Abstract
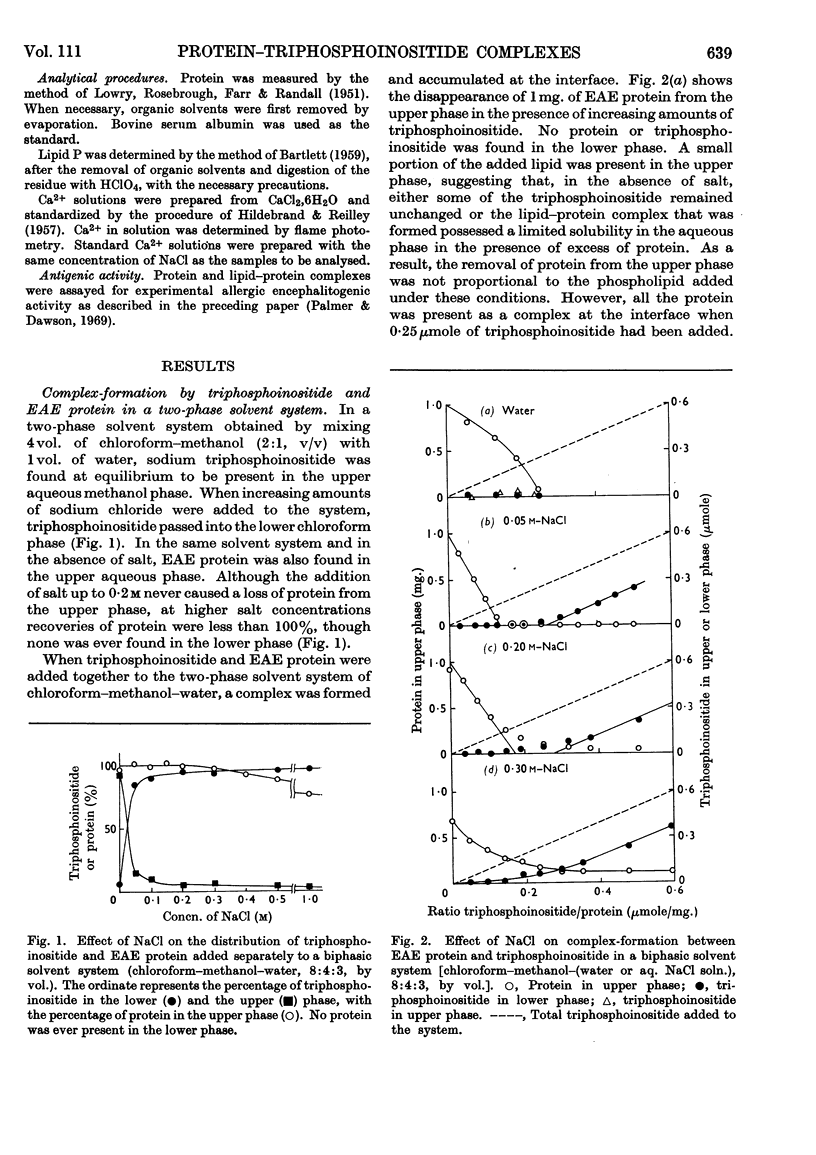
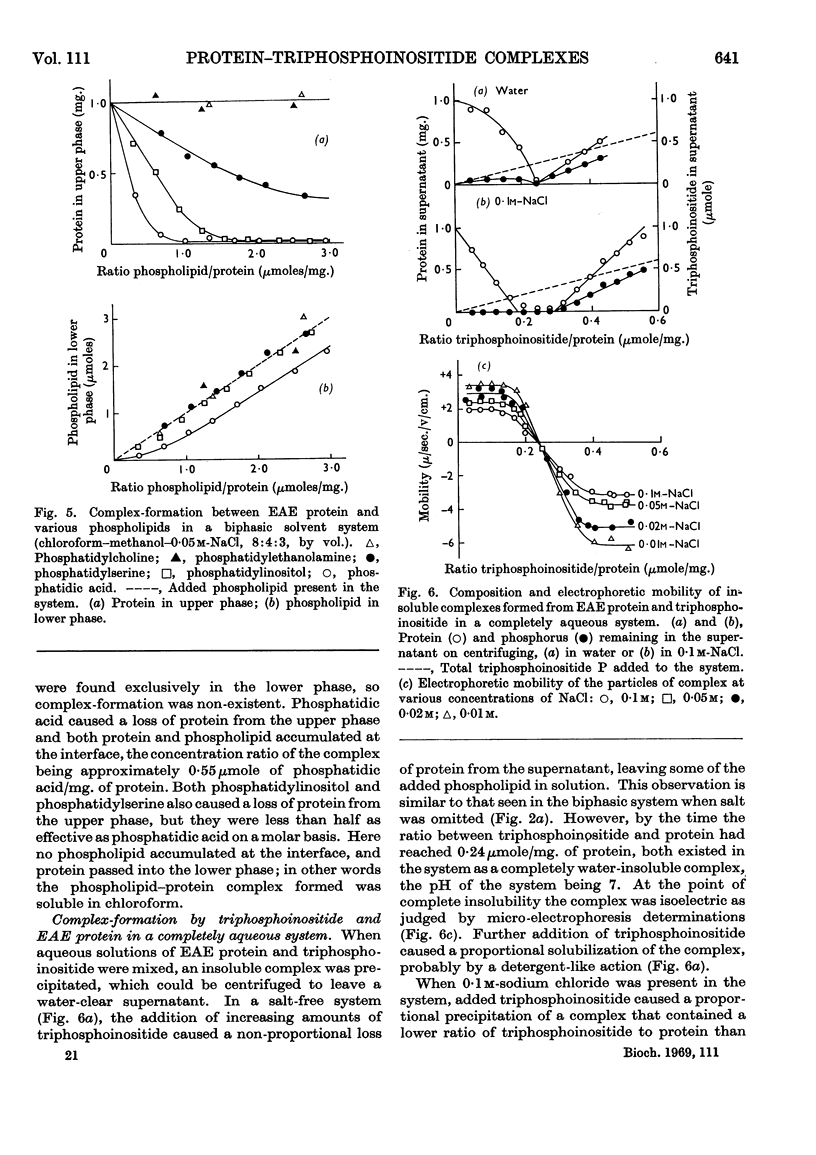
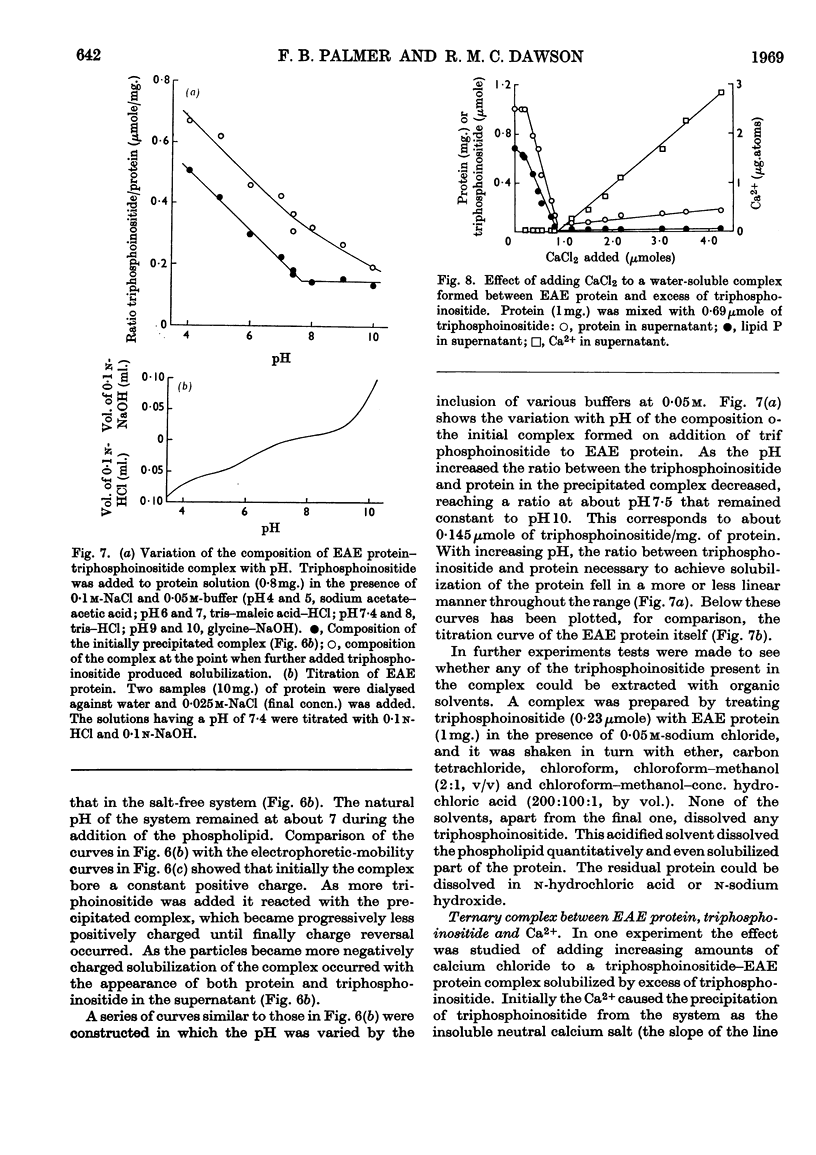
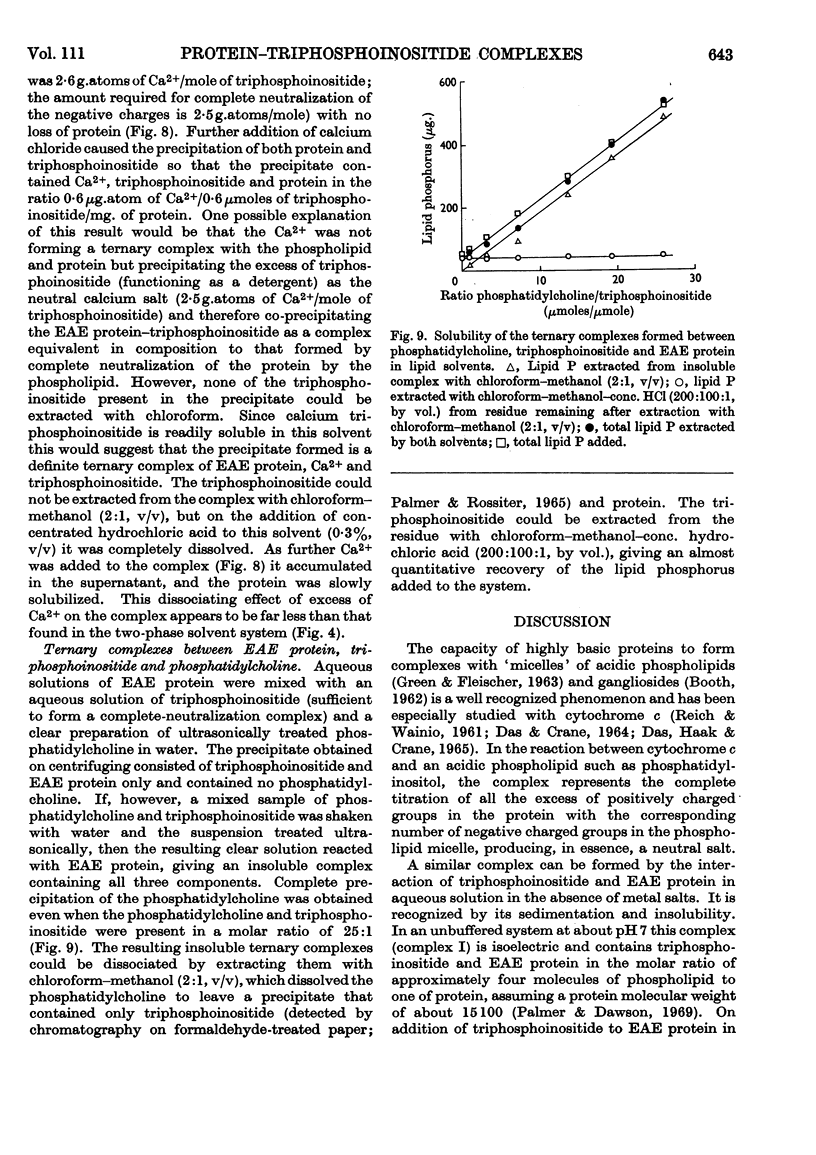
1. Reactions between triphosphoinositide and the basic experimental allergic encephalitogenic (EAE) protein were examined in aqueous solution and in a biphasic solvent system (chloroform–methanol–water, 8:4:3, by vol.). 2. In the absence of salt an insoluble complex (I) is formed containing triphosphoinositide and EAE protein in proportions that represent complete neutralization of lipid and protein at the pH concerned. 3. In the presence of a low concentration (0·05m) of sodium chloride an insoluble positively charged complex (II) forms. It contains triphosphoinositide and EAE protein in a lower concentration ratio than complex I. This complex, which has a constant composition between pH7·5 and pH10, can take up additional micellar triphosphoinositide producing complex I, which can then be solubilized by excess of triphosphoinositide. 4. The complexes are dissociated by more concentrated sodium chloride solutions and low concentrations of calcium chloride, suggesting that they are largely stabilized by electrostatic bonds. The protein recovered after dissociation is immunologically active and has the same electrophoretic mobility as the original. 5. Water-insoluble ternary complexes containing triphosphoinositide, EAE protein and large amounts of phosphatidylcholine can be prepared. From these, chloroform–methanol (2:1, v/v) extracts only phosphatidylcholine. 6. An insoluble ternary complex of Ca2+ ion, EAE protein and triphosphoinositide can be prepared by adding calcium chloride to a complex I preparation solubilized by excess of triphosphoinositide. 7. EAE protein will also form complexes with other acidic phospholipids, e.g. phosphatidic acid, phosphatidylserine and phosphatidylinositol, but not with phosphatidylcholine or phosphatidylethanolamine. The phosphatidylinositol and phosphatidylserine complexes are chloroform soluble, i.e. proteolipids. 8. The possibility that complexes between EAE protein and acidic phospholipids occur in vivo is discussed. Triphosphoinositide and EAE protein occur in ox brain myelin in approximately the same concentration ratios as they do in complex II, formed at physiological salt concentration and pH.
Full text
PDF

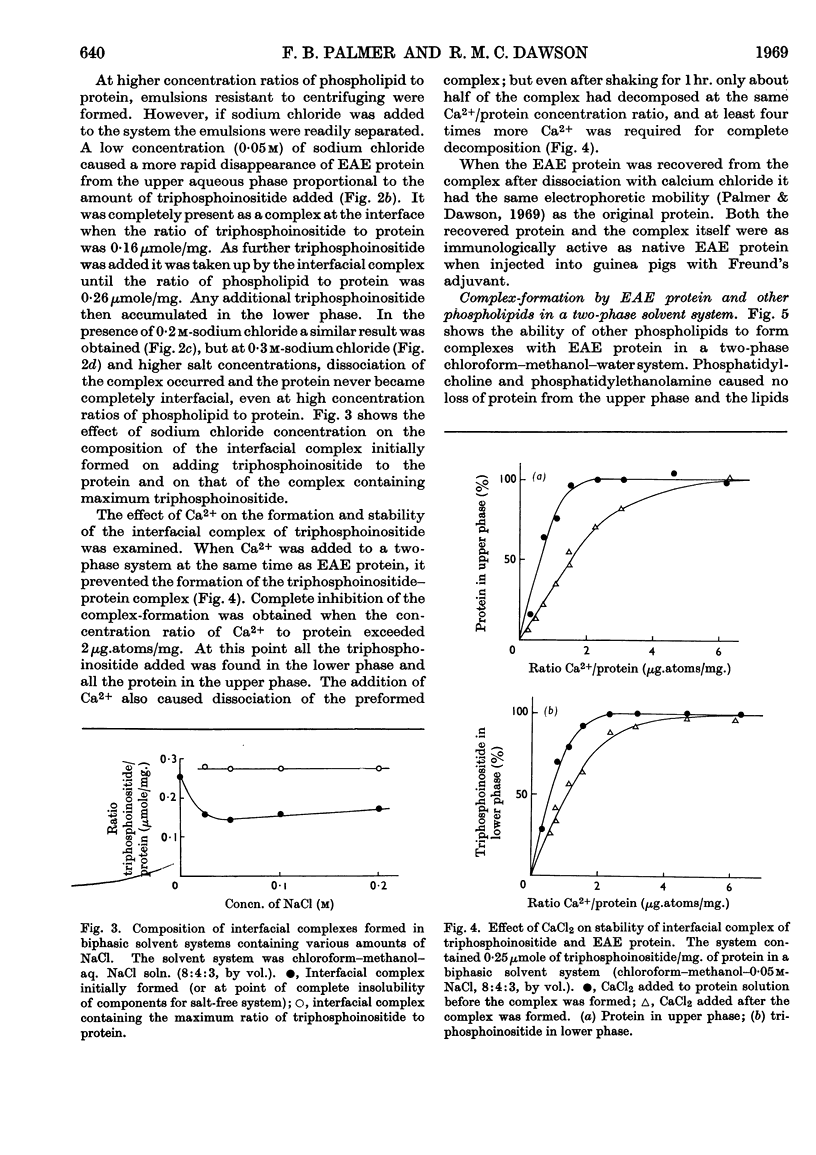



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM A. D., DAWSON R. M. The relation between the activity of a lecithinase and the electrophoretic charge of the substrate. Biochem J. 1959 Jul;72:486–492. doi: 10.1042/bj0720486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BOOTH D. A. The isolation and assay of gangliosides and their interactions with basic proteins. J Neurochem. 1962 May-Jun;9:265–276. doi: 10.1111/j.1471-4159.1962.tb09448.x. [DOI] [PubMed] [Google Scholar]
- DAS M. L., CRANE F. L. PROTEOLIPIDS. I. FORMATION OF PHOSPHOLIPID-CYTOCHROME C COMPLEXES. Biochemistry. 1964 May;3:696–700. doi: 10.1021/bi00893a017. [DOI] [PubMed] [Google Scholar]
- DAS M. L., HAAK E. D., CRANE F. L. PROTEOLIPIDS. IV. FORMATION OF COMPLEXES BETWEEN CYTOCHROME C AND PURIFIED PHOSPHOLIPIDS. Biochemistry. 1965 May;4:859–865. doi: 10.1021/bi00881a010. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. ON THE MECHANISM OF ACTION OF PHOSPHOLIPASE A. Biochem J. 1963 Sep;88:414–423. doi: 10.1042/bj0880414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., DAWSON R. M. The isolation of a new lipid, triphosphoinositide, and monophosphoinositide from ox brain. Biochem J. 1961 Dec;81:535–540. doi: 10.1042/bj0810535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M. 'Phosphatido-peptide'-like complexes formed by the interaction of calcium triphosphoinositide with protein. Biochem J. 1965 Oct;97(1):134–138. doi: 10.1042/bj0970134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Eichberg J. Diphosphoinositide and triphosphoinositide in animal tissues. Extraction, estimation and changes post mortem. Biochem J. 1965 Sep;96(3):634–643. doi: 10.1042/bj0960634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. Some properties of purified phospholipase D and especially the effect of amphipathic substances. Biochem J. 1967 Jan;102(1):76–86. doi: 10.1042/bj1020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberg J., Dawson R. M. Polyphosphoinositides in myelin. Biochem J. 1965 Sep;96(3):644–650. doi: 10.1042/bj0960644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Imai Y., Sato R. Conversion of P-450 to P-420 by neutral salts and some other reagents. Eur J Biochem. 1967 Jun;1(4):419–426. doi: 10.1007/978-3-662-25813-2_57. [DOI] [PubMed] [Google Scholar]
- Kornguth S. E., Anderson J. W. Localization of a basic protein in the myelin of various species with the aid of fluorescence and electron microscopy. J Cell Biol. 1965 Jul;26(1):157–166. doi: 10.1083/jcb.26.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lumsden C. E., Robertson D. M., Blight R. Chemical studies on experimental allergic encephalomyelitis. Peptide as the common denominator in all encephalitogenic 'antigens'. J Neurochem. 1966 Mar;13(3):127–162. doi: 10.1111/j.1471-4159.1966.tb07507.x. [DOI] [PubMed] [Google Scholar]
- Martenson R. E., LeBaron F. N. Studies on the acid-extractable proteins of bovine brain white matter. J Neurochem. 1966 Dec;13(12):1469–1479. doi: 10.1111/j.1471-4159.1966.tb04308.x. [DOI] [PubMed] [Google Scholar]
- Palmer F. B., Dawson R. M. The isolation and properties of experimental allergic encephalitogenic protein. Biochem J. 1969 Mar;111(5):629–636. doi: 10.1042/bj1110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer F. B., Rossiter R. J. A simple procedure for the study of inositol phosphatides in cat brain slices. Can J Biochem. 1965 Jun;43(6):671–683. doi: 10.1139/o65-078. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Miller N. Phospholipid model membranes. I. Structural characteristics of hydrated liquid crystals. Biochim Biophys Acta. 1967 Sep 9;135(4):624–638. doi: 10.1016/0005-2736(67)90094-6. [DOI] [PubMed] [Google Scholar]
- REICH M., WAINIO W. A cytochrome c-phospholipid complex. J Biol Chem. 1961 Nov;236:3058–3061. [PubMed] [Google Scholar]
- Sheltawy A., Dawson R. M. The polyphosphoinositides and other lipids of peripheral nerves. Biochem J. 1966 Jul;100(1):12–18. doi: 10.1042/bj1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON E. B., KIES M. W. CURRENT STUDIES ON THE LIPIDS AND PROTEINS OF MYELIN. Ann N Y Acad Sci. 1965 Mar 31;122:129–147. doi: 10.1111/j.1749-6632.1965.tb20198.x. [DOI] [PubMed] [Google Scholar]
- VON HIPPEL P. H., WONG K. Y. The effect of ions on the kinetics of formation and the stability of the collagenfold. Biochemistry. 1962 Jul;1:664–674. doi: 10.1021/bi00910a020. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., Wong K. Y. On the conformational stability of globular proteins. The effects of various electrolytes and nonelectrolytes on the thermal ribonuclease transition. J Biol Chem. 1965 Oct;240(10):3909–3923. [PubMed] [Google Scholar]


