated to Alexa Fluor 488 (green). DAPI (Thermo Fisher) was used for nuclear counterstaining. Cell images were captured using a fluorescence microscope (Olympus, Japan).
2.7. Enzyme-linked immunosorbent assay (ELISA)
After collecting the supernatants from the co-culture system of osteosarcoma cells and MSCs, the concentration of TGF-β1 in the supernatants was evaluated using an ELISA kit (ab100647, Abcam). The optical density (OD) values of the supernatants were measured using a microplate reader at a wavelength of 450 nm.
2.8. RNA stability test
The osteosarcoma cells transfected with sh-NC or sh-METTL3 were treated with actinomycin D (ActD; Sigma-Aldrich, USA) at a dose of 5 μg/mL for 3 and 6 h to inhibit transcription. Then, the cells were subjected to qPCR to measure the levels of TGF-β1 mRNA, allowing for the evaluation of the stability of TGF-β1 mRNA.
2.9. Xenograft assay
All in vivo animal experiments and procedures were conducted with the approval of the Ethics Committee of Yijishan Hospital of Wannan Medical College. Five-week-old BALB-c mice were raised in a pathogen-free environment and were allowed free access to food and water. The animal subjects were then randomly divided into 4 groups (n = 5) based on the type of cell they would be injected with: sh-NC MG-63 (MG-63 cells transfected with sh-NC), sh-NC MG-63 plus MSCs, sh-METTL3 MG-63 (MG-63 cells transfected with sh-METTL3) and sh-METTL3 MG-63 plus MSCs. Then, osteosarcoma xenograft models were established with the corresponding cells (MG-63 cells (1 × 106) and/or MSCs (1 × 106) subcutaneously injected into the left flank. After these cells developed into palpable tumors, the volumes of tumors were measured every seven days. The animal subjects were sacrificed, and the corresponding samples were collected for further evaluation after the observation for 28 days.
2.10. Immunohistochemistry
Tissues were fixed with 4 % paraformaldehyde, embedded in paraffin, and sectioned into 4 μm slices. Following deparaffinization, the sections were hydrated in a gradient of ethanol. Antigen retrieval was performed using 10 mM sodium citrate (pH 6.0) for 10 min at 95 °C. The sections were then blocked with 3 % bovine serum albumin (BSA) and incubated overnight at 4 °C with α-SMA (1:100, ab5694, Abcam) and TGF-β1 (1:500, ab215715, Abcam). After incubation with the second antibody, a DAB chromogenic agent was applied, and hematoxylin was used for nuclear counterstaining.
2.11. Statistical analysis
All data presented in this study are expressed as mean ± standard deviation (SD). To assess statistical differences, GraphPad Prism 8.0 (GraphPad Software, CA) was utilized. For comparisons involving more than two independent groups, we conducted a one-way analysis of variance followed by Tukey's post hoc test. For the analysis of two independent groups, we employed Student’s t-test. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Osteosarcoma cells induce the differentiation of human bone marrow MSCs into CAFs
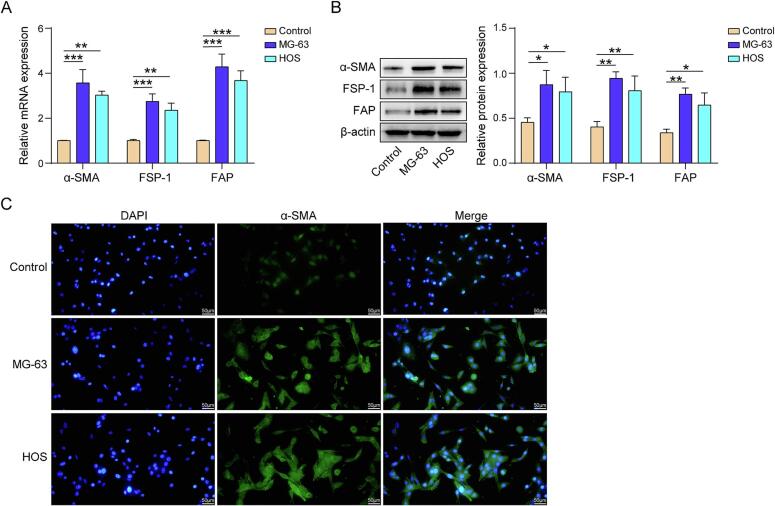
To identify the effect of human osteosarcoma cells on the differentiation process of human bone marrow MSCs, human bone marrow MSCs were co-cultured with MG-63 or HOS cells. The mRNA expression and protein levels of α-SMA, FSP-1 and FAP in MSCs were significantly upregulated following co-culture with MG-63 and HOS cells (Fig. 1A &and B). In addition, immunofluorescence results demonstrated that the addition of MG-63 and HOS significantly increased the expression of the CAF marker α-SMA in MSCs (Fig. 1C). These results indicate that both the MG-63 and HOS can stimulate MSCs to differentiate into CAFs.
Fig. 1.
Osteosarcoma cells induce the differentiation of human bone marrow MSCs into CAFs. (A) qPCR detection of α-SMA, FSP-1, and FAP mRNA expression (B) Detection of α-SMA, FSP-1, and FAP protein levels by western blot. (C) Immunofluorescence detection of α-SMA. n = 3. Data were displayed as mean ± SD and analyzed with one-way ANOVA followed by Turkey post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001.
3.2. TGF-β1 enhances the ability of osteosarcoma cells to induce the differentiate of MSCs into CAFs
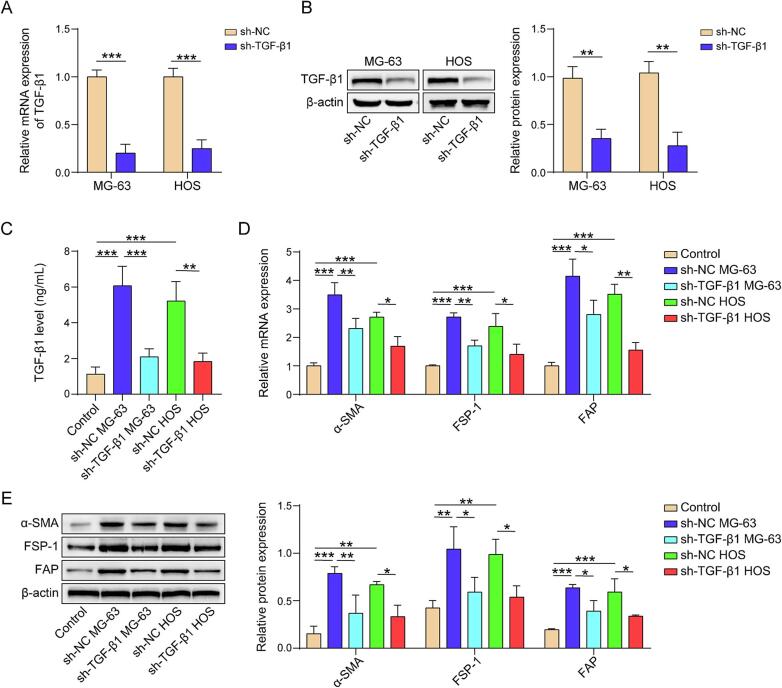
TGF-β1 gene in osteosarcoma cells (MG-63 and HOS) was silenced using TGF-β1 shRNA (Fig. 2A and B). Bone marrow MSCs were cultured alongside these osteosarcoma cells using co-culture technology. The levels of TGF-β1 in the co-culture medium were measured using ELISA, revealing that osteosarcoma cells induced TGF-β1 production compared to the blank control, while sh-TGF-β1 partly reversed the effect of osteosarcoma cells on TGF-β1 secretion (Fig. 2C). Similarly, osteosarcoma cells upregulated both the protein levels and mRNA expression of CAF markers in MSCs, but TGF-β1 shRNA inhibited the effect of osteosarcoma cells on CAF marker expression (Fig. 2D and E). These results demonstrat that the differentiation of MSCs into CAFs is induced by osteosarcoma cells through the secretion of TGF-β1, which is essential for this differentiation process.
Fig. 2.
TGF-β1 enhances the ability of osteosarcoma cells to induce the differentiation of MSCs into CAFs. (A) TGF-β1 mRNA expression evaluated by qPCR. (B) TGF-β1 protein level examined by western blot. (C) TGF-β1 level in culture medium measured by ELISA. (D) α-SMA, FSP-1, and FAP mRNA expression evaluated by qPCR. (E) α-SMA, FSP-1, and FAP protein level examined by western blot. n = 3. Data were displayed as mean ± SD and analyzed with Student’s t-test or one-way ANOVA followed by Turkey post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001.
3.3. METTL3 induces the m6A modification on TGF-β1 mRNA to enhance its stability and expression
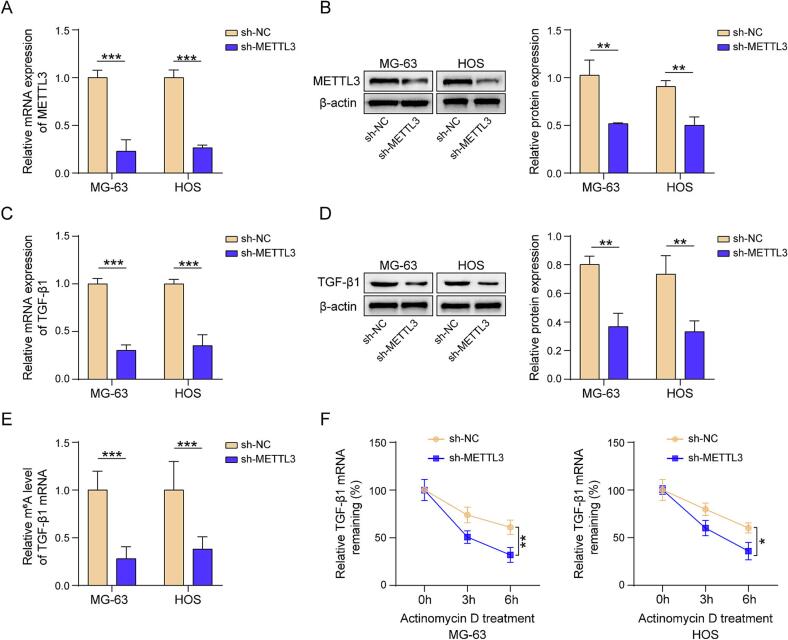
The qPCR results demonstrated that METTL3 shRNA significantly downregulated METTL3 expression level (Fig. 3A and B). Moreover, METTL3 inhibition reduced the TGF-β1 expression at both the mRNA and protein level (Fig. 3C and D). The results of MeRIP-qPCR demonstrated that the knockdown of METTL3 reduced the m6A levels of TGF-β1 mRNA (Fig. 3E). Furthermore, following the treatment with ActD, an RNA expression inhibitor, the existing mRNA stability was assessed by measuring its residual quantity. The results showed that the stability of TGF-β1 mRNA was notably suppressed due to METTL3 silencing (Fig. 3F). Taken together, METTL3 could increase the stability and expression levels of TGF-β1 through m6A modification of its mRNA.
Fig. 3.
METTL3 induces the m6A modification on TGF-β1 mRNA, enhancing its stability and expression. (A) METTL3 mRNA expression level evaluated by qPCR. (B) METTL3 protein examined by western blot. (C) TGF-β1 mRNA expression level evaluated by qPCR. (D) TGF-β1 protein examined by western blot. (E) TGF-β1 mRNA m6A mediation status evaluated by MeRIP-qPCR. (F) TGF-β1 mRNA stability evaluated by qPCR. n = 3. Data were displayed as mean ± SD and analyzed with Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001.
3.4. METTL3 enhances the ability of osteosarcoma cells to induce the differentiation of MSCs into CAFs via TGF-β1
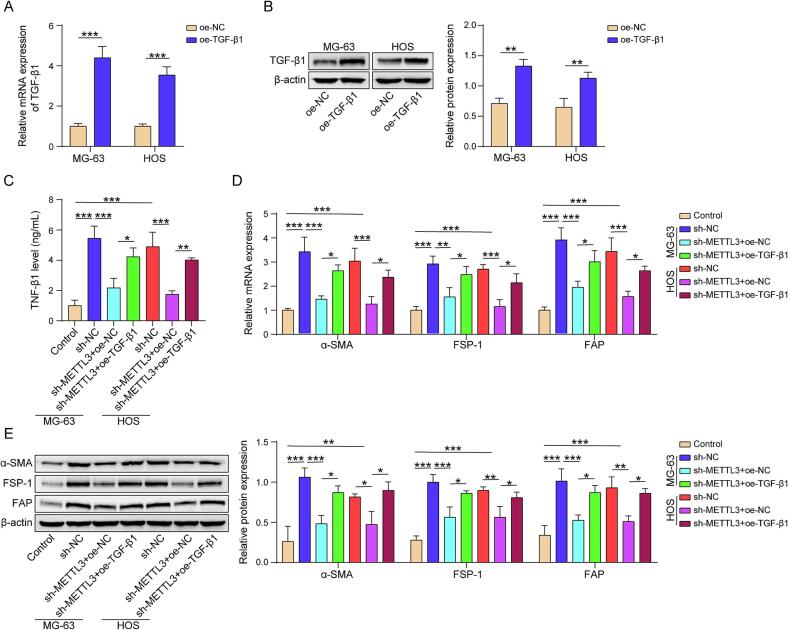
The transcription and translation levels of TGF-β1 in osteosarcoma cells were significantly upregulated after transfection with a TGF-β1 overexpression plasmid (Fig. 4A and B). We also measured the TGF-β1 levels in the medium using ELISA. ELISA results indicated that knocking down METTL3 could reduce TGF-β1 secretion in osteosarcoma cells, and the oe-TGF-β1 plasmid could partially reverse this reduction (Fig. 4C). Concurrently, downregulating METTL3 in osteosarcoma cells could reduce the expression of marker proteins for CAFs including α-SMA, FSP-1, and FAP, in co-cultured MSCs, while TGF-β1 overexpression was able to reverse this reduction (Fig. 4D and E). Therefore, METTL3 promotes the effect of osteosarcoma cells on the differentiation of MSCs into CAFs via TGF-β1.
Fig. 4.
METTL3 enhances the ability of osteosarcoma cells to induce the differentiation of MSCs into CAFs via TGF-β1. (A) TGF-β1 mRNA expression evaluated by qPCR. (B) TGF-β1 protein level examined by western blot. (C) TGF-β1 level in culture medium measured by ELISA. (D) α-SMA, FSP-1, and FAP mRNA expression evaluated by qPCR. (E) α-SMA, FSP-1, and FAP protein levels examined by western blot. n = 3. Data were displayed as mean ± SD and analyzed with Student’s t-test or one-way ANOVA followed by Turkey post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001.
3.5. METTL3/ TGF-β1 signaling axis promotes the progression of osteosarcoma induced by MSCs
MG-63 OS cells with various modifications were utilized to establish xenograft osteosarcoma models with or without MSCs in nude mice. The results indicated that MSCs could promote tumor growth, whereas the knockdown of METTL3 inhibited tumor growth. Additionally, this knockdown also mitigated the stimulatory effects of MSCs on tumor growth (Fig. 5A–C). The results from western blotting and qPCR also demonstrated that MSCs could stimulate the expression of Ki-67, TGF-β1, and METTL3. Conversely, the inhibition of METTL3 led to a downregulation of these molecular expressions and diminished the impact of MCSs on their expression levels (Fig. 5D and E). The CAF marker (α-SMA, FSP-1 and FAP) expression levels showed similar trends in response to MSCs and/or METTL3 inhibition in tumors derived from these xenograft models (Fig. 5F and G). Moreover, immunohistochemistry results demonstrated that MSCs had the ability to increase TGF-β1 and α-SMA expression, but the knockdown of METTL3 decreased these protein levels, which could be partially reversed by MSCs (Fig. 5H). Collectively, the METTL3/TGF-β1 axis may promote MSC-induced tumor progression in osteosarcoma.
Fig. 5.
METTL3/TGF-β1 signaling axis promotes the progression of osteosarcoma induced by MSCs. (A) Size of xenograft tumor tissues. (B) Volume growth curve of xenograft tumor tissues. (C) Weight of xenograft tumor tissues. (D) METTL3, TGF-β1 and Ki-67 mRNA expression level evaluated by qPCR. (E) METTL3, TGF-β1 and Ki-67 protein level examined by western blot. (F) α-SMA, FSP-1, and FAP mRNA expression evaluated by qPCR. (G) α-SMA, FSP-1, and FAP protein level examined by western blot. (H) Immunohistochemical detection of TGF-β1 and α-SMA. n = 5. Data were displayed as mean ± SD and analyzed with one-way ANOVA followed by Turkey post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001.
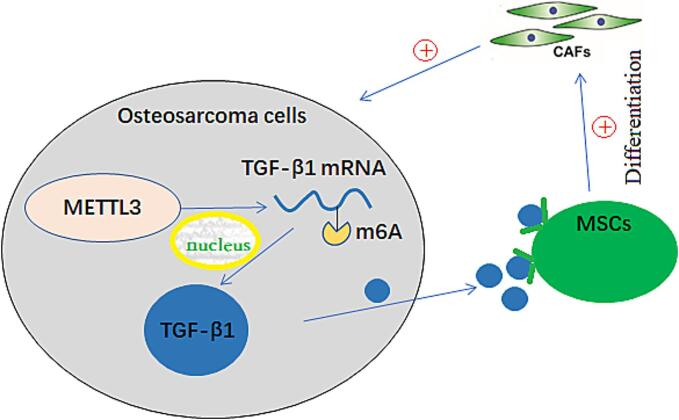
In summary, the results indicated that osteosarcoma cells could induce MSCs to differentiate into CAFs by secreting TGF-β1. Furthermore, METTL3 was found to enhance the stability and expression of TGF-β1 mRNA by mediating its m6A modification. A detailed summary of the results is shown in Fig. 6.
Fig. 6.
A schematic diagram illustrating the mechanism by which METTL3 mediates m6A modification of TGF-β1 mRNA in osteosarcoma cells, leading to the differentiation of mesenchymal stem cells into cancer-associated fibroblasts that promote the progression of osteosarcoma.
4. Discussion
Osteosarcoma is one of the most prevalent malignancies of the skeletal system [28]. Currently, the standard treatment involves a combination of surgery and multi-drug chemotherapy, which has remained unchanged [4], [29]. However, these strategies are primarily effective for early-stage tumors [30]. Therefore, there is an urgent need to identify effective treatments for advanced osteosarcoma. As one of the most prevalent and significant cell types in the bone matrix, human bone marrow MSCs possess the potential to differentiate into CAFs. Previous studies have demonstrated that TGF-β1 can promote this process in tumors [31], [32]. In addition, numerous studies have indicated that CAFs play a crucial role in tumor progression [33], [34]. Therefore, elucidating the interaction mechanisms between osteosarcoma cells and MSCs may pivotal in developing new treatment strategies. We investigated the combined impact of TGF-β1 and METTL3 on the key process by which osteosarcoma cells induced MSCs to differentiate into CAFs using a co-culture technique. Additionally, we analyzed the effects of the METTL3/TGF-β1 signaling axis on promoting MSC-mediated osteosarcoma progression through in vivo experiments in a xenograft model, highlighting directions for future research on potential treatments.
CAFs are the most abundant non-malignant cells in TME. Under normal conditions, CAFs remain in a quiescent state as mesenchymal cells; however, they become activated in response to the TME or during tissue repair processes [35]. High expression levels of CAF markers, such as α-SMA, FSP-1 and FAP, indicate extensive the large-scale production and activation of CAFs, which may correlate with a poor prognosis for the tumor [36], [37]. TGF-β1 derived from tumor cells in adenocarcinoma can facilitate the transformation of MSCs into CAFs. Furthermore, inbiting TGF-β1 in MSCs can effectively inpede this transformation process [22], [23], [32]. Previous studies have shown that TGF-β1 can induce the transformation of bone marrow MSCs into CAFs and enhance the stability of CAFs by promoting the secretion of inflammatory cytokines such as IL-6 [38]. In the present study, we demonstrated that osteosarcoma cells possess the ability to induce human MSCs to differentiate into CAFs through the overexpression and secretion of TGF-β1. Following co-culturing with human osteosarcoma cells, the expression of CAF marker proteins in bone marrow MSCs was significantly upregulated, confirming that differentiation into CAFs occurred in MSCs. Furthermore, our experiments indicated that knocking down TGF-β1 in osteosarcoma cells could partially reverse this induction effect.
Multiple studies have demonstrated that m6A modification is crucial for the upregulation of TGF-β1 expression [39], [40]. Currently, it has been established that the upregulation of TGF-β1 is closely linked to the upregulation of METTL3/METTL14 and global m6A hypermethylation in activated Kupffer cells [39]. Lu et al. indicated that METTL3 can promote the differentiation of lung-resident MSCs into myofibroblasts through the regulation of m6A RNA methylation [41]. In osteosarcoma cells, there is a general upregulation of METTL3 expression [42]. In addition, studies have demonstrated that METTL3 can promote the migration and proliferation of osteosarcoma cells by editing m6A modifications on various oncogenes [24], [43]. Therefore, this study hypothesizes that METTL3 may also induce the differentiation of MSCs in the TME of osteosarcoma into CAFs through the modification of specific, thereby stimulating the growth and migration of osteosarcoma. Our results provided strong evidence that METTL3 induced the differentiation of MSCs into CAFs within the osteosarcoma TME by directly mediating the m6A modification of TGF-β1 mRNA. This findings aligns with previous studies conducted in other sites, including the gastrointestinal tract and lung [41], [44]. In addition, it is consistent with multiple prior studies demonstrating that in vivo xenograft experiments indicate the METTL3/TGF-β1 signaling axis is crucial for osteosarcoma progression by promoting the differentiation of MSCs into CAFs. Furthermore, METTL14 can also regulate the expression of TGF-β1 [39]; however, it remains unclear whether METTL14 facilitates the transformation of MSCs into CAFs by regulating TGF-β1 in an m6A manner. This uncertainty presents a potential avenue for future research.
In summary, METTL3 expression is upregulated in osteosarcoma cells, promoting the differentiation of bone marrow MSCs into CAFs within the TME through the m6A methylation modification of TGF-β1 mRNA, which in turn, promote the progression and exacerbation of osteosarcoma. These findings offer new insights into the mechanisms of m6A gene expression regulation mediated by METTL3 and highlight the role of the TME in the promotion of osteosarcoma. Furthermore, this study presents novel approaches for the non-surgical treatment of advanced osteosarcoma.
Funding
This research was supported by Major Natural Science Research Project of Anhui Provincial Department of Education (2024AH040249), Talent Introduction Science Foundation of Yijishan Hospital, Wannan Medical College (YR20220214), Inflammation and Immune Mediated Diseases Laboratory of Anhui Province (IMMDL202408) and 2022 Anhui Provincial Institute of Translational Medicine Research Project (2022zhyx-C48).
CRediT authorship contribution statement
Jin Qi: Writing – review & editing, Writing – original draft, Methodology, Funding acquisition, Conceptualization. Sihang Liu: Validation, Methodology, Formal analysis. Baomin Wu: Writing – review & editing, Validation. Gang Xue: Visualization, Supervision, Resources, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study has received strong support from the BM.WU laboratory. We would like to express our gratitude to the BM-WU team for their significant assistance in manuscript revision and funding support, which has enabled the smooth progress of this study. Meanwhile, we would like to express our gratitude to Tanxi Biotechnology Company for their thoughtful design and assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2025.100662.
Contributor Information
Baomin Wu, Email: 617894573@qq.com.
Gang Xue, Email: xg1582281142@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
METTL3 promotes the effect of osteosarcoma cells on the differentiation of MSCs into CAFs via TGF-β1. (A) TGF-β1 mRNA expression level evaluated by qPCR. (B) TGF-β1 level in culture medium measured by ELISA. (C) The mRNA expression of α-SMA and FAP evaluated by qPCR. (D) METTL3 mRNA expression evaluated by qPCR. (E) TGF-β1 mRNA expression examined by qPCR. (E) TGF-β1 level in culture medium measured by ELISA. (F). α-SMA and FAP mRNA expression levels determined by qPCR. n = 3. Data were displayed as mean ± SD and analyzed with Student’s t-test or one-way ANOVA followed by Turkey post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001.
References
- 1.Tabatabaei S.H., Jahanshahi G., Dehghan Marvasti F. Diagnostic challenges of low-grade central osteosarcoma of jaw: a literature review. J. Dent. (Shiraz) 2015;16(2):62–67. [PMC free article] [PubMed] [Google Scholar]
- 2.Berhe S., Danzer E., Meyers P.A., Behr G., LaQuaglia M.P., Price A.P. Unusual abdominal metastases in osteosarcoma. J. Pediatr. Surg. Case Rep. 2018;28:13–16. doi: 10.1016/j.epsc.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge R., Huang G.M. Targeting transforming growth factor beta signaling in metastatic osteosarcoma. J. Bone Oncol. 2023;43 doi: 10.1016/j.jbo.2023.100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweetnam R. Osteosarcoma. Br. J. Hosp. Med. 1982;28(2) 112, 116–121. [PubMed] [Google Scholar]
- 5.Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyers P.A. Systemic therapy for osteosarcoma and Ewing sarcoma. Am. Soc. Clin. Oncol. Educ. Book. 2015:e644–e647. doi: 10.14694/EdBook_AM.2015.35.e644. [DOI] [PubMed] [Google Scholar]
- 7.Misaghi A., Goldin A., Awad M., Kulidjian A.A. Osteosarcoma: a comprehensive review. Sicot J. 2018;4:12. doi: 10.1051/sicotj/2017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C., Xie L., Ren T., Huang Y., Xu J., Guo W. Immunotherapy for osteosarcoma: fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021;500:1–10. doi: 10.1016/j.canlet.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Gaebler M., Silvestri A., Haybaeck J., Reichardt P., Lowery C.D., Stancato L.F., Zybarth G., Regenbrecht C.R.A. Three-dimensional patient-derived in vitro sarcoma models: promising tools for improving clinical tumor management. Front. Oncol. 2017;7:203. doi: 10.3389/fonc.2017.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller M.M., Fusenig N.E. Friends or foes – bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer. 2004;4(11):839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 11.Liotta L.A., Kohn E.C. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 12.Turley S.J., Cremasco V., Astarita J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015;15(11):669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 13.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 14.Ishii G., Ochiai A., Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv. Drug Deliv. Rev. 2016;99(Pt B):186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhihao Z., Cheng J., Xiaoshuang Z., Yangguang M., Tingyu W., Yongyong Y., Zhou Y., Jie Z., Tao Z., Xueyu H., Zhe W. Cancer-associated fibroblast infiltration in osteosarcoma: the discrepancy in subtypes pathways and immunosuppression. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1136960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W., Shao Z. Research trends and hotspots in the immune microenvironment related to osteosarcoma and tumor cell aging: a bibliometric and visualization study. Front. Endocrinol. (Lausanne) 2023;14 doi: 10.3389/fendo.2023.1289319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y.M., Wang W., Qiu E.D. Osteosarcoma cells induce differentiation of mesenchymal stem cells into cancer associated fibroblasts through Notch and Akt signaling pathway. Int. J. Clin. Exp. Path. 2017;10(8):8479–8486. [PMC free article] [PubMed] [Google Scholar]
- 18.Pan C., Liu P., Ma D., Zhang S., Ni M., Fang Q., Wang J. Bone marrow mesenchymal stem cells in microenvironment transform into cancer-associated fibroblasts to promote the progression of B-cell acute lymphoblastic leukemia. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110610. [DOI] [PubMed] [Google Scholar]
- 19.Pietrovito L., Leo A., Gori V., Lulli M., Parri M., Becherucci V., Piccini L., Bambi F., Taddei M.L., Chiarugi P. Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Mol. Oncol. 2018;12(5):659–676. doi: 10.1002/1878-0261.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massagué J. TGFbeta in cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padgett R.W., Reiss M. TGFbeta superfamily signaling: notes from the desert. Development. 2007;134(20):3565–3569. doi: 10.1242/dev.005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan H.X., Cao Z.B., He T.T., Huang T., Xiang C.L., Liu Y. TGFβ1 is essential for MSCs-CAFs differentiation and promotes HCT116 cells migration and invasion via JAK/STAT3 signaling. OncoTargets Ther. 2019;12:5323–5334. doi: 10.2147/OTT.S178618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan H.X., Xiao Z.G., Huang T., Fang Z.X., Liu Y., Huang Z.C. CXCR4/TGF-β1 mediated self-differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and promoted colorectal carcinoma development. Cancer Biol. Ther. 2020;21(3):248–257. doi: 10.1080/15384047.2019.1685156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Xu Y., Qiu G., Luo Y., Bao Y., Lu J., Wang T., Wang Y. METTL3 mediated MALAT1 m6A modification promotes proliferation and metastasis in osteosarcoma cells. Mol. Biotechnol. 2023 doi: 10.1007/s12033-023-00953-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X., Yang Y., Li Y., Liang G., Kang D., Zhou B., Li Q. METTL3 contributes to osteosarcoma progression by increasing DANCR mRNA stability via m6A modification. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.784719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q., Geng W., Guo H., Wang Z., Xu K., Chen C., Wang S. Emerging role of RNA methyltransferase METTL3 in gastrointestinal cancer. J. Hematol. Oncol. 2020;13(1):57. doi: 10.1186/s13045-020-00895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song C., Zhou C. HOXA10 mediates epithelial-mesenchymal transition to promote gastric cancer metastasis partly via modulation of TGFB2/Smad/METTL3 signaling axis. J. Exp. Clin. Cancer Res. 2021;40(1):62. doi: 10.1186/s13046-021-01859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison D.J., Geller D.S., Gill J.D., Lewis V.O., Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 2018;18(1):39–50. doi: 10.1080/14737140.2018.1413939. [DOI] [PubMed] [Google Scholar]
- 29.Isakoff M.S., Bielack S.S., Meltzer P., Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J. Clin. Oncol. 2015;33(27):3029–3035. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pu F., Guo H., Shi D., Chen F., Peng Y., Huang X., Liu J., Zhang Z., Shao Z. The generation and use of animal models of osteosarcoma in cancer research. Genes Dis. 2024;11(2):664–674. doi: 10.1016/j.gendis.2022.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber C.E., Kothari A.N., Wai P.Y., Li N.Y., Driver J., Zapf M.A., Franzen C.A., Gupta G.N., Osipo C., Zlobin A., Syn W.K., Zhang J., Kuo P.C., Mi Z. Osteopontin mediates an MZF1-TGF-β1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene. 2015;34(37):4821–4833. doi: 10.1038/onc.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barcellos-de-Souza P., Comito G., Pons-Segura C., Taddei M.L., Gori V., Becherucci V., Bambi F., Margheri F., Laurenzana A., Del Rosso M., Chiarugi P. Mesenchymal stem cells are recruited and activated into carcinoma-associated fibroblasts by prostate cancer microenvironment-derived TGF-β1. Stem Cells. 2016;34(10):2536–2547. doi: 10.1002/stem.2412. [DOI] [PubMed] [Google Scholar]
- 33.Thorlacius-Ussing J., Jensen C., Nissen N.I., Cox T.R., Kalluri R., Karsdal M., Willumsen N. The collagen landscape in cancer: profiling collagens in tumors and in circulation reveals novel markers of cancer-associated fibroblast subtypes. J. Pathol. 2024;262(1):22–36. doi: 10.1002/path.6207. [DOI] [PubMed] [Google Scholar]
- 34.Akinjiyan F.A., Ibitoye Z., Zhao P., Shriver L.P., Patti G.J., Longmore G.D., Fuh K.C. DDR2-regulated arginase activity in ovarian cancer-associated fibroblasts promotes collagen production and tumor progression. Oncogene. 2023 doi: 10.1038/s41388-023-02884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saxena N., Chakraborty S., Dutta S., Bhardwaj G., Karnik N., Shetty O., Jadhav S., Zafar H., Sen S. Stiffness-dependent MSC homing and their differentiation to CAFs: implications in breast cancer invasion. J. Cell Sci. 2023 doi: 10.1242/jcs.261145. [DOI] [PubMed] [Google Scholar]
- 36.Sandberg T.P., Stuart M.P., Oosting J., Tollenaar R.A., Sier C.F., Mesker W.E. Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumor-stroma ratio in colorectal cancer. BMC Cancer. 2019;19(1):1–9. doi: 10.1186/s12885-019-5462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz-Otero N., Clinch A.B., Hope J., Wang W., Reinhart-King C.A., King M.R. Cancer associated fibroblasts confer shear resistance to circulating tumor cells during prostate cancer metastatic progression. Oncotarget. 2020;11(12):1037. doi: 10.18632/oncotarget.27510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louault K., Porras T., Lee M.H., Muthugounder S., Kennedy R.J., Blavier L., Sarte E., Fernandez G.E., Yang F., Pawel B.R., Shimada H., Asgharzadeh S., DeClerck Y.A. Fibroblasts and macrophages cooperate to create a pro-tumorigenic and immune resistant environment via activation of TGF-β/IL-6 pathway in neuroblastoma. Oncoimmunology. 2022;11(1) doi: 10.1080/2162402X.2022.2146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng Y., Dong H., Sun B., Hu Y., Yang Y., Jia Y., Jia L., Zhong X., Zhao R. METTL3/METTL14 transactivation and m(6)A-dependent TGF-β1 translation in activated Kupffer cells. Cell. Mol. Gastroenterol. Hepatol. 2021;12(3):839–856. doi: 10.1016/j.jcmgh.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Chen F., Peng Y., Lv Z., Lin X., Chen Z., Wang H. N6-methyladenosine regulates the expression and secretion of TGFβ1 to affect the epithelial-mesenchymal transition of cancer cells. Cells. 2020;9(2) doi: 10.3390/cells9020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y., Liu Z., Zhang Y., Wu X., Bian W., Shan S., Yang D., Ren T. METTL3-mediated m6A RNA methylation induces the differentiation of lung resident mesenchymal stem cells into myofibroblasts via the miR-21/PTEN pathway. Respir. Res. 2023;24(1):300. doi: 10.1186/s12931-023-02606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An X., Wu W., Yang L., Dong J., Liu B., Guo J., Chen J., Guo B., Cao W., Jiang Q. ZBTB7C m6A modification incurred by METTL3 aberration promotes osteosarcoma progression. Transl. Res. 2023;259:62–71. doi: 10.1016/j.trsl.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Zhou L., Yang C., Zhang N., Zhang X., Zhao T., Yu J. Silencing METTL3 inhibits the proliferation and invasion of osteosarcoma by regulating ATAD2. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109964. [DOI] [PubMed] [Google Scholar]
- 44.Li M., Zha X., Wang S. The role of N6-methyladenosine mRNA in the tumor microenvironment. Biochim. Biophys. Acta. 2021;1875(2) doi: 10.1016/j.bbcan.2021.188522. [DOI] [PubMed] [Google Scholar]