Abstract
The parasagittal dura (PSD) is a thin channel along the sagittal sinus vein at the brain’s upper convexities. Previous studies have shown that cerebrospinal fluid (CSF) flows directly into the PSD, with PSD dimensions and tracer clearance rates associated with aging and brain disorders. Since slow lymphatic drainage is sensitive to water diffusion, PSD circulation may be evaluated using diffusion-weighted imaging (DWI). However, traditional echo-planar DWI (EP-DWI) suffers from low resolution and image distortion, limiting its application to PSD assessment. This study employed high-resolution Multiplexed Sensitivity Encoding (MUSE) DWI and Periodically Rotated Overlapping Parallel Lines with Enhanced Reconstruction (PROPELLER) DWI to investigate PSD water diffusion. These advanced techniques reduce image distortion while enhancing spatial resolution. Our results demonstrated that PSD structures are clearly visible on high-resolution DWI and apparent diffusion coefficient (ADC) maps, correlating with PSD locations identified on T2 FLAIR imaging. In addition, mean ADC values of PSD (1843.1–2062.2 × 10− 6 mm2/sec) were higher than those of gray and white matter but lower than CSF. These findings highlight the potential of MUSE and PROPELLER DWI for assessing PSD diffusion, offering a promising non-invasive tool for studying PSD circulation and its role in neurological disorders.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-91751-0.
Keywords: MUSE, PROPELLER, DWI, Meningeal lymphatic vessels, Parasagittal dura
Subject terms: Magnetic resonance imaging, Neuroimmunology
Introduction
The lymphatic system is a circulation that contributes to facilitating the clearance of excess fluid and macromolecules from the interstitium. It accomplishes this by passing through lymph nodes to remove bacteria, abnormal cells, and other matter1. In brain, the brain glymphatic system (GS)2 and the meningeal lymphatic vessels (MLVs)3 have been discovered since 2012. The brain GS collects cerebrospinal fluid (CSF) from the subarachnoid space and brain interstitial fluid (ISF) through aquaporin-4 (AQP4) water channels. The MLVs situated in the dorsal and basal regions serve as downstream channels, responsible for draining ISF, macromolecules, and immune cells out of the cranial cavity. Ultimately, the brain GS fluid is drained through the MLVs into the deep cervical lymph nodes4. Additionally, they play a crucial role in regulating immune responses in the brain. Recent research has shown that ISF, CSF, MLVs, and the brain GS are important factors impacting brain homeostasis3. A numerous studies have reported the association between brain disorders and impaired GS and MLVs, such as cerebral small‑vessel disease5, ischemic stroke6, and dementia7.
Using high-resolution 3D T2-Fluid Attenuated Inversion Recovery (FLAIR) magnetic resonance imaging, dural lymphatic structures were identified along the dural venous sinuses in the dorsal regions and along the cranial nerves in the ventral regions of the human brain8. Whole-brain high-resolution 3D T2 FLAIR images showed that the parasagittal dura (PSD) space is the largest region for observing MLVs9. The function of parasagittal MLVs and the flux rate of cerebrospinal fluid to the PSD have been evaluated using the propagation of a tracer with multi-phase T1-weighted imaging (T1WI) after the injection of a contrast agent10. However, the observation period in multi-phase T1WIs exceeds four hours because of the extremely slow fluid drainage in the PSD space.
The function of PSD can potentially be evaluated using echo-planar diffusion-weighted imaging (EP-DWI) since the slow lymphatic drainage is sensitive to water diffusion. The ability of DWI to assess water mobility makes it a valuable tool for evaluating how well lymphatic drainage functions in these areas, potentially offering diagnostic information for various central nervous system pathologies. Unfortunately, due to the characteristics of low resolution and image distortion11, EP-DWI cannot be effectively applied to the PSD. As a result, to our knowledge, diffusion MRI has not yet been directly applied to PSD for diffusion measurement in the literature. In contrast, either multiplexed sensitivity encoding (MUSE) technique12 or periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER)13 can provide diffusion weighted image (DWI) with high spatial resolution and reduced image distortion. In this study, MUSE DWI and PROPELLER DWI with high resolution were used to investigate water diffusion in the PSD space.
Results
The representative high-resolution T2 FLAIR image and 3D reconstruction of PSD were shown in Fig. 1. The arrows pointed the PSD locations with similar present with previous studies8. Figure 2 displayed sagittal view images, including T2 FLAIR, MUSE DWI, PROPELLER DWI, MUSE apparent diffusion coefficient (ADC) map, and PROPELLER ADC map. Figure 3 displayed the coronal view images. Table 1 presented the signal intensity (mean ± SD) of MUSE DWI and PROPELLER DWI for white matter (WM), gray matter (GM), PSD, and CSF in both the sagittal and coronal views. Table 2 listed ADC values (mean ± SD) of WM, GM, PSD, and CSF, measured using the pixel-wise method (PWM) and the ROI-based method (RBM) across sagittal and coronal view. In the study, we compared the ADC values between the different categories PSD, CSF, GM, and WM. This box plot visualized the distribution of DWI intensity and ADC values of WM, GM, PSD, and CSF across PWM and RBM measurements in sagittal and coronal view (Figs. 4 and 5).
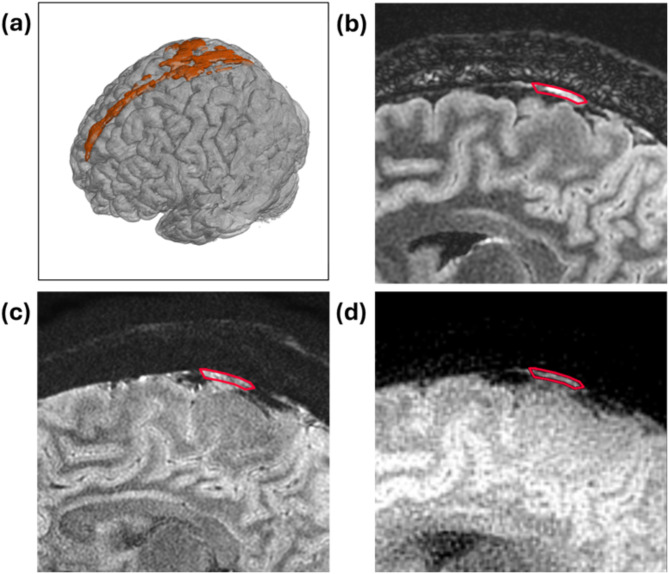
Fig. 1.
(a) 3D FLAIR reconstruction showing the PSD structure in orange, (b) 2D T2 FLAIR image, (c) MUSE DWI, and (d) PROP DWI. The red contour outlines the PSD region, as identified on DWI, corresponding to the high signal in the PSD on FLAIR images. This overlay enhances geometric accuracy visualization and highlights the improved delineation of the PSD structure using MUSE and PROP techniques.
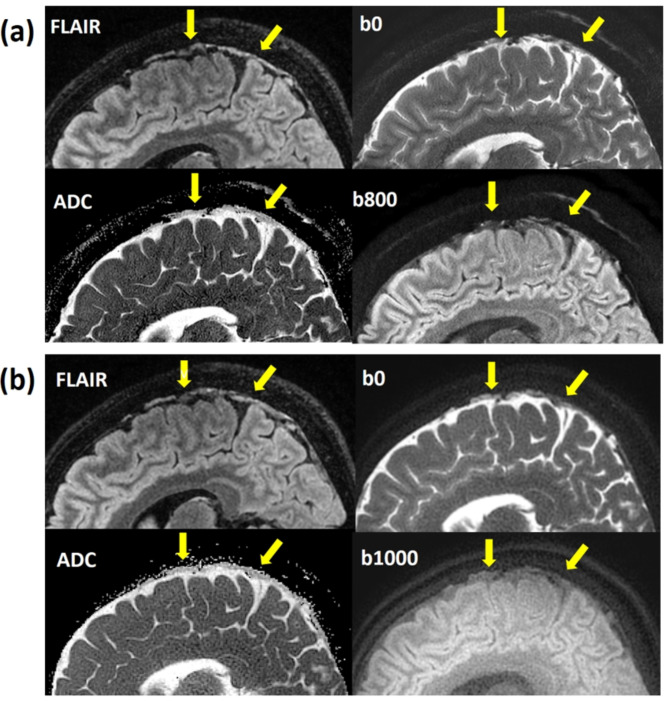
Fig. 2.
(a) The sagittal view displayed the PSD on T2 FLAIR image (up left), MUSE DWI b0 (up right), MUSE DWI b800 (down right), and MUSE ADC map (down left) as indicated by arrows. (b) The sagittal view displayed the PSD on T2 FLAIR image (up left), PROPELLER DWI b0 (up right), PROPELLER DWI b1000 (down right), and PROPELLER ADC map (down left) as indicated by arrows. The ADC of the PSD appeared darker than that of the CSF.
Fig. 3.
(a) The coronal view displayed the PSD on T2 FLAIR image (up left), MUSE DWI b0 (up right), MUSE DWI b800 (down right), and MUSE ADC map (down left) as indicated by arrows. (b) The coronal view displayed the PSD on T2 FLAIR image (up left), PROPELLER DWI b0 (up right), PROPELLER DWI b1000 (down right), and PROPELLER ADC map (down left) as indicated by arrows. The ADC of the PSD appeared darker than that of the CSF.
Table 1.
DWI signal intensity (mean ± SD) of parasagittal dura (PSD), cerebrospinal fluid (CSF), gray matter (GM), and white matter (WM).
| PSD | CSF | GM | WM | ||
|---|---|---|---|---|---|
| MUSE (Sag) | b0 | 4202.9 ± 1136.8 | 7087.3 ± 830.6 | 3075.3 ± 280.0 | 2308.0 ± 255.8 |
| b500 | 1495.0 ± 390.2 | 1706.7 ± 218.9 | 1844.4 ± 153.1 | 1520.2 ± 163.6 | |
| b800 | 872.38 ± 217.56 | 749.62 ± 99.61 | 1442.92 ± 127.67 | 1218.52 ± 135.00 | |
| MUSE (Cor) | b0 | 4891.5 ± 1242.7 | 7185.2 ± 882.9 | 3103.7 ± 319.7 | 2278.6 ± 201.7 |
| b500 | 1827.2 ± 471.2 | 1759.1 ± 163.9 | 1822.2 ± 150.4 | 1508.9 ± 117.4 | |
| b800 | 1085.0 ± 274.3 | 776.1 ± 72.9 | 1436.8 ± 114.7 | 1240.3 ± 92.2 | |
| PROP (Sag) | b0 | 502.4 ± 112.9 | 967.7 ± 112.3 | 375.9 ± 50.5 | 321.6 ± 27.1 |
| b1000 | 79.1 ± 17.8 | 76.0 ± 13.8 | 132.4 ± 17.2 | 137.3 ± 13.7 | |
| PROP (Cor) | b0 | 517.9 ± 54.0 | 977.6 ± 163.7 | 351.0 ± 67.8 | 293.7 ± 38.9 |
| b1000 | 81.5 ± 9.0 | 75.7 ± 13.1 | 127.9 ± 17.8 | 130.5 ± 18.2 | |
MUSE multiplexed sensitivity encoding, PROPELLER parallel lines with enhanced reconstruction, DWI diffusion weighted image, Sag sagittal, Cor coronal.
Table 2.
ADC values (mean ± SD) of parasagittal dura (PSD), cerebrospinal fluid (CSF), Gray matter (GM), and white matter (WM).
| ×10− 6 mm2/sec | PSD | CSF | GM | WM | ||
|---|---|---|---|---|---|---|
| MUSE ADC (b = 500) | Sag |
PWM RBM |
2048.7 ± 116.5 2062.2 ± 110.9 |
2852.6 ± 177.5 2849.8 ± 173.4 |
991.1 ± 76.9 1021.1 ± 97.3 |
837.6 ± 52.9 834.4 ± 58.3 |
| Cor |
PWM RBM |
1966.1 ± 113.3 1972.3 ± 110.9 |
2818.8 ± 158.6 2808.2 ± 143.6 |
1024.1 ± 125.5 1061.8 ± 143.4 |
821.9 ± 102.4 822.9 ± 105.9 |
|
| MUSE ADC (b = 800) | Sag |
PWM RBM |
1942.0 ± 115.7 1957.2 ± 106.3 |
2810.0 ± 127.4 2810.1 ± 122.1 |
926.1 ± 65.9 945.7 ± 71.2 |
789.2 ± 43.1 798.4 ± 45.6 |
| Cor |
PWM RBM |
1868.4 ± 84.7 1882.1 ± 84.0 |
2785.9 ± 138.7 2778.2 ± 133.0 |
931.7 ± 95.8 960.3 ± 107.7 |
750.2 ± 77.6 759.0 ± 77.6 |
|
| PROP ADC (b = 1000) | Sag |
PWM RBM |
1851.7 ± 105.9 1850.5 ± 103.7 |
2564.0 ± 178.7 2553.9 ± 169.7 |
1020.8 ± 76.5 1042.0 ± 91.0 |
847.8 ± 48.2 852.2 ± 48.8 |
| Cor |
PWM RBM |
1843.1 ± 117.9 1849.1 ± 111.5 |
2568.7 ± 171.9 2561.3 ± 167.1 |
965.3 ± 123.5 1000.7 ± 141.4 |
807.1 ± 71.2 812.0 ± 69.5 |
MUSE multiplexed sensitivity encoding, PROPELLER parallel lines with enhanced reconstruction, DWI diffusion weighted image, Sag sagittal, Cor coronal.
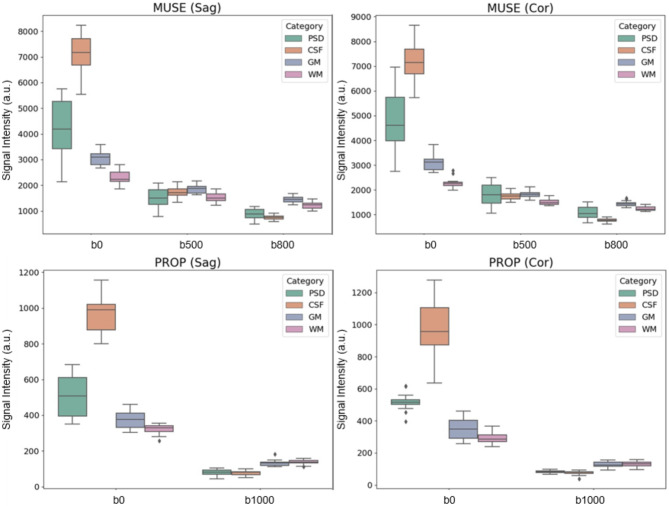
Fig. 4.
Box plots illustrating the DWI signal intensity of PSD, CSF, GM, and WM across b-values of 0, 500, 800, and 1000 in both sagittal and coronal views. (a) MUSE DWI in the sagittal plane, (b) MUSE DWI in the coronal plane, (c) PROPELLER DWI in the sagittal plane, and (d) PROPELLER DWI in the coronal plane.
Fig. 5.
The box plot illustrates the ADC values of PSD, CSF, GM, and WM based on b-values of 500, 800, and 1000, using the PWM and RBM methods in both sagittal (PWMsag, RBMsag) and coronal (PWMcor, RBMcor) views. Each label on the x-axis represents a specific condition: PSD500, PSD800, and PSD1000 represent PSD ADC values measured with b = 0 combined with b = 500 in the MUSE scan, b = 0 combined with b = 800 in the MUSE scan, and b = 0 combined with b = 1000 in the PROPELLER scan, respectively. Similarly, CSF500, CSF800, CSF1000 correspond to CSF measurements; GM500, GM800, GM1000 correspond to GM measurements; and WM500, WM800, WM1000 correspond to WM measurements under the same conditions.
Figure 4 presented box plots illustrating the DWI signal intensity of PSD, CSF, GM, and WM across b-values of 0, 500, 800, and 1000 in both the sagittal and coronal views. In Fig. 5, the ADC values of PSD measured by b = 0 and b = 500 in the MUSE DWI scan are labeled as PSD500, by b = 0 and b = 800 in the MUSE DWI scan as PSD800, and by b = 0 and b = 1000 in the PROPELLER DWI scan as PSD1000. Similarly, CSF500, CSF800, and CSF1000 correspond to the ADCs of CSF; GM500, GM800, and GM1000 correspond to ADCs of GM; and WM500, WM800, and WM1000 correspond to ADCs of WM produced by two MUSE DWI and one PROPELLER DWI scans. The PWM and RBM measurements appear to have similar distributions across most measures, and Kruskal-Wallis H test suggested consistency between the two methods (p > 0.05).
ADC values of PSD gradually decreased with increasing b-values. The mean ADC values were slightly above 2000 × 10–6 mm²/sec in PSD500, slightly lower in PSD800, and around 1850 × 10–6 mm2/sec in PSD1000. Similarly, mean ADC values of CSF showed a gradual decrease from low b-values to high b-values. The ADC values were slightly below 2900 × 10–6 mm2/sec in CSF500, slightly lower in CSF800, and around 2550 × 10–6 mm2/sec in CSF1000. However, ADC values of GM and WM decreased from b = 500 to b = 800 in the MUSE scan but slightly increased at b = 1000 in the PROPELLER scan. Mean ADC values of GM were slightly above 1000 × 10–6 mm2/sec in GM500, slightly below 1000 × 10–6 mm2/sec in GM800, and returned to around 1000 × 10–6 mm2/sec in GM1000. Similarly, mean ADC values of WM were slightly above 800 × 10–6 mm2/sec in WM500, slightly below 800 × 10–6 mm2/sec in WM800, and returned to above 800 × 10–6 mm2/sec in WM1000. The Kruskal-Wallis H test and subsequent pairwise comparisons using the Mann-Whitney U test with Dunn–Bonferroni post hoc correction indicated that the ADC of PSD significantly differed from those of CSF, GM, and WM (p < 0.001).
Discussion
In this study, high-resolution 3D T2 FLAIR was used as the standard reference for investigating the feasibility of visualizing the PSD using high-resolution MUSE DWI and PROPELLER DWI. The 3D T2 FLAIR MRI technique is particularly effective at visualizing certain brain structures and pathologies by suppressing the signal from CSF14–16. By nullifying the bright CSF signal within the ventricular compartment and subarachnoid space, T2 FLAIR enhances the contrast between the PSD with its high signal and surrounding structures with darker signals, facilitating the differentiation and visualization of small structures such as MLVs and perineural spaces in coronal and sagittal views10.
Utility and limitations of T2 FLAIR imaging for visualizing PSD
Previous studies have utilized high-resolution T2 FLAIR to explore MLVs10,17 and arachnoid granulations18 in the PSD. Furthermore, T2 FLAIR has been extended to investigate extracranial lymphatic structures from intracranial regions, tracing the pathways of MLVs around venous sinuses and cranial nerves8, as well as periarterial fluid drainage along the vessel walls of the internal carotid arteries, which then connect to the cervical lymph nodes19. Some findings are corroborated by a study using contrast agent administration20. In summary, T2 FLAIR’s ability to suppress CSF signals, enhance contrast, and provide high-resolution images makes it an excellent tool for visualizing MLVs and related drainage pathways in the brain. Additionally, T2 FLAIR provides high-resolution images that are essential for evaluating PSD thickness in aging8 and PSD volume in various CSF disorders21. The benefits of T2 FLAIR MRI include its noninvasive nature and the lack of need for contrast agents, making it suitable for patients who cannot receive contrast media and enabling longitudinal studies. However, although high-resolution FLAIR can clearly depict the PSD structure, it only provides limited information on functional and pathological characteristics.
Challenges and advances in DWI techniques for PSD imaging
The PSD is located on both sides of the superior sagittal sinus and contains abundant arachnoid granulations and parasagittal MLVs21. These vessels play a crucial role in draining interstitial fluids out of the brain and contributing to the glymphatic system22. In this study, we evaluated the feasibility of measuring the ADC of the PSD using high-resolution MUSE DWI and PROPELLER DWI. Single-shot EP-DWI (SS-EP-DWI) is the most widely used DWI sequence in clinical practice, with typical voxel sizes around (1.5—1.8)×(1.5—1.8)×(5—6) mm3 in routine SS-EP-DWI. However, SS-EP-DWI is prone to high geometric distortion and low spatial resolution due to its sensitivity to magnetic susceptibility artifacts, especially near air and bone interface11. For example, the geometric distortion of the parotid glands can result in body surface length errors of 7–12%23, affecting 27–30% of a population24. The PSD, like the parotid glands, is located at brain convexities near the skull, making it susceptible to geometric distortion from magnetic susceptibility artifacts. Additionally, MLVs are predominantly located in the PSD22, and the dura mater is a flimsy structure with varying thickness. One study reported a mean thickness ranging from 0.35 mm to 1.1 mm25, while the other measured a mean thickness of 1.14 ± 0.06 mm26. Consequently, the PSD space may be missed in SS-EP-DWI due to image distortion and low spatial resolution, highlighting the need for DWI techniques with less distortion and higher resolution to accurately observe the PSD space.
Numerous studies have indicated that MUSE DWI and PROPELLER DWI outperform SS-EP-DWI by providing less distorted images and higher spatial resolution, as both are multi-shot diffusion-weighted imaging techniques based on different principles. MUSE DWI has been proposed to improve spatial resolution and geometric fidelity in brain DWI27, while PROPELLER DWI offers better quality in terms of distortion, susceptibility-related changes, and lesion conspicuity28. Our results showed that the PSD space was clearly visible on both MUSE and PROPELLER imaging with high-resolution DWI and their ADC maps, corresponding to the T2 FLAIR image in both sagittal and coronal views. In this study, MUSE DWI with a voxel size of 0.65 × 0.65 × 3 mm3 and PROPELLER DWI with a voxel size of 0.78 × 0.78 × 3 mm3 were used to evaluate water diffusion in the PSD space. Therefore, high spatial resolution DWI with minimal distortion is important for accurate ADC measurement in the PSD. Note that further reduction of geometric distortion or improvement of spatial resolution in MUSE DWI may necessitate using a higher number of shots (e.g., eight shots or more), but the maximum achievable number of shots for MUSE DWI is typically limited by the availability of receiver channels. On the other hand, although PROPELLER DWI with fast-spin echo readout can produce distortion-free images, it is generally less scan-efficient compared to MUSE DWI (two b-values versus three b-values acquired with comparable scan time). The better scan efficiency of MUSE DWI may allow for ADC measurement with multiple b-values. Nevertheless, PROPELLER DWI is less susceptible to head motions (e.g., head tremor) and may be more suitable for assessing PSD space in challenging subjects. Ultimately, the choice between the two techniques may depend on their availability on the MRI scanner, the desired number of b-values, and the target patient populations.
Role of ADC in characterizing water diffusion in the PSD
ADC is a quantitative metric that reflects the microscopic environment by measuring the diffusibility of water in brain tissue. ADC plays an important role in diagnosing diseases that affect various tissue types and involve different pathological processes, such as ischemic stroke, neoplasms, intracranial infections, traumatic brain injury, and demyelinating conditions29. Our study showed that the ADC values in the PSD space are intermediate between those of brain parenchyma (GM and WM) and CSF, as diffusion is more restricted in bound water, such as within solid tissue or macromolecules. The protein-rich fluid, which includes waste or macromolecules draining from the CSF-ISF-CSF washout process originating from the perivenous space, eventually drains into the PSD, which has a thin film space30. Consequently, the concentration of macromolecules in the PSD is higher than in CSF due to macromolecular accumulation and the narrow space within the PSD. Therefore, our study revealed that ADCs in the PSD are higher than in solid tissues like GM and WM but lower than in the free fluid of CSF.
Controversies and advancements in PSD imaging: insights into neuroimmune function and circulation efficiency
Some studies refer to the bright signal region in the meninges on T2 FLAIR images as MLVs8,10,17. However, other research challenges this interpretation31,32. The primary contradictions arise from the fact that hyperintense FLAIR signals in the PSD may be attributed not only to lymphatic tissue but also to other tissues. Additionally, MLVs may be influenced by partial volume effects, as they are often too small to be reliably identified at a 1 mm spatial resolution on T2 FLAIR images. Although the identification of a lymphatic signal in the PSD on FLAIR MRI remains heavily contested31–34, there is strong evidence suggesting that the PSD serves as a potential neuroimmune interface. Numerous immune-related tissues and brain metabolites have been found within the PSD space18,35. Dural channels within the PSD also act as reservoirs for cerebrospinal fluid drainage36. Moreover, the rate of contrast agent wash-in and wash-out in the PSD is influenced by factors such as aging22,37, brain parenchymal fraction38, and brain diseases5,39. Increasingly, studies reveal that the PSD harbors rich immune elements and that circulation within the PSD is connected to the efficiency of brain waste metabolism and immune function. However, most of these studies rely on contrast agent administration, a procedure that requires several hours to measure the accumulation and drainage of the contrast agent. Our study demonstrated that ADC measurements in the PSD have potential as a rapid metric for assessing PSD circulation efficiency, which can be completed in a few minutes.
Limitations
Although our study successfully demonstrated the feasibility of measuring PSD ADC using multi-shot high-resolution DWI techniques and confirmed its distinctiveness from WM, GM, and CSF in healthy young subjects, further investigation is needed to enhance clinical applicability. First, the sample size was small, and all subjects were healthy and young, with a mean age of 23 years. While this group was sufficient for testing the reliability of ADC measurements in the PSD, it may not be representative of the broader population.
Second, the use of 2D MUSE DWI and PROPELLER DWI with a 3 mm slice thickness may introduce partial volume effects in the ADC measurements of the PSD. This challenge arose from both the flimsy and fragmented structure of the PSD with varying thickness25,26 and the inherent slice thickness limitation of 2D DWI. However, this was a necessary trade-off between slice thickness and sufficient signal-to-noise ratio for 2D acquisition. While we mitigated partial volume contamination by measuring ADC only in thicker PSD regions, this also limited our ability to trace ADC variations across the PSD along the sagittal sinus vein. To address this limitation, future studies could consider using 3D MUSE DWI for more precise PSD ADC measurement40, potentially minimizing partial volume effects and achieving higher SNR.
Third, we tested two sequences (MUSE DWI and PROPELLER DWI) and three b-values (500 and 800 for MUSE DWI, and 1000 for PROPELLER DWI) in this study. SNR analysis revealed that high b-values resulted in low SNR in CSF due to fast flow. Although increasing NEX could improve SNR, it would also lengthen the already extensive scan time (over 60 min per participant for acquiring high-resolution DWI with different b-values and scan planes). Therefore, we used b = 800 for MUSE DWI to optimize the trade-off between SNR and scan efficiency. As a result, we could not evaluate the variability of PSD ADC sensitivity across different b-values. Although evaluating PSD ADC variability with multiple b-values is important, this remains an area for future research. Recent studies have explored the intravoxel incoherent motion (IVIM), a DWI technique with multiple b-values, in brain disorders41, and our findings suggest its potential for PSD IVIM measurement.
Fourth, our study only showed that PSD ADC values differ from those of other brain tissues (WM, GM, and CSF) previously established in healthy young subjects. Recent studies have explored PSD and MLV morphology and drainage in brain disorders and aging5,8,21, revealing variations in volume and lymphatic drainage patterns in pathological conditions. Future studies should validate the reliability and sensitivity of PSD ADC measurements in larger populations, particularly in aging and specific brain disorders.
Additionally, our study did not account for pulsation effects on ADC measurements in the PSD caused by surrounding veins and CSF. Future research should investigate potential biases in PSD ADC values resulting from pulsation effects due to vein and CSF contamination, using techniques such as FLAIR-DWI42 and cardiac gating43.
Conclusions
The study successfully demonstrated the feasibility of assessing ADC measurements in the PSD using MUSE DWI and PROPELLER DWI, both of which offer high spatial resolution and minimal image distortion. The high resolution and distortion insensitivity of MUSE DWI and PROPELLER DWI enable the visualization of PSD regions corresponding to their locations on high-resolution T2 FLAIR MRI, supporting the feasibility of measuring diffusion in the PSD in both sagittal and coronal views. Additionally, the ADC values in the PSD are higher than those in GM and WM, but lower than in CSF. The ADC values are consistent whether using the PWM or RBM measurement methods.
Materials and methods
Study participants
This study recruited 6 male and 2 female volunteers with a mean age of 23 years (range: 20 to 28 years) who did not exhibit any symptoms related to brain or cerebral circulation. This prospective study was approved by the local institutional review board at China Medical University Hospital (CMUH111-REC1-033). All methods were performed in accordance with relevant guidelines and regulations, and written informed consent was obtained from all participants.
MRI scans
MR studies were conducted using 3.0T scanners (SIGNA Architect, GE Healthcare) with head and neck coils. A high-resolution 3D T2-FLAIR sequence was applied to locate the PSD, with imaging parameters as follows: repetition time (TR) of 10,000 ms, echo time (TE) of 156 to 160 ms, inversion time (TI) of 2,350 to 2,410 ms, field of view (FOV) of 190 to 200 × 190 to 200 mm, matrix size of 224 × 224, number of excitations (NEX) of 1, and slice thickness of 1 mm. MUSE DWI was performed with 4 shots, using scanning parameters as follows: TR of 5,000 ms, TE of 91 to 95 ms, b-values of 0, 500, and 800 s/mm2, 2 NEXs (b = 0), 3 NEXs (b = 500), and 6 NEXs (b = 800), FOV of 190 to 200 × 190 to 200 mm, matrix size of 288 to 320 × 288 to 320, and slice thickness of 3 mm. PROPELLER DWI was also performed, using scanning parameters as follows: TR of 5,000 ms, TE of 91 to 95 ms, ETL 32, b-values of 0 and 1000 s/mm2, 2 NEXs (b = 0), and 6 NEXs (b = 1000), FOV of 190 to 200 × 190 to 200 mm, matrix size of 256 × 256, and slice thickness of 3 mm. The protocol parameters for the 3D T2-FLAIR, MUSE DWI, and PROPELLER DWI sequences are summarized in Table 3.
Table 3.
The protocol parameters for T2 FLAIR, MUSE DWI, and PROPELLER DWI sequences.
| T2 FLAIR (Sag.) | MUSE DWI (Sag.) | PROPELLER DWI (Sag.) | MUSE DWI (Cor.) | PROPELLER DWI (Cor.) | |
|---|---|---|---|---|---|
| TR | 10,000 ms | 5000 ms | 5000 ms | 5000 ms | 5000 ms |
| TE | 157 ms | 95 ms | 81 ms | 95 ms | 81 ms |
| TI | 2358 ms | NA | NA | NA | NA |
| b value | NA | 0, 500, 800 | 0,1000 | 0, 500, 800 | 0, 1000 |
| number of shots | NA | 4 | NA | 4 | NA |
| NEX | 1 |
2 (b = 0) 3 (b = 500) 6 (b = 800) |
1.5 (b = 0) 6 (b = 1000) |
2 (b = 0) 3 (b = 500) 6 (b = 800) |
1.5 (b = 0) 6 (b = 1000) |
| FOV | 190 × 190 mm | 200 × 200 mm | 200 × 200 mm | 190 × 190 mm | 190 × 190 mm |
| Matrix Size | 224 × 224 | 320 × 320 | 256 × 256 | 288 × 288 | 256 × 256 |
| Slice Thickness | 1 mm | 3 mm | 3 mm | 3 mm | 3 mm |
| Scan Time | 9:44 | 10:20 | 9:50 | 10:20 | 9:50 |
MUSE multiplexed sensitivity encoding, PROPELLER parallel lines with enhanced reconstruction, DWI diffusion weighted image, Sag sagittal, Cor coronal.
The total scan time was approximately 60–70 min, including the localizer scan, 3D high-resolution FLAIR scan, high-resolution 2D MUSE DWI and 2D PROPELLER DWI in both the sagittal and coronal planes, as well as the reconstruction of three-plane FLAIR images (coronal, sagittal, and axial) for PSD slice localization. These high-resolution 2D DWI scans, consisting of 12–15 slices in the sagittal and coronal planes, were positioned as perpendicular as possible to the PSD channel, targeting clear and thicker PSD regions, based on the three-plane FLAIR images.
Data processing and analysis
Apparent diffusion coefficient (ADC) maps were generated through pixel-by-pixel computation from DWI images using the Stejskal–Tanner formula:  . ADC values were measured within regions of interest (ROI) encompassing four tissues: the PSD, gray matter (GM), white matter (WM), and CSF. ROIs on the ADC map, corresponding to areas on FLAIR images, were placed to avoid partial volume effects and extra-tissue noise. The ROIs were drawn by a neuroradiologist with 3 years of experience in neuroradiology. For each subject, two ROIs with clear and sufficiently large regions for PSD, CSF, GM, and WM were selected in both sagittal and coronal slices. The mean ADC values were generated using both a pixel-wise method (PWM) and an ROI-based method (RBM) in sagittal and coronal views for comparison, denoted as PWMsag, RBMsag, PWMcor, and RBMcor. Finally, the mean values and standard deviations for the four tissues across all subjects were calculated for analysis. The signal-to-noise ratio (SNR) analysis was conducted using the dual acquisition (subtraction) technique in the study. Detailed methods and results are provided in the supplementary information.
. ADC values were measured within regions of interest (ROI) encompassing four tissues: the PSD, gray matter (GM), white matter (WM), and CSF. ROIs on the ADC map, corresponding to areas on FLAIR images, were placed to avoid partial volume effects and extra-tissue noise. The ROIs were drawn by a neuroradiologist with 3 years of experience in neuroradiology. For each subject, two ROIs with clear and sufficiently large regions for PSD, CSF, GM, and WM were selected in both sagittal and coronal slices. The mean ADC values were generated using both a pixel-wise method (PWM) and an ROI-based method (RBM) in sagittal and coronal views for comparison, denoted as PWMsag, RBMsag, PWMcor, and RBMcor. Finally, the mean values and standard deviations for the four tissues across all subjects were calculated for analysis. The signal-to-noise ratio (SNR) analysis was conducted using the dual acquisition (subtraction) technique in the study. Detailed methods and results are provided in the supplementary information.
Statistical analysis
Statistical analysis was performed by using SPSS software (IBM SPSS Statistics for Windows, Version 24.0; IBM Corp., Armonk, NY). The Kruskal-Wallis H test was used to compare the four groups (PWMsag, RBMsag, PWMcor, RBMcor) across the different categories (e.g., PSD, CSF, GM, WM), and significant difference among the four tissue types (PSD, CSF, GM, WM). When the significant difference in Kruskal-Wallis H test was found in the group comparison, then Mann-Whitney U test applying a Dunn–Bonferroni post hoc correction was used to compare each pair. A p value less than 0.05 was considered to indicate statistical significance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful for the financial support. Yi-Jui Liu received financial support partly from National Science and Technology Council of Taiwan (111–2314-B-035–001-MY3). Ya-Hui Li received financial support partly from National Science and Technology Council of Taiwan (113-2314-B-039-011) and China Medical University Hsinchu Hospital (CMUHCH-DMR-114-003). Hing-Chiu Chang received financial support partly from Hong Kong Research Grant Council (GRF 17106820, GRF 17125321, and ECS 24213522).
Abbreviations
- PSD
Parasagittal dura
- GS
Glymphatic system
- ISF
Interstitial fluid
- MLV
Meningeal lymphatic vessel
- MUSE
Multiplexed sensitivity encoding
- PROPELLER
Periodically rotated overlapping parallel lines with enhanced reconstruction
Author contributions
YJ Liu and HC Chang designed the research study. YJ Liu, SC Lin, CH Liao, SL Peng, YX Lu, and YH Li performed the research. CF Hsieh, CH Lee, MT Tsai, and CJ Juan provided assistance and advice for the study’s experiments. YJ Liu, SC Lin, YX Lu, and YH Li analyzed the data. YJ Liu drafted the manuscript. All authors read and approved the final manuscript. Additionally, all authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Data availability
The data analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ya-Hui Li, Email: yahuili860916@gmail.com.
Hing-Chiu Chang, Email: hcchang@cuhk.edu.hk.
References
- 1.Secker, G. A. & Harvey, N. L. VEGFR signaling during lymphatic vascular development: from progenitor cells to functional vessels. Dev. Dyn.244, 323–331. 10.1002/dvdy.24227 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl Med.4, 147ra111. 10.1126/scitranslmed.3003748 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature523, 337–341. 10.1038/nature14432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature572, 62–66. 10.1038/s41586-019-1419-5 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Zhang, M. et al. Evaluation of glymphatic-meningeal lymphatic system with intravenous gadolinium-based contrast-enhancement in cerebral small-vessel disease. Eur. Radiol.33, 6096–6106. 10.1007/s00330-023-09796-6 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Mestre, H. et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science367. 10.1126/science.aax7171 (2020). [DOI] [PMC free article] [PubMed]
- 7.Nedergaard, M. & Goldman, S. A. Glymphatic failure as a final common pathway to dementia. Science370, 50–56. 10.1126/science.abb8739 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albayram, M. S. et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat. Commun.13, 203. 10.1038/s41467-021-27887-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, D. S., Suh, M., Sarker, A. & Choi, Y. Brain Glymphatic/Lymphatic imaging by MRI and PET. Nucl. Med. Mol. Imaging. 54, 207–223. 10.1007/s13139-020-00665-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ringstad, G. & Eide, P. K. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat. Commun.11, 354. 10.1038/s41467-019-14195-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Bihan, D., Poupon, C., Amadon, A. & Lethimonnier, F. Artifacts and pitfalls in diffusion MRI. J. Magn. Reson. Imaging. 24, 478–488. 10.1002/jmri.20683 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Chen, N. K., Guidon, A., Chang, H. C. & Song, A. W. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage72, 41–47. 10.1016/j.neuroimage.2013.01.038 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pipe, J. G., Farthing, V. G. & Forbes, K. P. Multishot diffusion-weighted FSE using PROPELLER MRI. Magn. Reson. Med.47, 42–52. 10.1002/mrm.10014 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Laajava, J. & Korja, M. Peritumoral T2/FLAIR hyperintense MRI findings of meningiomas are not necessarily edema and May persist permanently: a systematic review. Neurosurg. Rev.46, 193. 10.1007/s10143-023-02094-1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Titelbaum, D. S. et al. Leptomeningeal enhancement on 3D-FLAIR MRI in multiple sclerosis: systematic observations in clinical practice. J. Neuroimaging. 30, 917–929. 10.1111/jon.12774 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Vaswani, A. K. et al. Diagnostic accuracy of contrast-enhanced FLAIR magnetic resonance imaging in diagnosis of meningitis correlated with CSF analysis. ISRN Radiol 578986. 10.1155/2014/578986 (2014). [DOI] [PMC free article] [PubMed]
- 17.Kuo, P. H., Stuehm, C., Squire, S. & Johnson, K. Meningeal lymphatic vessel flow runs countercurrent to venous flow in the superior sagittal sinus of the human brain. Tomography4, 99–104. 10.18383/j.tom.2018.00013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah, T. et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J. Exp. Med.220. 10.1084/jem.20220618 (2023). [DOI] [PMC free article] [PubMed]
- 19.Keser, Z. et al. High-resolution MRI to noninvasively characterize drainage around the carotid artery into the cervical lymph nodes. J. Neuroimaging. 33, 102–108. 10.1111/jon.13056 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Jacob, L. et al. Conserved meningeal lymphatic drainage circuits in mice and humans. J. Exp. Med.219. 10.1084/jem.20220035 (2022). [DOI] [PMC free article] [PubMed]
- 21.Melin, E., Ringstad, G., Valnes, L. M. & Eide, P. K. Human parasagittal dura is a potential neuroimmune interface. Commun. Biol.6, 260. 10.1038/s42003-023-04634-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joo, B., Park, M., Ahn, S. J. & Suh, S. H. Assessment of meningeal lymphatics in the parasagittal dural space: A prospective feasibility study using dynamic Contrast-Enhanced magnetic resonance imaging. Korean J. Radiol.24, 444–453. 10.3348/kjr.2022.0980 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirata, K. et al. Comparison of the image quality of turbo spin echo- and echo-planar diffusion-weighted images of the oral cavity. Med. (Baltim).97, e0447. 10.1097/MD.0000000000010447 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juan, C. J. et al. Salivary glands: echo-planar versus PROPELLER Diffusion-weighted MR imaging for assessment of ADCs. Radiology253, 144–152. 10.1148/radiol.2531082228 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Walsh, D. R. et al. Mechanical properties of the cranial meninges: A systematic review. J. Neurotrauma. 38, 1748–1761. 10.1089/neu.2020.7288 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Walsh, D. R. et al. Mechanical characterisation of the human dura mater, Falx cerebri and superior sagittal sinus. Acta Biomater.134, 388–400. 10.1016/j.actbio.2021.07.043 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Chang, H. C., Guhaniyogi, S. & Chen, N. K. Interleaved diffusion-weighted improved by adaptive partial-Fourier and multiband multiplexed sensitivity-encoding reconstruction. Magn. Reson. Med.73, 1872–1884. 10.1002/mrm.25318 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, T. H. et al. Comparison of DWI methods in the pediatric brain: PROPELLER Turbo Spin-Echo imaging versus Readout-Segmented Echo-Planar imaging versus Single-Shot Echo-Planar imaging. AJR Am. J. Roentgenol.210, 1352–1358. 10.2214/AJR.17.18796 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Schaefer, P. W., Grant, P. E. & Gonzalez, R. G. Diffusion-weighted MR imaging of the brain. Radiology217, 331–345. 10.1148/radiology.217.2.r00nv24331 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Yankova, G., Bogomyakova, O. & Tulupov, A. The glymphatic system and meningeal lymphatics of the brain: new Understanding of brain clearance. Rev. Neurosci.32, 693–705. 10.1515/revneuro-2020-0106 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Ringstad, G. & Eide, P. K. The pitfalls of interpreting hyperintense FLAIR signal as lymph outside the human brain. Nat. Commun.14, 4913. 10.1038/s41467-023-40508-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ringstad, G. & Eide, P. K. Glymphatic-lymphatic coupling: assessment of the evidence from magnetic resonance imaging of humans. Cell. Mol. Life Sci.81, 131. 10.1007/s00018-024-05141-2 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naganawa, S., Kato, Y., Yoshida, T. & Sone, M. Fluid signal suppression characteristics of 3D-FLAIR with a T2 selective inversion pulse in the skull base. Nat. Commun.14, 4915. 10.1038/s41467-023-40507-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albayram, M. S., Smith, G. & Albayram, O. Reply to: the pitfalls of interpreting hyperintense FLAIR signal as lymph outside the human brain. Nat. Commun.14, 4912. 10.1038/s41467-023-40510-8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vera Quesada, C. L., Rao, S. B., Torp, R. & Eide, P. K. Immunohistochemical visualization of lymphatic vessels in human dura mater: methodological perspectives. Fluids Barriers CNS. 20, 23. 10.1186/s12987-023-00426-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, M., Park, J. P., Kim, S. H. & Cha, Y. J. Evaluation of dural channels in the human parasagittal dural space and dura mater. Ann. Anat.244, 151974. 10.1016/j.aanat.2022.151974 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Zhou, Y. et al. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann. Neurol.87, 357–369. 10.1002/ana.25670 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Gabr, R. E., Lincoln, J. A., Hasan, K. M. & Kramer, L. A. Functional assessment of the dural lymphatic vessels using dynamic contrast MRI in multiple sclerosis. Brain Behav.13, e3042. 10.1002/brb3.3042 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou, Y. et al. Impaired meningeal lymphatics and glymphatic pathway in patients with white matter hyperintensity. Adv. Sci. (Weinh). 11, e2402059. 10.1002/advs.202402059 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang, H. C., Hui, E. S., Chiu, P. W., Liu, X. & Chen, N. K. Phase correction for three-dimensional (3D) diffusion-weighted interleaved EPI using 3D multiplexed sensitivity encoding and reconstruction (3D-MUSER). Magn. Reson. Med.79, 2702–2712. 10.1002/mrm.26944 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Yamada, S. et al. Usefulness of intravoxel incoherent motion MRI for visualizing slow cerebrospinal fluid motion. Fluids Barriers CNS. 20, 16. 10.1186/s12987-023-00415-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falconer, J. C. & Narayana, P. A. Cerebrospinal fluid-suppressed high-resolution diffusion imaging of human brain. Magn. Reson. Med.37, 119–123. 10.1002/mrm.1910370117 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Skare, S. & Andersson, J. L. On the effects of gating in diffusion imaging of the brain using single shot EPI. Magn. Reson. Imaging. 19, 1125–1128. 10.1016/s0730-725x(01)00415-5 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed during the current study are available from the corresponding author on reasonable request.