Abstract
1. The incorporation of [32P]phosphate into phospholipids was measured in slices cut from the pial surface of guinea-pig cerebral cortex; incorporation into the phosphorus of some water-soluble precursors of phospholipid was measured under similar conditions. 2. Slices subjected to overall electrical stimulation at a frequency of 5pulses/sec. differed from control slices in their pattern of phospholipid labelling. After 1hr. of stimulation, incorporation of [32P]phosphate into phosphatidylcholine, ethanolamine phospholipid and cardiolipin was respectively 54, 55 and 58% of the control value, and that into phosphatidylinositol was 186% of control. Phosphatidic acid labelling tended to increase with electrical stimulation, but the statistical significance of this change was marginal. Labelling of phosphatidylglycerol and di- and tri-phosphoinositides was not affected significantly by electrical stimulation. 3. Electrical stimulation of the tissue altered the specific radioactivities of water-soluble precursors of phospholipid. 4. The turnover rates of the phosphate groups of phospholipids were estimated approximately from the specific radioactivities of phospholipids and their precursors. Phosphatidylinositol (and its lipid-soluble precursors) showed the largest change in turnover rate in response to electrical stimulation of the tissue; the turnover rates of other lipids were also affected. Changes in the specific radioactivity of phospholipids did not correspond to changes in turnover in these experiments.
Full text
PDF

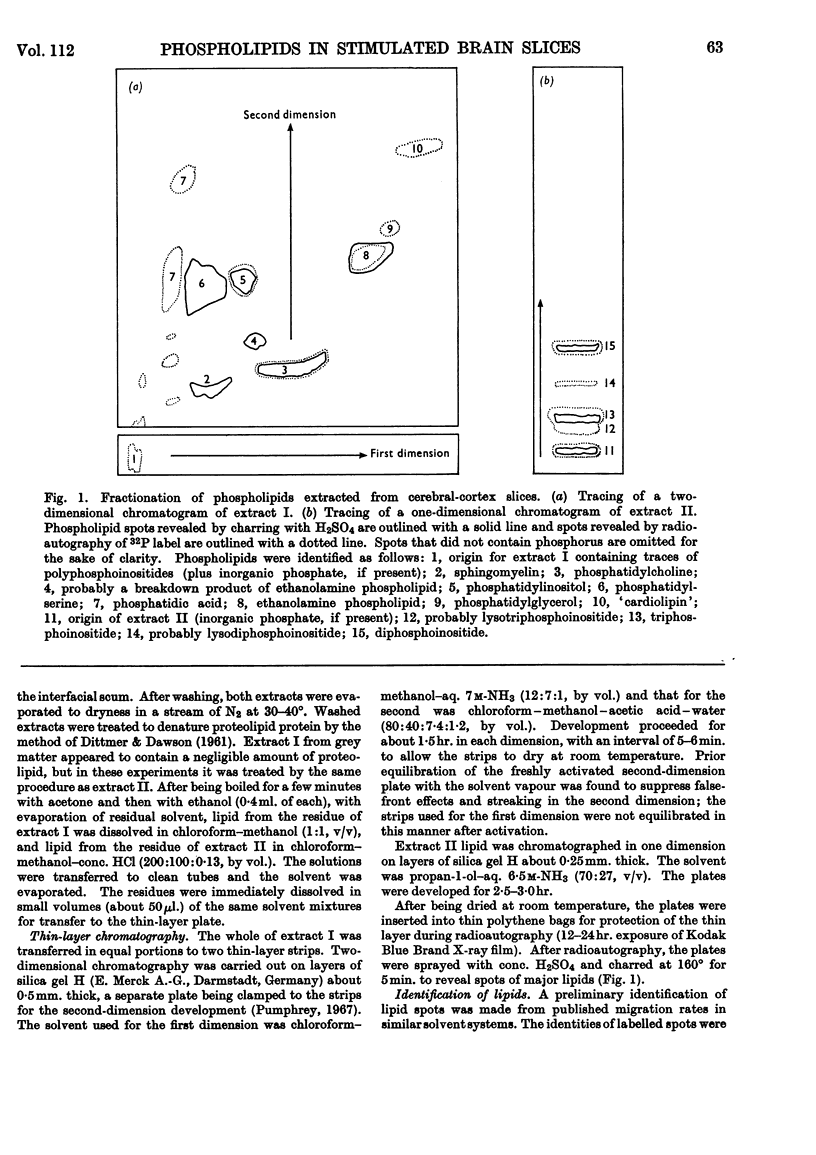


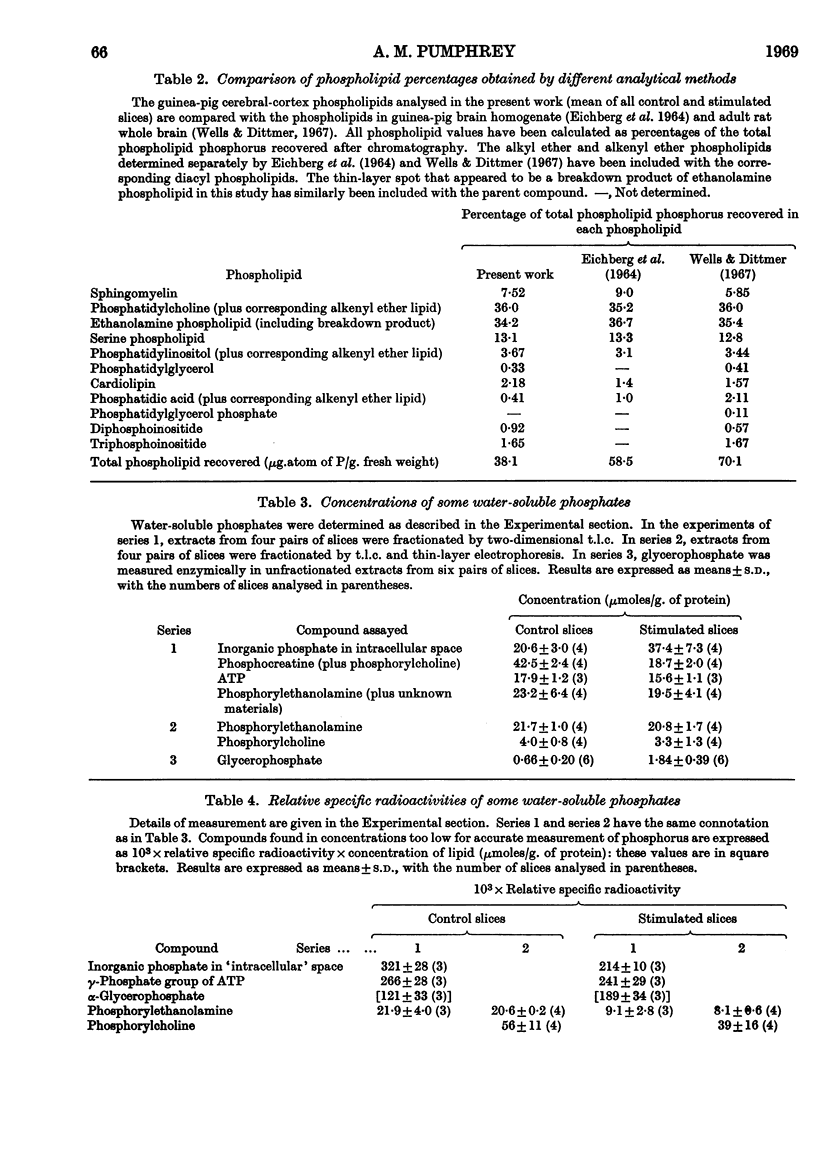
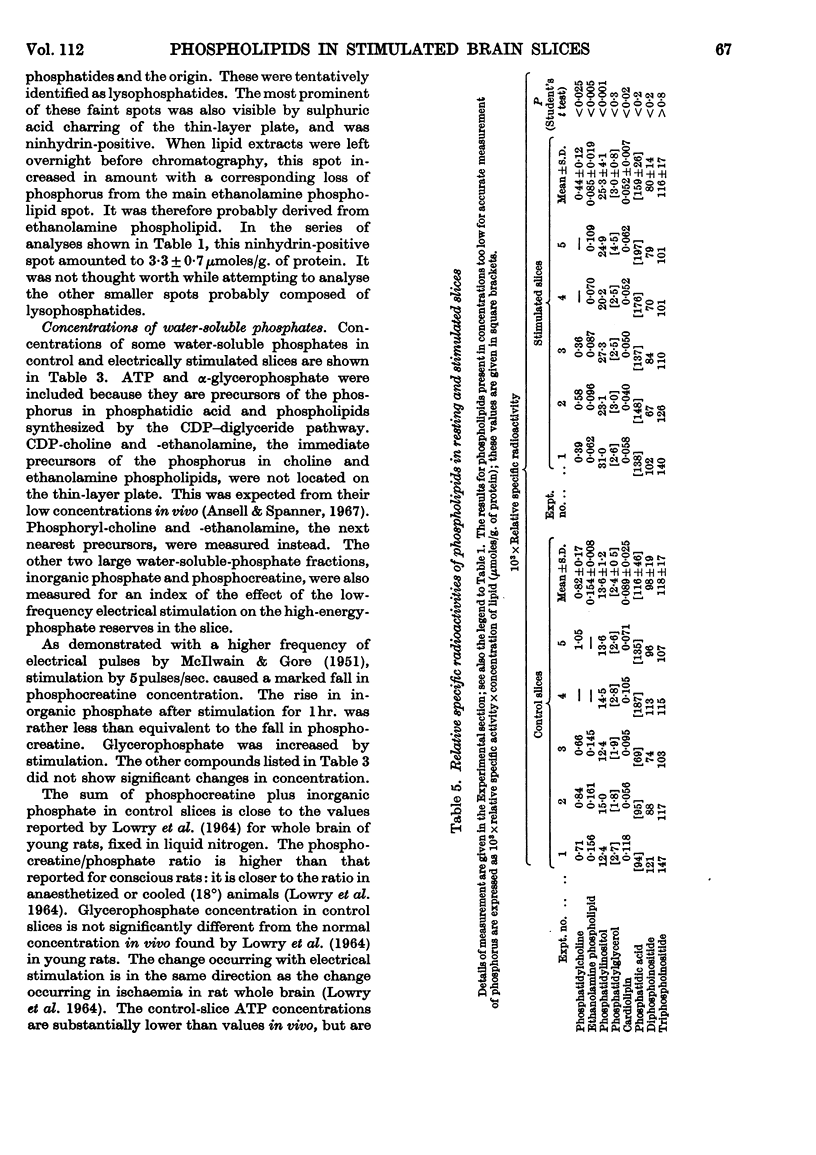



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABOOD L. G., GOLDMAN E., LIPMAN V. Metabolic studies on phospholipids and nucleic acid of frog nerve during excitation. J Neurochem. 1958;2(4):318–325. doi: 10.1111/j.1471-4159.1958.tb12381.x. [DOI] [PubMed] [Google Scholar]
- ANSELL G. B., SPANNER S. The effect of insulin on the formation of phosphorylcholine and phosphorylethanolamine in the brain. J Neurochem. 1959 Oct;4:325–331. doi: 10.1111/j.1471-4159.1959.tb13213.x. [DOI] [PubMed] [Google Scholar]
- Ansell G. B., Spanner S. The metabolism of labelled ethanolamine in the brain of the rat in vivo. J Neurochem. 1967 Sep;14(9):873–885. doi: 10.1111/j.1471-4159.1967.tb09576.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BIELESKI R. L. SEPARATION OF PHOSPHATE ESTERS BY THIN-LAYER CHROMATOGRAPHY AND ELECTROPHORESIS. Anal Biochem. 1965 Aug;12:230–234. doi: 10.1016/0003-2697(65)90086-2. [DOI] [PubMed] [Google Scholar]
- BROCKERHOFF H., BALLOU C. E. Phosphate incorporation in brain phosphionositides. J Biol Chem. 1962 Jan;237:49–52. [PubMed] [Google Scholar]
- Bjørnstad P., Bremer J. In vivo studies on pathways for the biosynthesis of lecithin in the rat. J Lipid Res. 1966 Jan;7(1):38–45. [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., DAVENPORT J. B. Improvements in the method of determining individual phospholipids in a complex mixture by successive chemical hydrolyses. Biochem J. 1962 Sep;84:497–501. doi: 10.1042/bj0840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., DAWSON R. M. The isolation of a new lipid, triphosphoinositide, and monophosphoinositide from ox brain. Biochem J. 1961 Dec;81:535–540. doi: 10.1042/bj0810535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberg J., Whittaker V. P., Dawson R. M. Distribution of lipids in subcellular particles of guinea-pig brain. Biochem J. 1964 Jul;92(1):91–100. doi: 10.1042/bj0920091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEALD P. J. Analysis of radioactive phosphates in extracts of cerebral tissues. Biochem J. 1956 Jun;63(2):235–242. doi: 10.1042/bj0630235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. The mechanism of phosphate exchange in phosphatidic acid in response to acetylcholine. J Biol Chem. 1959 Jun;234(6):1387–1390. [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Acyl dihydroxyacetone phosphate. Characterization of a 32P-labeled lipid from guinea pig liver mitochondria. J Biol Chem. 1968 Apr 10;243(7):1617–1622. [PubMed] [Google Scholar]
- KEESEY J. C., WALLGREN H., MCILWAIN H. THE SODIUM, POTASSIUM AND CHLORIDE OF CEREBRAL TISSUES: MAINTENANCE, CHANGE ON STIMULATION AND SUBSEQUENT RECOVERY. Biochem J. 1965 May;95:289–300. doi: 10.1042/bj0950289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARRABEE M. G., KLINGMAN J. D., LEICHT W. S. EFFECTS OF TEMPERATURE, CALCIUM AND ACTIVITY ON PHOSPHOLIPID METABOLISM IN A SYMPATHETIC GANGLION. J Neurochem. 1963 Aug;10:549–570. doi: 10.1111/j.1471-4159.1963.tb05053.x. [DOI] [PubMed] [Google Scholar]
- LARRABEE M. G., LEICHT W. S. METABOLISM OF PHOSPHATIDYL INOSITOL AND OTHER LIPIDS IN ACTIVE NEURONES OF SYMPATHETIC GANGLIA AND OTHER PERIPHERAL NERVOUS TISSUES. THE SITE OF THE INOSITIDE EFFECT. J Neurochem. 1965 Jan;12:1–13. doi: 10.1111/j.1471-4159.1965.tb10245.x. [DOI] [PubMed] [Google Scholar]
- LOLLEY R. N. THE CALCIUM CONTENT OF ISOLATED CEREBRAL TISSUES AND THEIR STEADY-STATE EXCHANGE OF CALCIUM. J Neurochem. 1963 Sep;10:665–676. doi: 10.1111/j.1471-4159.1963.tb08938.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- MAJNO G., GASTEIGER E. L., LA GATTUTA M., KARNOVSKY M. L. Lipid biosynthesis in vitro by electrically stimulated rat sciatic nerves. J Neurochem. 1958 Dec;3(2):127–131. doi: 10.1111/j.1471-4159.1958.tb12618.x. [DOI] [PubMed] [Google Scholar]
- McILWAIN H., GORE M. B. R. Actions of electrical stimulation and of 2:4-dinitrophenol on the phosphates in sections of mammalian brain in vitro. Biochem J. 1951 Nov;50(1):24–28. doi: 10.1042/bj0500024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Sercombe R. Electrical activity observed in guinea-pig olfactory cortex maintained in vitro and its modification by changes in the ionic composition of the bathing medium. J Physiol. 1968 May;196(2):94P–95P. [PubMed] [Google Scholar]
- Rodnight R., Hems D. A., Lavin B. E. Phosphate binding by cerebral microsomes in relation to adenosine-triphosphatase activity. Biochem J. 1966 Nov;101(2):502–515. doi: 10.1042/bj1010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Control of glycolysis in cerebral cortex slices. Biochem J. 1967 Aug;104(2):524–533. doi: 10.1042/bj1040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltawy A., Dawson R. M. The polyphosphoinositides and other lipids of peripheral nerves. Biochem J. 1966 Jul;100(1):12–18. doi: 10.1042/bj1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARON S., McILWAIN H. Fluid content and compartments in isolated cerebral tissues. J Neurochem. 1961 Dec;8:262–275. doi: 10.1111/j.1471-4159.1961.tb13552.x. [DOI] [PubMed] [Google Scholar]
- WADE H. E., MORGAN D. M. Detection of phosphate esters on paper chromatograms. Nature. 1953 Mar 21;171(4351):529–530. doi: 10.1038/171529a0. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Dittmer J. C. A comprehensive study of the postnatal changes in the concentration of the lipids of developing rat brain. Biochemistry. 1967 Oct;6(10):3169–3175. doi: 10.1021/bi00862a026. [DOI] [PubMed] [Google Scholar]


