Abstract
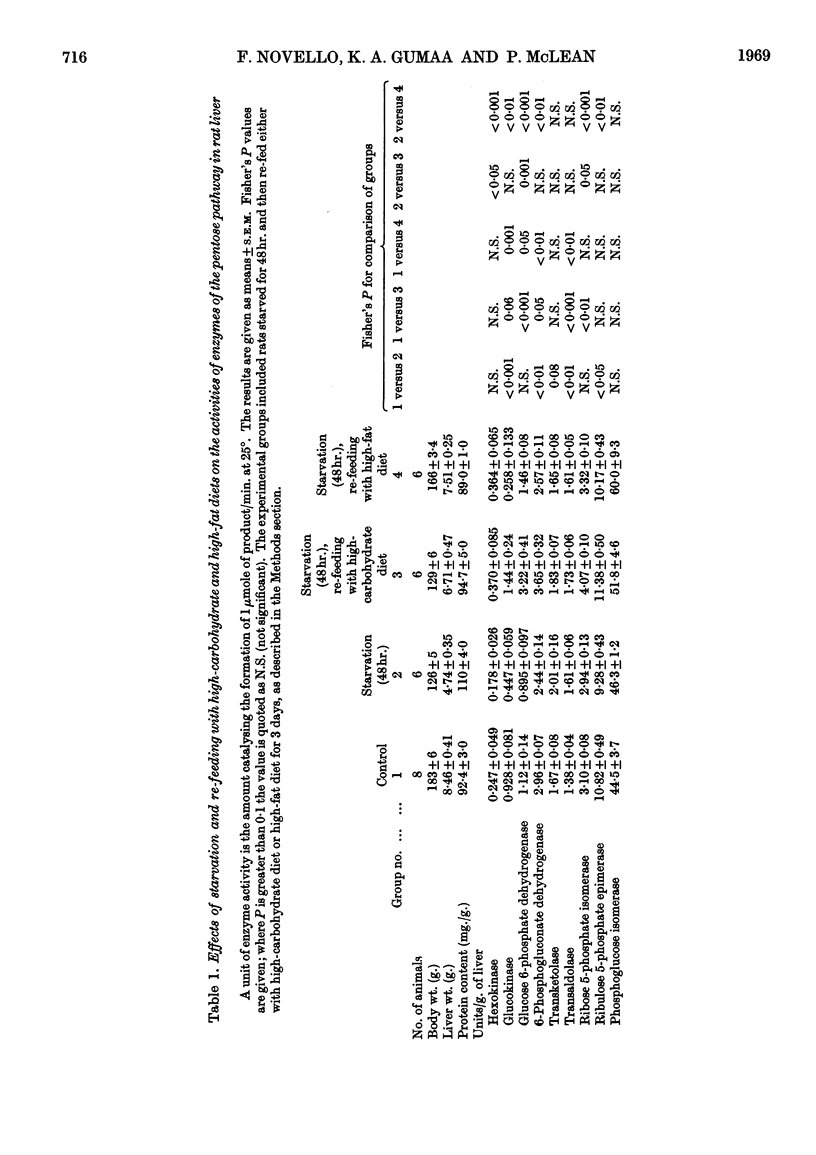
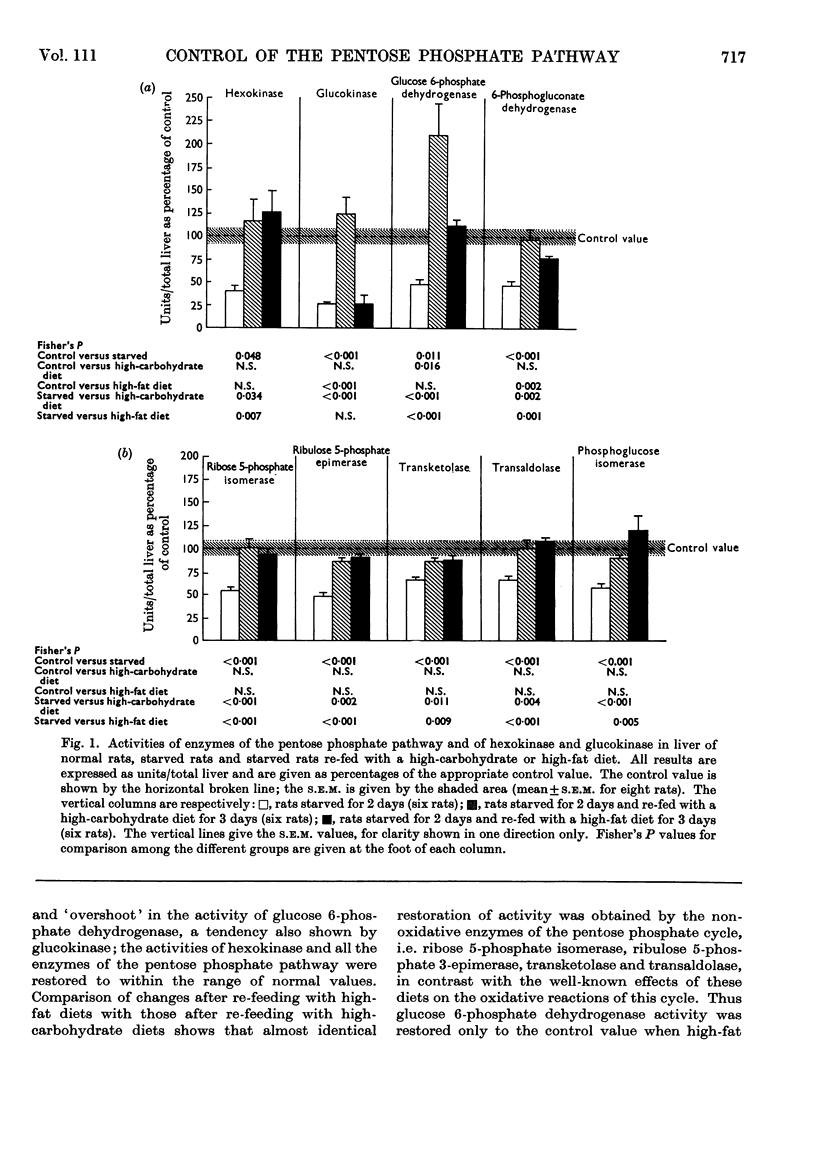
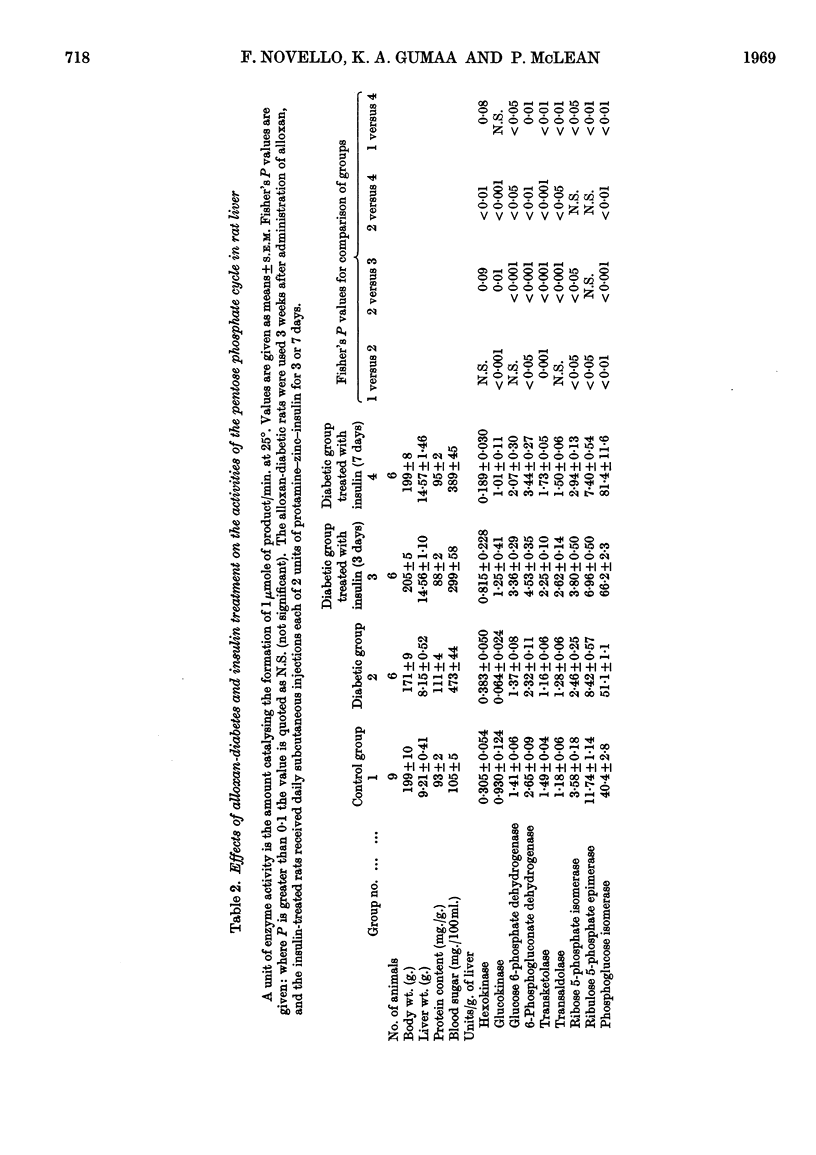
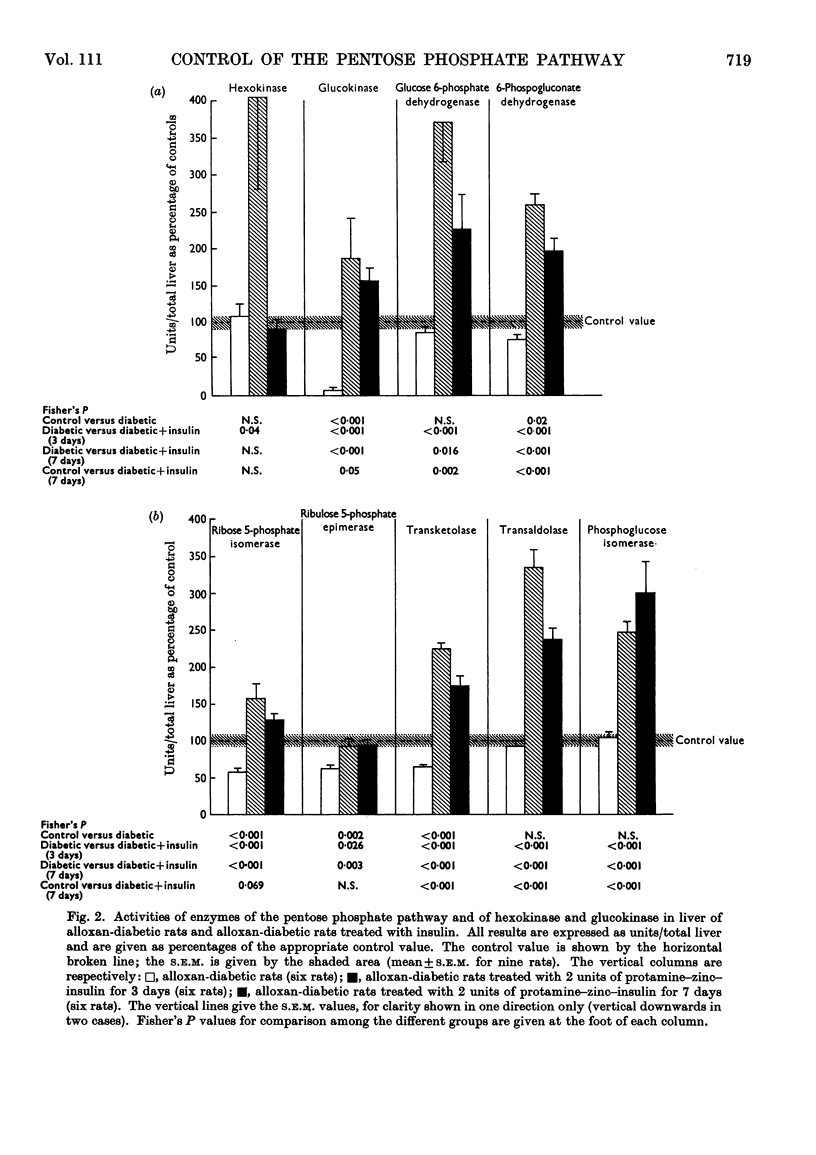
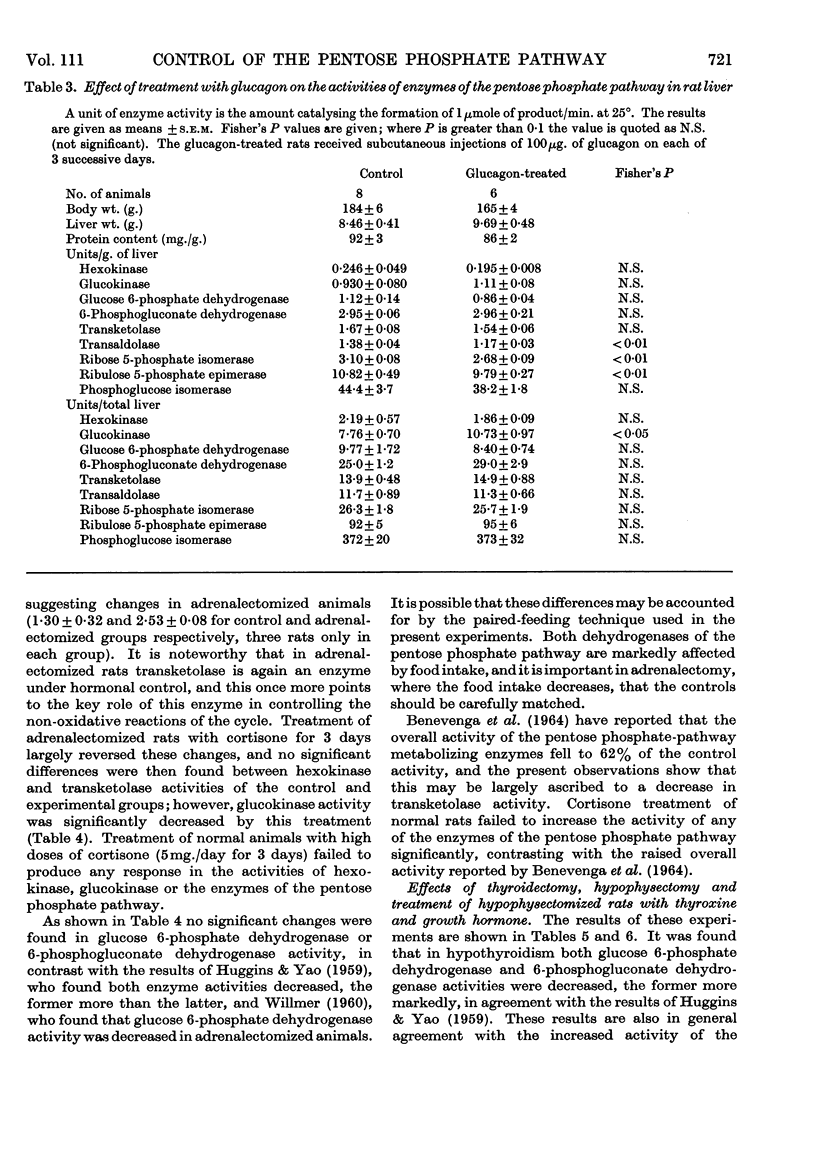
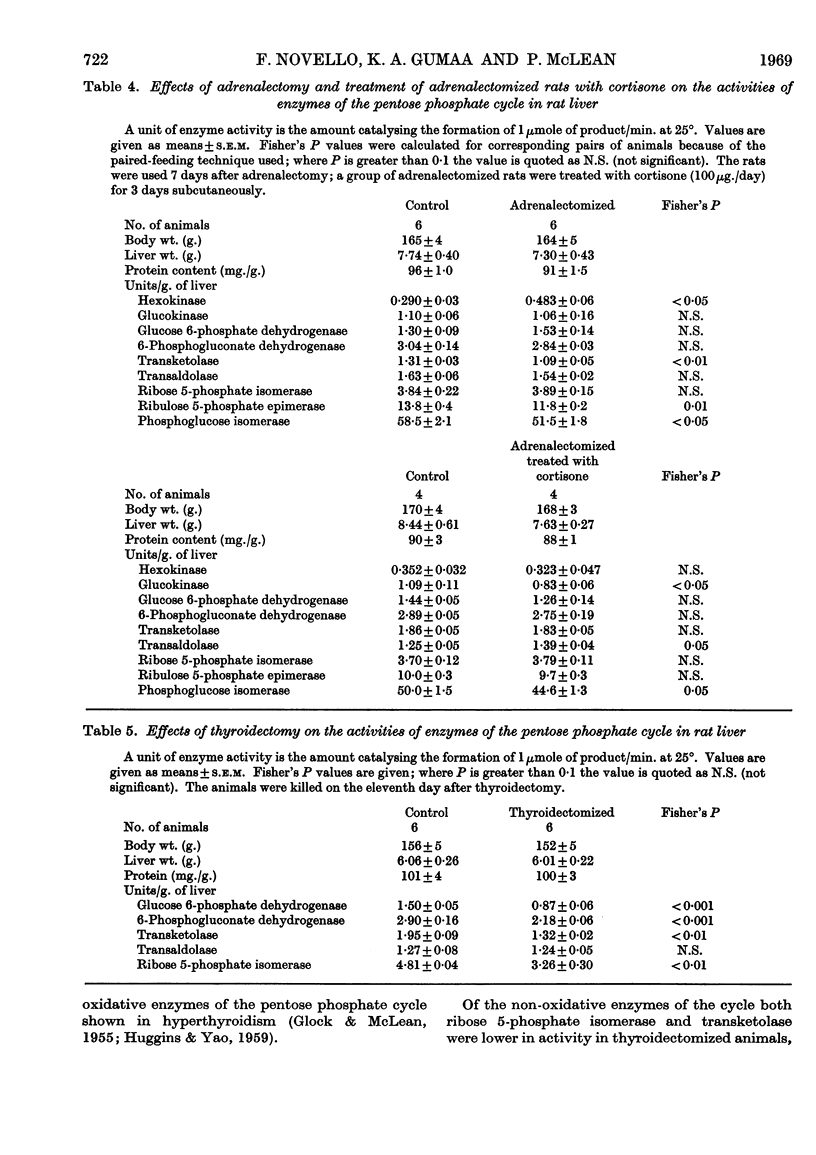
1. Measurements were made of the non-oxidative reactions of the pentose phosphate cycle in liver (transketolase, transaldolase, ribulose 5-phosphate epimerase and ribose 5-phosphate isomerase activities) in a variety of hormonal and nutritional conditions. In addition, glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase activities were measured for comparison with the oxidative reactions of the cycle; hexokinase, glucokinase and phosphoglucose isomerase activities were also included. Starvation for 2 days caused significant lowering of activity of all the enzymes of the pentose phosphate cycle based on activity in the whole liver. Re-feeding with a high-carbohydrate diet restored all the enzyme activities to the range of the control values with the exception of that of glucose 6-phosphate dehydrogenase, which showed the well-known `overshoot' effect. Re-feeding with a high-fat diet also restored the activities of all the enzymes of the pentose phosphate cycle and of hexokinase; glucokinase activity alone remained unchanged. Expressed as units/g. of liver or units/mg. of protein hexokinase, glucose 6-phosphate dehydrogenase, transketolase and pentose phosphate isomerase activities were unchanged by starvation; both 6-phosphogluconate dehydrogenase and ribulose 5-phosphate epimerase activities decreased faster than the liver weight or protein content. 2. Alloxan-diabetes resulted in a decrease of approx. 30–40% in the activities of 6-phosphogluconate dehydrogenase, ribose 5-phosphate isomerase, ribulose 5-phosphate epimerase and transketolase; in contrast with this glucose 6-phosphate dehydrogenase, transaldolase and phosphoglucose isomerase activities were unchanged. Treatment of alloxan-diabetic rats with protamine–zinc–insulin for 3 days caused a very marked increase to above normal levels of activity in all the enzymes of the pentose phosphate pathway except ribulose 5-phosphate epimerase, which was restored to the control value. Hexokinase activity was also raised by this treatment. After 7 days treatment of alloxan-diabetic rats with protamine–zinc–insulin the enzyme activities returned towards the control values. 3. In adrenalectomized rats the two most important changes were the rise in hexokinase activity and the fall in transketolase activity; in addition, ribulose 5-phosphate epimerase activity was also decreased. These effects were reversed by cortisone treatment. In addition, in cortisone-treated adrenalectomized rats glucokinase activity was significantly lower than the control value. 4. In thyroidectomized rats both ribose 5-phosphate isomerase and transketolase activities were decreased; in contrast with this transaldolase activity did not change significantly. Hypophysectomy caused a 50% fall in transketolase activity that was partially reversed by treatment with thyroxine and almost fully reversed by treatment with growth hormone for 8 days. 5. The results are discussed in relation to the hormonal control of the non-oxidative reactions of the pentose phosphate cycle, the marked changes in transketolase activity being particularly outstanding.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENEVENGA N. J., STIELAU W. J., FREEDLAND R. A. FACTORS AFFECTING THE ACTIVITY OF PENTOSE PHOSPHATE-METABOLIZING ENZYMES IN RAT LIVER. J Nutr. 1964 Dec;84:345–350. doi: 10.1093/jn/84.4.345. [DOI] [PubMed] [Google Scholar]
- BLUMENTHAL M. D., ABRAHAM S., CHAIKOFF I. L. ADAPTIVE BEHAVIOR OF HEPATIC GLUCOKINASE IN THE ALLOXAN-DIABETIC RAT. Arch Biochem Biophys. 1964 Feb;104:225–230. doi: 10.1016/s0003-9861(64)80007-2. [DOI] [PubMed] [Google Scholar]
- FITCH W. M., CHAIKOFF I. L. Extent and patterns of adaptation of enzyme activities in livers of normal rats fed diets high in glucose and fructose. J Biol Chem. 1960 Mar;235:554–557. [PubMed] [Google Scholar]
- FITCH W. M., HILL R., CHAIKOFF I. L. Hepatic glycolytic enzyme activities in the alloxan-diabetic rat: response to glucose and fructose feeding. J Biol Chem. 1959 Nov;234:2811–2813. [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. A preliminary investigation of the hormonal control of the hexose monophosphate oxidative pathway. Biochem J. 1955 Nov;61(3):390–397. doi: 10.1042/bj0610390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGINS C., YAO F. O. Influence of hormones on liver. I. Effects of steroids and thyroxine on pyridine nucleotide-linked dehydrogenases. J Exp Med. 1959 Dec 1;110:899–919. doi: 10.1084/jem.110.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyin V. S. Hormonal regulation of liver hexokinase activity. Adv Enzyme Regul. 1964;2:151–175. doi: 10.1016/s0065-2571(64)80011-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCLEAN P., NOVELLO F. INFLUENCE OF PANCREATIC HORMONES ON ENZYMES CONCERNED WITH UREA SYNTHESIS IN RAT LIVER. Biochem J. 1965 Feb;94:410–422. doi: 10.1042/bj0940410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P., Brown J. Activities of some enzymes concerned with citrate and glucose metabolism in transplanted rat hepatomas. Biochem J. 1966 Mar;98(3):874–882. doi: 10.1042/bj0980874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer H., Pérez N., Codoceo R. Liver glucokinase induction in acute and chronic insulin insufficiency in rats. J Biol Chem. 1967 Mar 10;242(5):860–864. [PubMed] [Google Scholar]
- Novello F., McLean P. The pentose phosphate pathway of glucose metabolism. Measurement of the non-oxidative reactions of the cycle. Biochem J. 1968 May;107(6):775–791. doi: 10.1042/bj1070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETTE D., KLINGENBERG M., BUECHER T. Comparable and specific proportions in the mitochondrial enzyme activity pattern. Biochem Biophys Res Commun. 1962 Jun 4;7:425–429. doi: 10.1016/0006-291x(62)90328-5. [DOI] [PubMed] [Google Scholar]
- PETTE D., LUH W., BUECHER T. A constant-proportion group in the enzyme activity pattern of the Embden-Meyerhof chain. Biochem Biophys Res Commun. 1962 Jun 4;7:419–424. doi: 10.1016/0006-291x(62)90327-3. [DOI] [PubMed] [Google Scholar]
- POTTER V. R., ONO T. Enzyme patterns in rat liver and Morris hepatoma 5123 during metabolic transitions. Cold Spring Harb Symp Quant Biol. 1961;26:355–362. doi: 10.1101/sqb.1961.026.01.043. [DOI] [PubMed] [Google Scholar]
- SALAS M., VINUELA E., SOLS A. INSULIN-DEPENDENT SYNTHESIS OF LIVER GLUCOKINASE IN THE RAT. J Biol Chem. 1963 Nov;238:3535–3538. [PubMed] [Google Scholar]
- SHARMA C., MANJESHWAR R., WEINHOUSE S. EFFECTS OF DIET AND INSULIN ON GLUCOSE-ADENOSINE TRIPHOSPHATE PHOSPHOTRANSFERASES OF RAT LIVER. J Biol Chem. 1963 Dec;238:3840–3845. [PubMed] [Google Scholar]
- Sharma C., Manjeshwar R., Weinhouse S. Hormonal and dietary regulation of hepatic glucokinase. Adv Enzyme Regul. 1964;2:189–200. doi: 10.1016/s0065-2571(64)80013-3. [DOI] [PubMed] [Google Scholar]
- Srivastava L. M., Hübscher G. Glucose metabolism in the mucosa of the small intestine. Enzymes of the pentose phosphate pathway. Biochem J. 1966 Oct;101(1):48–55. doi: 10.1042/bj1010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEPPERMAN H. M., TEPPERMAN J. ON THE RESPONSE OF HEPATIC GLUCOSE-6-PHOSPHATE DEHYDROGENASE ACTIVITY TO CHANGES IN DIET COMPOSITION AND FOOD INTAKE PATTERN. Adv Enzyme Regul. 1963;1:121–136. doi: 10.1016/0065-2571(63)90013-x. [DOI] [PubMed] [Google Scholar]
- TEPPERMAN H. M., TEPPERMAN J. Role of hormones in glucose-6-phosphate dehydrogenase adaptation of rat liver. Am J Physiol. 1962 Mar;202:401–406. doi: 10.1152/ajplegacy.1962.202.3.401. [DOI] [PubMed] [Google Scholar]
- TEPPERMAN J., TEPPERMAN H. M. Effects of antecedent food intake pattern on hepatic lipogenesis. Am J Physiol. 1958 Apr;193(1):55–64. doi: 10.1152/ajplegacy.1958.193.1.55. [DOI] [PubMed] [Google Scholar]
- WEBER G., MACDONALD H. Role of enzymes in metabolic homeostasis. I. Depletion and restoration of liver enzymes involved in glycolysis, glucogenesis and hexosemonophosphate shunt in normal and hypophysectomized rats. Exp Cell Res. 1961 Jan;22:292–302. doi: 10.1016/0014-4827(61)90108-2. [DOI] [PubMed] [Google Scholar]
- WILLMER J. S. Changes in hepatic enzyme levels after adrenalectomy. II. Glucose-6-phosphatase, glucose-6-phosphate dehydrogenase, and 6-phosphogluconate dehydrogenase. Can J Biochem Physiol. 1960 Dec;38:1449–1456. [PubMed] [Google Scholar]
- Walker D. G., Rao S. The role of glucokinase in the phosphorylation of glucose by rat liver. Biochem J. 1964 Feb;90(2):360–368. doi: 10.1042/bj0900360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters E., McLean P. The effect of anti-insulin serum and alloxan-diabetes on the distribution and multiple forms of hexokinase in lactating rat mammary gland. Biochem J. 1968 Oct;109(5):737–741. doi: 10.1042/bj1090737. [DOI] [PMC free article] [PubMed] [Google Scholar]


