Abstract
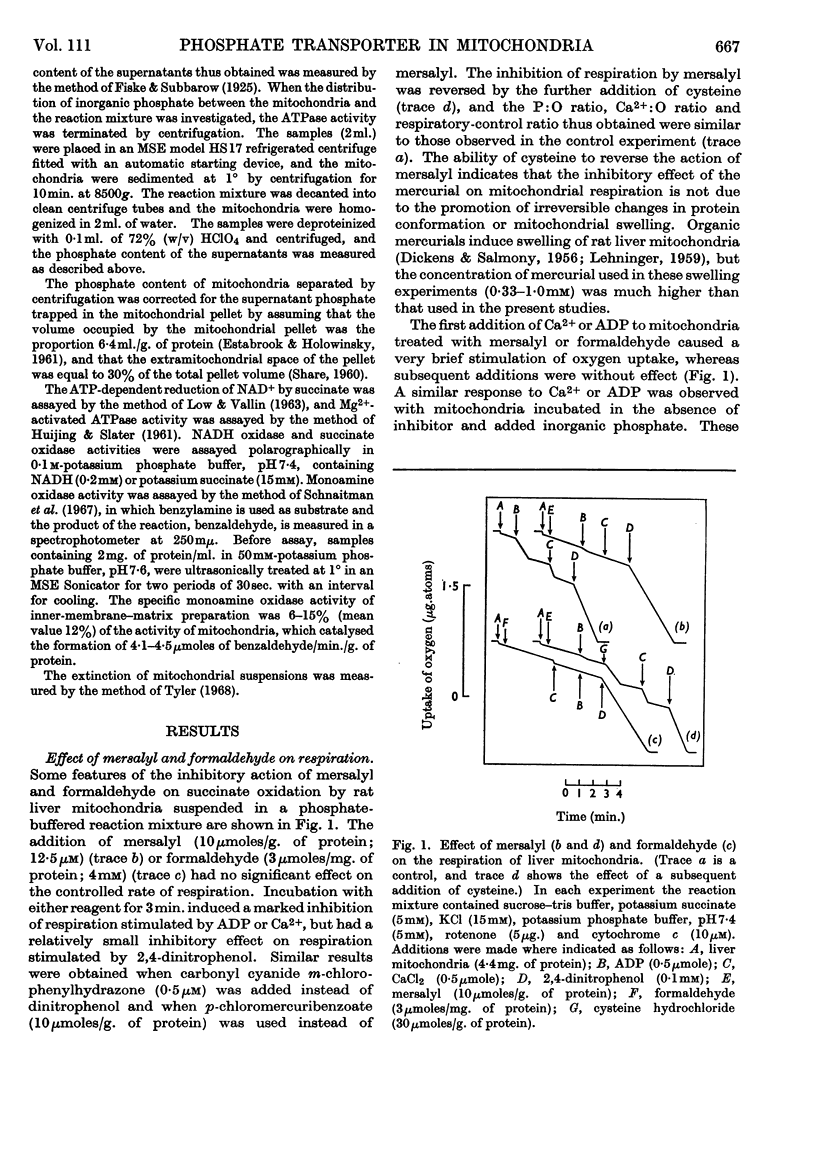
1. The organic mercurial sodium mersalyl, formaldehyde, dicyclohexylcarbodiimide and tributyltin each blocked respiratory-chain-linked ATP synthesis in rat liver mitochondria. 2. Mersalyl and formaldehyde also blocked a number of other processes dependent on the entry of inorganic phosphate into mitochondria, including mitochondrial respiration and swelling stimulated by cations and phosphate, the substrate-level phosphorylation reaction of the citric acid cycle, and swelling in ammonium phosphate. 3. Dicyclohexylcarbodi-imide and tributyltin did not inhibit the entry of phosphate into mitochondria. 4. Mersalyl and formaldehyde had a relatively slight effect on succinate oxidation and swelling stimulated by cations when phosphate was replaced by acetate, on succinate oxidation stimulated by uncoupling agents, and on swelling in solutions of ammonium salts other than phosphate or arsenate. 5. Formaldehyde blocked the oxidation of NAD-linked substrates in mitochondria treated with 2,4-dinitrophenol and the ATP-dependent reduction of NAD by succinate catalysed by ox heart submitochondrial particles. Both these effects appear to be due to an inhibition by formaldehyde of the NAD–flavin region of the respiratory chain. 6. Concentrations of dicyclohexylcarbodiimide or tributyltin sufficient to abolish ADP-stimulated respiration blocked the dinitrophenol-stimulated adenosine triphosphatase activity, whereas mersalyl and formaldehyde caused only partial inhibition of ATP hydrolysis. 7. When mitochondria were incubated with dinitrophenol and ATP, less than 10% of the total inorganic phosphate liberated was recovered in the mitochondria and no swelling occurred. In the presence of mersalyl or formaldehyde at least 80% of the total inorganic phosphate liberated was retained in the mitochondria and extensive swelling was observed. This swelling was inhibited by oligomycin but not by antimycin or rotenone. 8. The addition of mersalyl to mitochondria swollen by treatment with valinomycin, K+ and phosphate blocked the contraction induced by dinitrophenol and caused an increase in the phosphate content of the mitochondria, but had no effect on the contraction of mitochondria when phosphate was replaced by acetate. 9. It is concluded that mitochondria contain a phosphate-transporter system, which catalyses the movement of phosphate in either direction across the mitochondrial membrane, and that this system is inactivated by organic mercurials and by formaldehyde. Evidence is presented that the phosphate-transporter system is situated in the inner membrane of rat liver mitochondria and is also present in other types of mammalian mitochondria.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beechey R. B., Holloway C. T., Knight I. G., Roberton A. M. Dicyclohexylcarbodiimide--an inhibitor of oxidative phosphorylation. Biochem Biophys Res Commun. 1966 Apr 6;23(1):75–80. doi: 10.1016/0006-291x(66)90271-3. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Roberton A. M., Holloway C. T., Knight I. G. The properties of dicyclohexylcarbodiimide as an inhibitor of oxidative phosphorylation. Biochemistry. 1967 Dec;6(12):3867–3879. doi: 10.1021/bi00864a033. [DOI] [PubMed] [Google Scholar]
- Bygrave F. L., Lehninger A. L. The affinity of mitochondrial oxidative phosphorylation mechanisms for phosphate and adenosine diphosphate. Proc Natl Acad Sci U S A. 1967 May;57(5):1409–1415. doi: 10.1073/pnas.57.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPPELL J. B., GREVILLE G. D. Effects of oligomycin on respiration and swelling of isolated liver mitochondria. Nature. 1961 May 6;190:502–504. doi: 10.1038/190502a0. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B. The effect of alkylguanidines on mitochondrial metabolism. J Biol Chem. 1963 Jan;238:410–417. [PubMed] [Google Scholar]
- CRIDDLE R. S., BOCK R. M., GREEN D. E., TISDALE H. Physical characteristics of proteins of the electron transfer system and interpretation of the structure of the mitochondrion. Biochemistry. 1962 Sep;1:827–842. doi: 10.1021/bi00911a015. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKENS F., SALMONY D. Effects of thyroid hormones in vitro on tissue respiration, oxidative phosphorylation and the swelling of mitochondria. Biochem J. 1956 Dec;64(4):645–651. doi: 10.1042/bj0640645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W., HOLOWINSKY A. Studies on the content and organization of the respiratory enzymes of mitochondria. J Biophys Biochem Cytol. 1961 Jan;9:19–28. doi: 10.1083/jcb.9.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ-MORAN H. Cell-membrane ultrastructure. Low-temperature electron microsopy and x-ray diffraction studies of lipoprotein components in lamellar systems. Circulation. 1962 Nov;26:1039–1065. doi: 10.1161/01.cir.26.5.1039. [DOI] [PubMed] [Google Scholar]
- Fonyo A., Bessman S. P. The action of oligomycin and of para-hydroxymercuribenzoate on mitochondrial respiration stimulated by ADP, arsenate and calcium. Biochem Biophys Res Commun. 1966 Jul 6;24(1):61–66. doi: 10.1016/0006-291x(66)90410-4. [DOI] [PubMed] [Google Scholar]
- GAMBLE J. L., Jr ACCUMULATION OF CITRATE AND MALATE BY MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2668–2672. [PubMed] [Google Scholar]
- HULTIN H. O., RICHARDSON S. H. THE BINDING OF PHOSPHATE, PYROPHOSPHATE, AND NUCLEOTIDES TO THE STRUCTURAL PROTEIN OF BEEF HEART MITOCHONDRIA. Arch Biochem Biophys. 1964 May;105:288–296. doi: 10.1016/0003-9861(64)90009-8. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Höfer M. P., Pressman B. C. Stimulation of mitochondrial respiration and phosphorylation by transport-inducing antibiotics. Biochemistry. 1967 May;6(5):1348–1360. doi: 10.1021/bi00857a018. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. IX. Reconstruction of oligomycin-sensitive adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2467–2474. [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L. Reversal of various types of mitochondrial swelling by adenosine triphosphate. J Biol Chem. 1959 Sep;234:2465–2471. [PubMed] [Google Scholar]
- MINAKAMI S., SCHINDLER F. J., ESTABROOK R. W. HYDROGEN TRANSFER BETWEEN REDUCED DIPHOSPHOPYRIDINE NUCLEOTIDE DEHYDROGENASE AND THE RESPIRATORY CHAIN. I. EFFECT OF SULFHYDRYL INHIBITORS AND PHOSPHOLIPASE. J Biol Chem. 1964 Jun;239:2042–2048. [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Transport of phosphate across the osmotic barrier of Micrococcus pyogenes; specificity and kinetics. J Gen Microbiol. 1954 Aug;11(1):73–82. doi: 10.1099/00221287-11-1-73. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Transport of phosphate across the surface of Micrococcus pyogenes; nature of the cell inorganic phosphate. J Gen Microbiol. 1953 Oct;9(2):273–287. doi: 10.1099/00221287-9-2-273. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F., Klingenberg M. Inhibition of respiration under the control of azide uptake by mitochondria. Eur J Biochem. 1967 Jun;1(4):439–446. doi: 10.1007/978-3-662-25813-2_60. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER W. C. Biochemical constitution of mammalian mitochondria. J Histochem Cytochem. 1953 Jul;1(4):212–233. doi: 10.1177/1.4.212. [DOI] [PubMed] [Google Scholar]
- SHARE L. Volumes of distribution of hemoglobin, of [14C] carboxypolyglucose and of [14C]sucrose in pellets of rat-liver mitochondria. Biochim Biophys Acta. 1960 Feb 12;38:154–155. doi: 10.1016/0006-3002(60)91209-9. [DOI] [PubMed] [Google Scholar]
- Schatz G., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VII. Oxidative phosphorylation in the diphosphopyridine nucleotide-cytochrome b segment of the respiratory chain: assay and properties in submitochondrial particles. J Biol Chem. 1966 Mar 25;241(6):1429–1438. [PubMed] [Google Scholar]
- Schnaitman C. A., Pedersen P. L. Localization of oligomycin-sensitive ADP-ATP exchange activity in rat liver mitochondria. Biochem Biophys Res Commun. 1968 Feb 26;30(4):428–433. doi: 10.1016/0006-291x(68)90762-6. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYLER D. D., BUTOW R. A., GONZE J., ESTABROOK R. W. EVIDENCE FOR THE EXISTENCE AND FUNCTION OF AN OCCULT, HIGHLY REACTIVE SULPHYDRYL GROUP IN THE RESPIRATORY CHAIN DPNH DEHYDROGENASE. Biochem Biophys Res Commun. 1965 May 3;19:551–557. doi: 10.1016/0006-291x(65)90161-0. [DOI] [PubMed] [Google Scholar]
- Tyler D. D. The inhibition of phosphate entry into rat liver mitochondria by organic mercurials and by formaldehyde. Biochem J. 1968 Mar;107(1):121–123. doi: 10.1042/bj1070121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk J. J., Frisell W. R. Inhibition by formaldehyde of energy transfer and related processes in rat-liver mitochondria. Biochim Biophys Acta. 1967 Sep 6;143(2):292–298. doi: 10.1016/0005-2728(67)90083-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Chance B. Reversal of azide inhibition by uncouplers. Biochem Biophys Res Commun. 1966 Jun 13;23(5):751–756. doi: 10.1016/0006-291x(66)90465-7. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Bygrave F. L., Lehninger A. L. Characterization of the atractyloside-sensitive adenine nucleotide transport system in rat liver mitochondria. J Biol Chem. 1968 Jan 10;243(1):20–28. [PubMed] [Google Scholar]
- Woodward D. O., Munkres K. D. Alterations of a maternally inherited mitochondrial structural protein in respiratory-deficient strains of Neurospora. Proc Natl Acad Sci U S A. 1966 Apr;55(4):872–880. doi: 10.1073/pnas.55.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]


