Abstract
1. Inhibition of ox liver glutamate dehydrogenase with N-(N′-acetyl-4[35S]-sulphamoylphenyl)maleimide (ASPM) is more specific at pH7·3 than at pH6·9. At pH7·3 inhibition accompanies the incorporation at 1 mole of ASPM residues into about 53000g. of protein. 2. Digestion of the modified protein with chymotrypsin and trypsin yields a unique radioactive peptide. 3. Acid hydrolysis of 1 mole of this peptide yields 1 mole of N∈-succin-2-yl-lysine. The ∈-amino group of a lysyl residue is thus the site of modification of the protein. 4. The sequence containing the modified lysyl residue is: [Formula: see text] where Asx respresents either aspartic acid or asparagine.
Full text
PDF
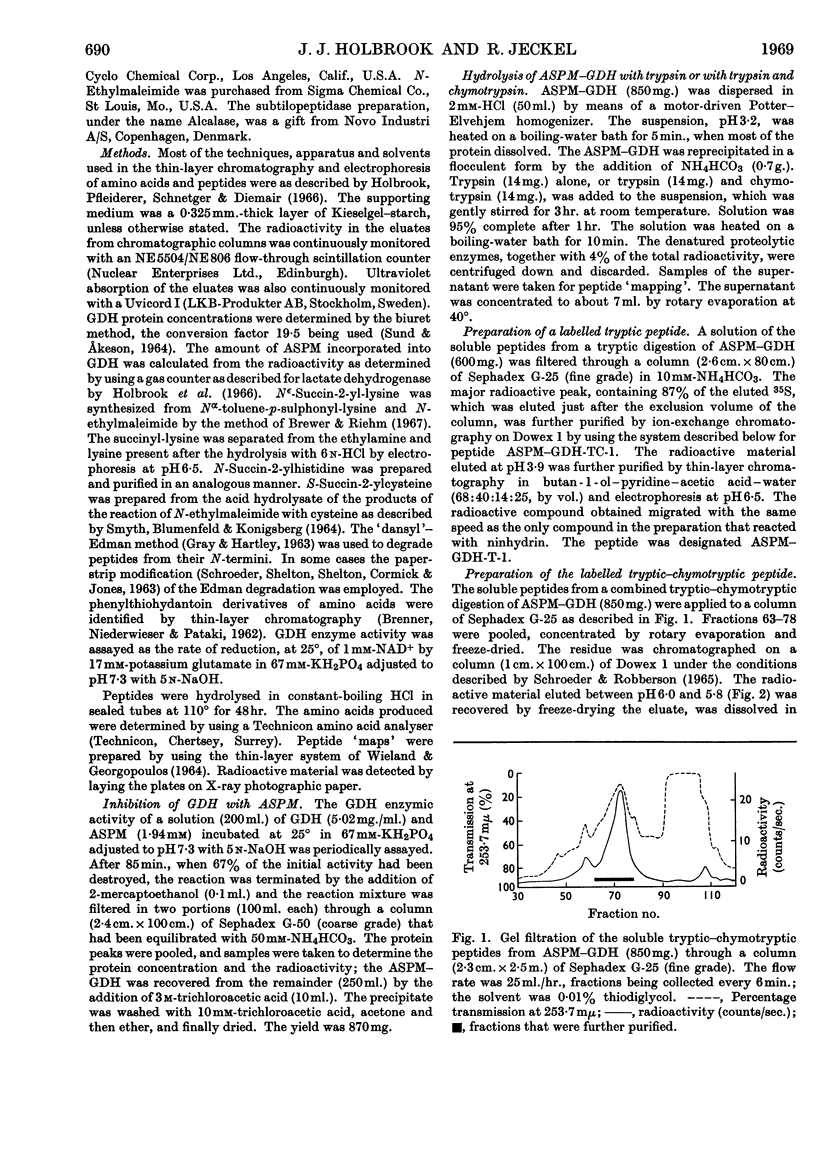
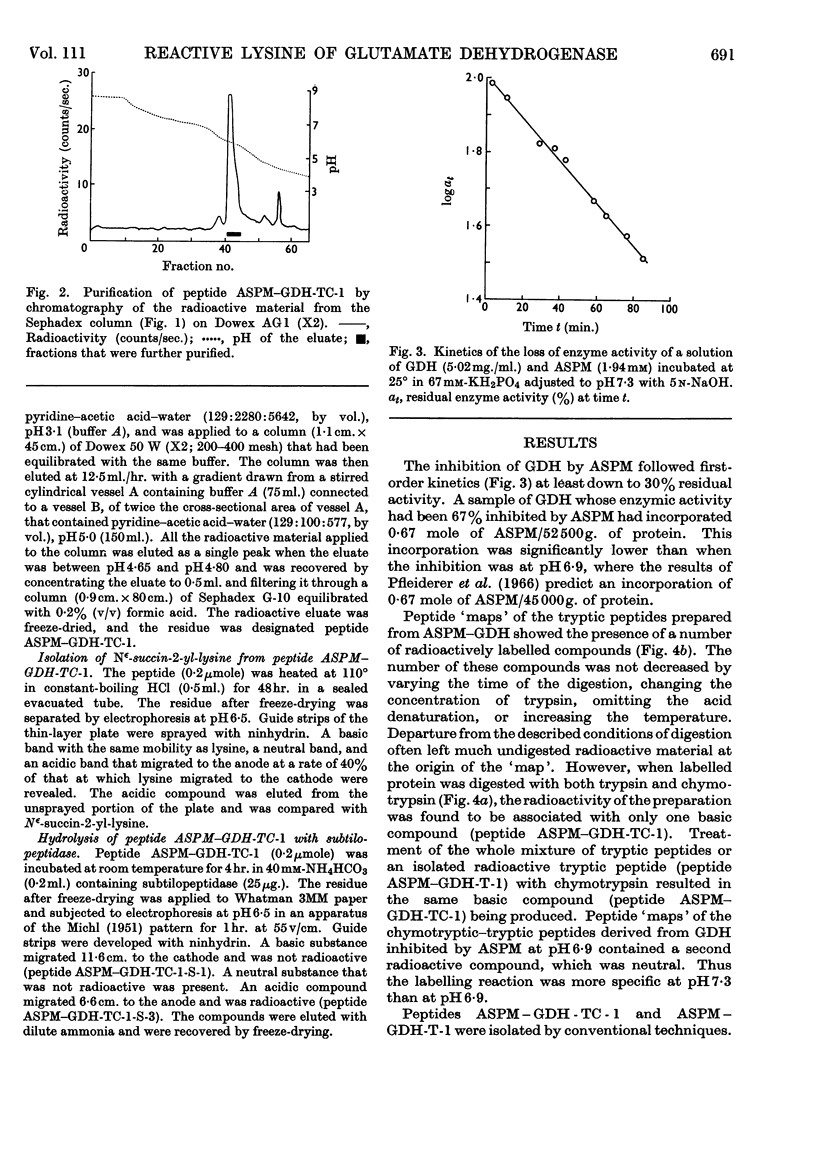
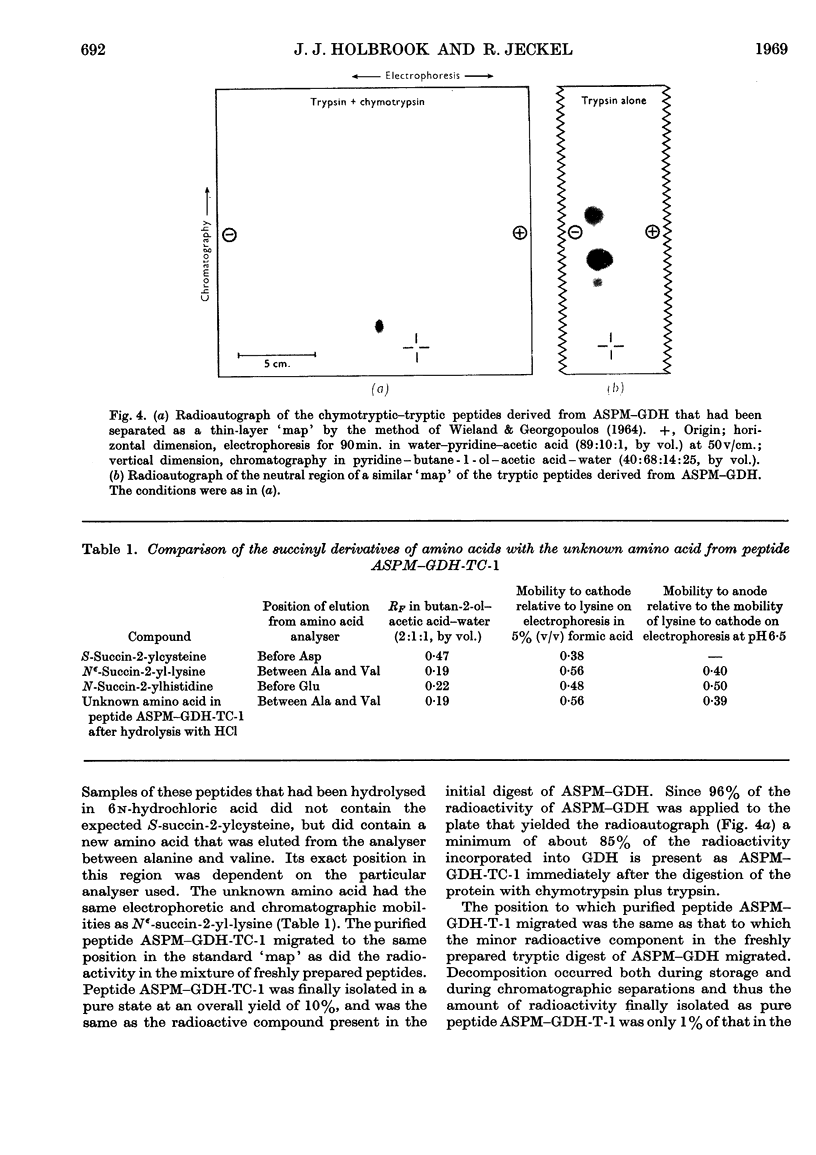
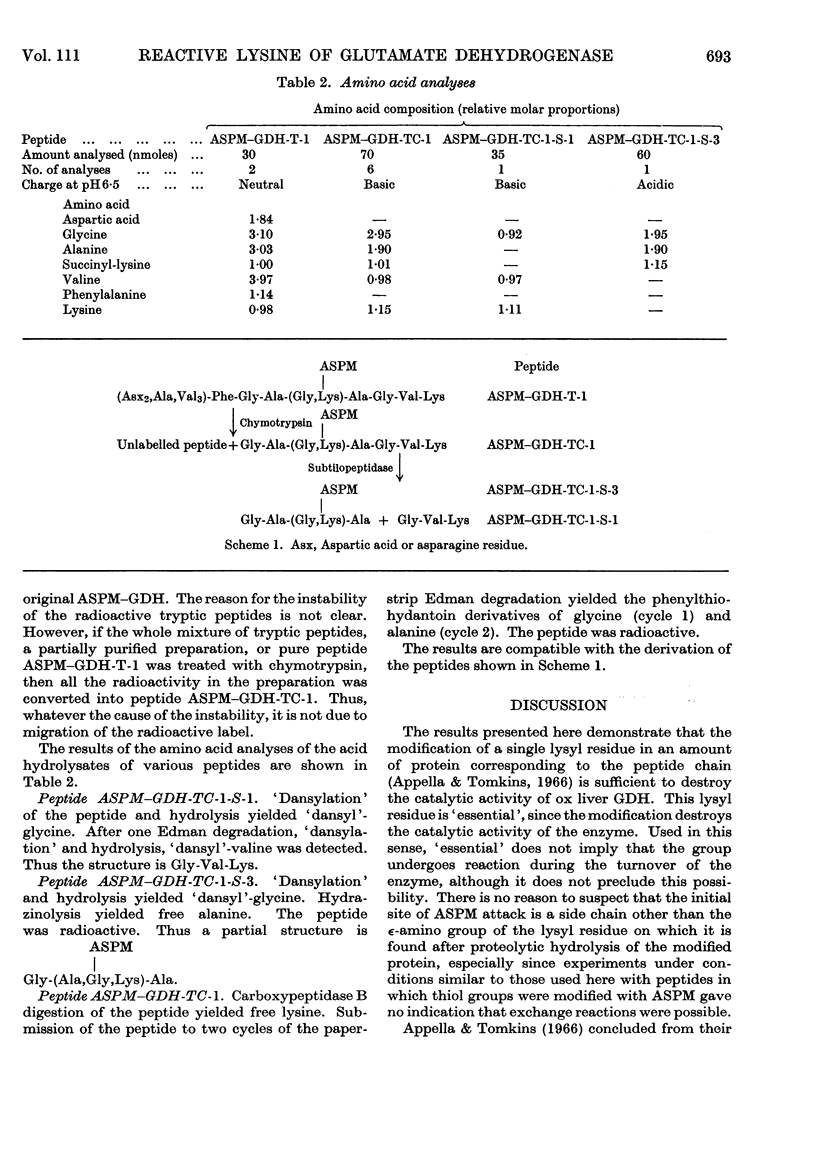

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. M., Anderson C. D., Churchich J. E. Inhibition of glutamic dehydrogenase by pyridoxal 5'-phosphate. Biochemistry. 1966 Sep;5(9):2893–2900. doi: 10.1021/bi00873a017. [DOI] [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. I. Effect of acetylation on catalytic and regulatory properties. J Biol Chem. 1966 Aug 25;241(16):3652–3660. [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. II. Effect of acetylation on molecular properties. J Biol Chem. 1966 Aug 25;241(16):3661–3670. [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Pfleiderer G., Mella K., Volz M., Leskowac W., Jeckel R. The importance of SH-groups for enzymic activity. 7. The amino acid sequence around the essential SH-group of pig heart lactate dehydrogenase, isoenzyme I. Eur J Biochem. 1967 Jun;1(4):476–481. doi: 10.1111/j.1432-1033.1967.tb00095.x. [DOI] [PubMed] [Google Scholar]
- SCHROEDER W. A., SHELTON J. R., SHELTON J. B., CORMICK J., JONES R. T. THE AMINO ACID SEQUENCE OF THE GAMMA CHAIN OF HUMAN FETAL HEMOGLOBIN. Biochemistry. 1963 Sep-Oct;2:992–1008. doi: 10.1021/bi00905a016. [DOI] [PubMed] [Google Scholar]
- SUND H., AKESON A. DIE AMINOSAEUREZUSAMMENSETZUNG DER GLUTAMINSAEUREDEHYDROGENASE AUS RINDERLEBER. Biochem Z. 1964 Sep 28;340:421–435. [PubMed] [Google Scholar]
- Schroeder W. A., Robberson B. An improved gradient for ion exchange chromatography of peptides on Dowex-1. Anal Chem. 1965 Nov;37(12):1583–1585. doi: 10.1021/ac60231a036. [DOI] [PubMed] [Google Scholar]
- Sharpless N. E., Flavin M. The reactions of amines and amino acids with maleimides. Structure of the reaction products deduced from infrared and nuclear magnetic resonance spectroscopy. Biochemistry. 1966 Sep;5(9):2963–2971. doi: 10.1021/bi00873a028. [DOI] [PubMed] [Google Scholar]
- Smyth D. G., Blumenfeld O. O., Konigsberg W. Reactions of N-ethylmaleimide with peptides and amino acids. Biochem J. 1964 Jun;91(3):589–595. doi: 10.1042/bj0910589. [DOI] [PMC free article] [PubMed] [Google Scholar]



