Abstract
Aeration is widely employed in water and wastewater treatment systems. Aerobic wastewater treatment relies significantly on maintaining adequate levels of dissolved oxygen (DO) in water to ensure optimal quality of treated wastewater. Conventional aeration systems, however, require considerable energy usage due to the ineffectiveness of oxygen mass transfer to the treated water. Adopting micronanobubbles (MNB) can improve this limitation due to their high gas-liquid mass transfer rates. This study used micro-nano air bubbles to experimentally determine the volumetric oxygen transfer coefficient (kLa), standard oxygen transfer rate (SOTR), and standard oxygen transfer efficiency (SOTE) of an MNB aeration system and compared them with those of a conventional aeration system. The MNB generation and bubble analysis results showed that the MNB concentration was 10^8–10^9 bubbles/mL, with an average diameter ranging from 100 nm to 2 μm. MNB aeration achieved a maximum kLa of 0.4204 min−1 at an airflow rate of 0.5 ± 0.1 LPM for a 25 L water volume, which was notably higher than the corresponding observed values for the conventional aeration system. Furthermore, MNB aeration demonstrated superior SOTR and SOTE values across all airflow rates tested, achieving a maximum of 139.78 mg/h and 54.33 %, respectively. The findings of this study suggest that MNB aeration, with its enhanced mass transfer characteristics, offers a more energy-efficient alternative to conventional aeration methods, with the potential for higher oxygen transfer rates and improved wastewater treatment performance. Results also indicate that optimal operation at specific airflow rates can increase oxygen transfer efficiency by two folds compared to conventional aeration systems.
Keywords: Aeration, Airflow rate, Micronanobubbles, Oxygen transfer dynamics, Water treatment
Highlights
-
•
The study identified an optimal air-to-water flow rate ratio for generating MNBs.
-
•
MNB bubble size 100 nm ‒ 2 μm showed enhancement in the efficiency of oxygen transfer rates than the bubbles produced by the diffuser aeration.
-
•
MNB showed higher kLa at a low airflow rate than that obtained using the diffuser aeration.
-
•
MNB is crucial for various applications involving efficient mass-transfer processes.
1. Introduction
Aeration is an essential process in treating wastewater that involves introducing air into water to maintain healthy biological growth in the system. Aeration accomplishes two main objectives: first, it delivers the essential oxygen needed by the metabolizing microorganisms, and second, it facilitates mixing, ensuring efficient interaction between the microorganisms and both dissolved and suspended organic matter within the wastewater [1]. Wastewater treatment technologies with high efficiencies and low costs are needed for efficient pollutant removal at wastewater treatment plants (WWTPs) before their discharge or reuse, thus contributing to water conservation. Micronanobubble (MNB) technology is a promising technology with the potential to transform traditional water treatment methods by improving efficiency, reducing energy consumption, and minimizing environmental impact [[2], [3], [4], [5], [6], [7]].
Dissolved oxygen (DO) is critical for assessing water quality in aerobic wastewater treatment plants. Adequate DO levels are essential for maintaining microbial growth, contributing to the increased removal of pollutants [1]. A major challenge in maintaining the required DO at wastewater plants is attributed to power consumption during the aeration process of the activated sludge process (ASP). Usually, aeration in ASP systems is achieved by bottom diffusers [8]. The efficiency of diffusers of macro bubbles is considerably low, ranging between 6 % and 30 %, which results in huge quantities of air that need to be pumped to achieve the required treatment level [9]. A critical factor in enhancing the efficiency of wastewater treatment is the transfer of oxygen in water. The aerobic biodegradation capacity is related to the growth of site-specific microorganisms, which are significantly affected by the oxygen content in water [10]. Thus, there is a need to improve oxygen transfer in the aeration tanks of ASP to reduce energy consumption and the carbon footprint associated with the process.
Conventional aeration methods can generate macrobubbles with diameters ranging from millimeters to centimeters. However, these larger bubbles have a brief lifespan in water and prove inefficient for the oxygen transfer process [11,12]. Conventional diffusion aeration exhibits limited oxygen transfer efficiency, ranging from 6 % to 10 % in applications such as ASP treatment plants [13]. This inefficiency leads to a limited zone of influence and reduced activity of aerobic microorganisms [2,14]. Studies by various researchers on MNBs have proposed this as an effective technique for enhancing oxygen transfer in water compared to traditional methods utilizing macro bubbles. The discovery of microbubbles with a radius of 30 μm was initially made by Turner in water in 1961, which were observed to persist for extended periods [[15], [16], [17], [18]]. Similarly, nanobubbles exhibit a prolonged presence in water, and their stability can be attributed to the electrically charged liquid-gas interface, generating repulsion forces that prevent bubble coalescence [19]. MNBs, characterized by their tiny diameters in the range of micrometers and nanometers, display significant potential for environmental remediation [11].
As a result, MNB technology, boasting an oxygen transfer efficiency spanning from 30 % to 100 %, has emerged and found applications across various fields, including biology, chemistry, and environmental science [9,20,21] However, a significant challenge arises from the lack of consistency in defining and categorizing MNBs. For instance Ref. [17], delineated microbubbles or fine bubbles as minute bubbles ranging from 10 to 50 μm or 10–60 μm, respectively. In contrast [2], classified bubbles smaller than 200 nm as nanobubbles. In contrast [22], defined nanobubbles as bubbles between 1 nm and 1 μm. Researchers have often amalgamated microbubbles and nanobubbles, collectively referring to them as “micronanobubbles” without strictly distinguishing between them.
Numerous aeration devices, such as rotors, agitators, sprayers, and diffusers, such as fine bubble diffusers, coarse diffusers, and dissolved air floatation (DAF) equipment, are employed in wastewater treatment to optimize a consistent distribution of DO throughout the aeration tanks [7,8,[23], [24], [25], [26]]. Such devices introduce oxygen into the water by emitting bubbles from a diffuser disc at the bottom of the aeration tanks. In the literature, the efficiency of aeration systems is assessed using criteria such as the standard oxygen transfer rate (SOTR) and standard aeration efficiency (SAE). The literature has documented that results may exhibit differences of up to 40 % when employing different aeration devices [[27], [28], [29], [30]]. SOTR represents the oxygen transfer rate of an aerator from deoxygenated levels to saturated levels under standard conditions. At the same time, SAE indicates the efficiency of an aerator in aerating the aeration tank relative to the energy required. SOTR and SAE values can also vary based on operating conditions [10,14,17,28]. While numerous aeration studies have been conducted to quantify the SOTR for various aerators, the existing experimental results lack a foundation to predict performance based on bubble characteristics. Therefore, there is a pressing need to develop models for MNB aeration by bridging the gap between theoretical and experimental oxygen transfer rates, considering bubble size characteristics. This study focused on the experimental characterization of oxygen transfer for the proposed MNB aeration method.
We hypothesized substituting conventional macro air bubbles with MNBs in the aeration tanks of ASP systems, leading to a reduced volume of air pumped into the reactor and a consequent decrease in aeration costs. MNBs are tiny bubbles with diameters on the order of micrometers and nanometers, showing great potential for environmental remediation [11]. Based on the differences in their sizes, bubbles are categorized as macrobubbles, microbubbles (MBs), and nanobubbles (NBs) for conventional or big bubbles, fine bubbles, and ultrafine bubbles, respectively [2]. Garibay et al. [31] employed the term conventional bubbles to describe bubbles with sizes ranging between 600 and 2500 μm. In 2017 [9], suggested the size boundaries of the MBs, with a minimum size of approximately 10 μm, while the maximum size suggested was 100 μm. The most typical tools used for measuring the size and size distribution of the MBs and NBs are the laser-diffraction particle-size analyzer [2], atomic force microscope [32], and image analysis [33]. The size and size distribution of the MBs and NBs were affected by the design and operation conditions. The size and fraction of the MBs mainly depend on the pressure differences across the nozzle system; for example, high pressure would produce smaller bubbles because of an increase in air density, and at pressures above approximately 3.5 atm, the sizes of the MBs were almost constant [33].
Despite significant research in the literature demonstrating the effectiveness of reducing bubble sizes for mass transfer in the aeration process, to the authors’ knowledge, most studies compared the DO concentration and mass transfer using ultrapure/pure water and synthetic wastewater. As a result, the oxygen transfer dynamics using real wastewater under different generation mechanisms remain with limited studies as well as the effect of real wastewater on the generation process [34]. Hence, the study used domestic wastewater to clarify the possibility of upgrading the current aeration mechanism. Thus, the novelty of this study was to compare the aeration parameters using MNBs and MBs in parallel systems. This was conducted by investigating the effect of different air flowrate on the oxygen transfer dynamics which are (1) the volumetric oxygen transfer coefficient (kLa), (2) the standard oxygen transfer rate (SOTR), and (3) the standard oxygen transfer efficiency (SOTE).
This study builds upon the extensive branch of research on MNB technology, focusing on its application to improve oxygen transfer efficiency under controlled lab conditions. While previous studies have established the benefits of MNBs for mass transfer processes, our work provides new insights into underexplored operational parameters, such as the effect of specific air-to-water flow rate ratios on MNB generation and subsequent oxygen transfer performance.
In addition to confirming known trends, such as the inverse relationship between bubble size and mass transfer efficiency, our study employs a novel MNB generation system, enabling precise control and measurement of key parameters. This approach offers a fresh perspective by quantitatively linking MNB concentration to enhancements in oxygen transfer efficiency, providing practical design recommendations that were previously underreported. These advancements, when compared to prior studies, underscore the potential of MNB technology for applications requiring efficient oxygen transfer, such as wastewater treatment and chemical processing. By presenting these findings, we aim to contribute to the ongoing development and optimization of MNB-based aeration technologies.
Therefore, this research studied the oxygen transfer dynamics of MNB aeration and diffuser aeration in clean water and wastewater across two different laboratory setups. Subsequent comparisons were made to assess the impacts of aeration mechanisms on the values that define the mass transfer efficiency of oxygen in water. The primary objective of the study involved experimentally illustrating the DO transfer efficiency of the proposed MNB generation system. This involved a dual approach. Initially, real-time measurement of parameters essential to the oxygen transfer model, such as airflow rate and bubble diameters, was necessary. A bench-scale aeration setup was assembled to facilitate experiments with simultaneous measurements of the required parameters. Next, kLa, SOTR, SOTE, oxygen uptake rate (OUR), and alpha (α) and beta (β) factors were determined and compared with the values obtained for the diffuser aeration. The experimental determination of these values involved utilizing the aeration setups to raise DO levels to saturation in water.
2. Materials and methods
2.1. Theoretical background of oxygen transfer dynamics in water
Oxygen transfer in water occurs when air bubbles are introduced into the water. The oxygen within the bubbles moves from the gas to the liquid phase. This process continues until an equilibrium state is reached. The interface between gas and liquid can be dissected into a liquid interface, a gas interface, and a surface interface. The origins of various models that explain mass transfer are film [35] and penetration [36] theories, and they only vary in their mathematical expressions for the transfer of mass across the interface surface. The rate of mass transfer is usually expressed as:
| (Equation 1) |
where KL is the liquid film coefficient (m/h), A is the cross-sectional area through which diffusion occurs (m2), Cs is the oxygen concentration in the water phase at saturation (mg/l), and Ct is the oxygen concentration in the water phase at time t (mg/l).
The movement of oxygen occurs in a reactor tank designed for aeration through the boundary between the gas and liquid phases, facilitated by both diffusion and bulk transport. The transfer of oxygen through the gas film from the bulk gas occurs at a rate proportional to the difference between the concentration in the bulk gas solution and the concentration at the gas–liquid interface. The speed at which oxygen is transferred is influenced by the concentration gradient between the current DO concentration in the water and the theoretical saturation level. Therefore, oxygen transport modeling within the aeration system holds significance as it serves as a benchmark for enhancing overall process performance, guiding process design, and facilitating simulation. Considering the volume of the tank in Equation (1), the mass balance equation can be represented as in Equation (2):
| (Equation 2) |
To serve the practical purposes, the overall gas transfer coefficient, KL a, is defined, and therefore, Equation (3) can be reqritten as the rate equation shown below:
| (Equation 3) |
where KL a is the overall transfer coefficient expressed in h−1, and a denotes the ratio of A to V.
In wastewater treatment, the gas transfer rate is commonly characterized by the α-factor that represents the correlation between wastewater and the oxygen transfer coefficient for clean water and is, therefore, usually selected to facilitate the comparison of oxygen transfer coefficients. This is the overall oxygen transfer coefficient ratio in wastewater to the oxygen transfer in clean water. In the design of oxygenation systems, it is vital to possess reliable values for α and β factors. These parameters play a crucial role in accurately estimating oxygen transfer rates and adjusting for the impact of dissolved solids on the solubility of oxygen in water, ensuring the effectiveness and efficiency of the oxygenation process.
Understanding the alpha factor, denoted as “α,” is crucial, as it represents the ratio between oxygen uptake rates in wastewater and clean water. Similarly, the presence of organic and inorganic dissolved solids in wastewater can diminish the solubility of DO in water. To account for this effect, the beta factor, represented by the symbol “β,” is employed to consider the solubility of oxygen in consideration of the concentration of dissolved solids in the wastewater. In the case of a wastewater treatment plant, particularly in the activated sludge reactor, the representation of the oxygen balance in the aeration tank is influenced by microbial respiration and DO concentrations in the influent and effluent, as outlined by the American Society of Civil Engineers [37,38]. Therefore, having a clear idea of the alpha and beta values while designing and operating aeration systems for ASP is imperative. The α factor is given using Equation (4).
| (Equation 4) |
Similarly, β factor is employed to adjust for the impact of dissolved solids concentration in wastewater on the solubility of oxygen. Typically, oxygen solubility in municipal wastewater treatment plants is less efficient than its transfer in clean water. The β factor is given using Equation (5).
| (Equation 5) |
In addition, the temperature correction for the KL a is given using Equation (6).
| (Equation 6) |
where the value of θ is estimated at 1.024 [39].
The efficiency of an aeration system in transferring oxygen to water is quantified using The oxygen transfer rate (OTR) and is defined by Equation (7). Knowledge of OTR helps in process optimization by ensuring sufficient oxygen is supplied for biological process like ASP. It also helps to evaluate oxygen supply in natural water bodies to support aquatic life.
| (Equation 7) |
Where C0 is the oxygen present in the water originally.
However, for the purpose of making it a reference value for system design and comparison thhe KL a determined under standard conditions can be used to calculate the SOTR and SOTE of an aeration system. SOTR is defined as the quantity of oxygen transferred at standard conditions per hour and is given using Equation (8).
| (Equation 8) |
SOTE is a measurement used to assess how efficiently an aeration system transfers oxygen from the gas to the liquid phases. It is expressed as a percentage and indicates the actual oxygen transfer rate ratio to the maximum theoretical oxygen transfer rate achievable under standardized conditions. A higher SOTE value suggests greater efficiency in oxygen transfer.
| (Equation 9) |
where W O2 is the mass flow rate of oxygen in the airstream used for aeration.
Another parameter determined in this study is OUR. OUR is a crucial parameter, particularly in wastewater treatment with an ASP. OUR represents the rate at which microorganisms consume oxygen for metabolic processes, providing valuable insights into microbial activity and overall ecosystem health. OUR is typically measured by monitoring the decrease in DO concentration over time in a closed system, where the consumption of oxygen by microorganisms leads to a decline in oxygen levels. OUR is expressed using Equation (10):
| (Equation 10) |
where is the change in DO concentration (in units of mass per unit volume, typically expressed as mg/L or g/m³) and is the change in time.
2.2. Experimental setup
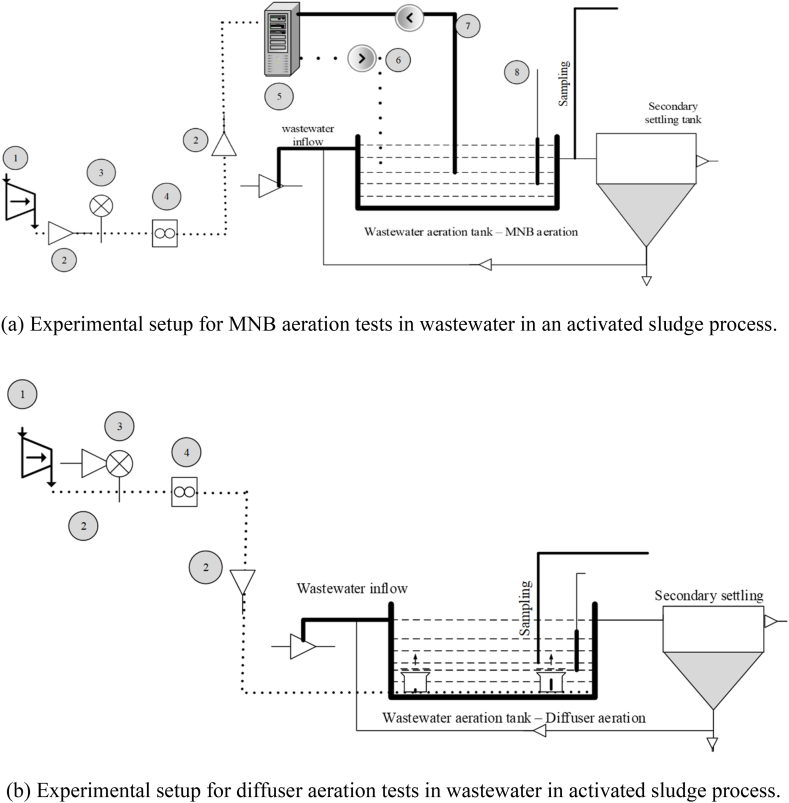
The experiments were conducted to study the dynamics of oxygen transfer in a bench-scale setup for MNB aeration in clean water and wastewater and to compare it with diffuser aeration. The experiments involved determining the volumetric DO transfer coefficient, alpha and beta factors, SOTR, and SOTE. The overall study included two stages: (1) aeration experiments with tap water in a glass tank setup and (2) aeration experiments with wastewater in an activated sludge setup. Two bench-scale aeration setups were established to conduct experiments under unsteady conditions for both cases, and observed values were employed to determine the dynamics parameters for each experiment.
The experimental setup for measuring the volumetric oxygen transfer coefficient comprised separate bench-scale water tanks constructed from glass, with dimensions of 35 cm in length, 22 cm in width, and 28 cm in height (l × b × h), and an aeration system. The MNB experiments used an MNB generation system (as discussed in Section 2.2.1). A suitable online flowmeter was employed to measure the airflow rate. Temperature readings were recorded at the beginning and end of the experiment, with a permissible maximum difference of 5 °C. This protocol maintained uniformity in experimental settings and reduced the potential impact of temperature changes on outcomes. The experiment followed a non-steady-state gas transfer to evaluate the effect of aeration methodology. The experiments were conducted by covering the headspace to avoid any natural air transfer.
2.2.1. MNB and diffuser aeration systems
MNB bubbles were generated in a continuous system, as illustrated in Fig. 1a. The main components of the MNB generator consisted of a self-priming pump working at a pressure of 3.5–4.5 bar, a bubble generator, and a nozzle or valve. The system injects controlled air by adjusting the flow rate using a rotameter and water into the pump, which is sheared by the impellers of the pump. This results in the formation of an air/water mixture, which is then subjected to a high-pressure condition inside the nanobubble generator, and the saturated air/water mixture is forced through a nozzle at the bottom of the aeration tank. Therefore, the MNB system works by recirculating the water volume in the tank at a fixed flow rate, allowing a fixed residence time in the system. The system is subject to a temperature rise. However, the temperature is controlled using ice packs and a water bath.
Fig. 1.
Schematic representation of experimental setup using clean water.
The diffuser aeration used compressed air into the system through diffusers installed in the tank in the experiments to study diffused aeration DO transfer. This feature provided equal spacing between diffusers, as shown in Fig. 1b. Ultrafine diffusers available in the market were used for the diffused aeration to deliver compressed air, and the diffusers were porous disc-type stones. The diffusers were placed near the bottom of the system, a common practice chosen for the optimal distribution of aeration throughout the water tank. The experimental conditions for both aeration systems were identical, differing only in the air delivery system.
2.2.2. Particle analyzer and other equipment for analysis
The particle size distribution of the bubbles generated was measured using a nanoparticle tracking analyzer, NANOTRAC Wave II Particle Analyzer manufactured by Microtrac (Germany), which works on the principle of dynamic light scattering (DLS). The analyzer uses laser beams of 780 nm and can measure particles in the range of 0.8 nm and 10 μm.
The DO in the water was measured using the Hach LDO101 model probe, which worked on the principle of luminescence. The probe can measure the DO levels in the range 0–20 mg/l and with an accuracy of ±0.1 mg/l for values less the 8 mg/l and 0.2 mg/l for values above 8 mg/l.
2.2.3. Samples and chemicals
Clean water experiments were conducted using potable water collected from the tap. The wastewater experiments were conducted on the wastewater after primary settling collected from the nearest municipal wastewater treatment plant (WWTP), Al Saad WWTP, in Al Ain, United Arab Emirates. If required, sodium sulfite (Na2SO4) was used to deoxygenate the DO levels between 0.5 and 0.75 mg/L.
2.3. Experiment methodology
2.3.1. Experiments with clean water
The tank was filled with clean water up to a volume of 25 L, as shown in Fig. 1a and b, to initiate each experimental run. Before conducting the aeration tests, the initial DO concentration of the water was measured, assuming uniformity throughout the water volume. The experiments involved deoxygenation, followed by reaeration to a saturation value. Therefore, deoxygenation of the water was conducted using a solution containing Na2SO4 and was continued until the DO content ranged from 0.5 to 0.75 mg/L. Subsequently, the respective aeration systems were operated to allow the DO content of the water to reach saturation. Saturation was achieved depending on the aeration system airflow rates used. The experiments were run with different airflow rates. The range of volumetric airflow rates investigated varied from 0.5 low air flow rate LPM (low air flow rate) to 2.5 LPM (medium flow rate) and 5 LPM (high flow rate), thus investigating the impact of low to high air flowrates for a selected volume of water. Aerated water samples were drawn from the tank to characterize the bubble size distribution using a nanoparticle tracking analyzer.
2.3.2. Experimental determination of oxygen transfer coefficients in wastewater
The wastewater experiments were conducted following the established guidelines for in-process oxygen transfer testing, as stipulated by Refs. [37,38]. Domestic wastewater, after post-primary settling collected from the nearby municipal wastewater treatment plant Al Saad WWTP in Al Ain, United Arab Emirates, was used. Oxygen transfer was affected by the oxygen uptake of the microorganisms in the aeration of the wastewater. The study was conducted using a method similar to tap water. The primary settled wastewater was stored in a tank and pumped into the aeration tank, which was also activated with RAS from the sludge settling tank (Fig. 2a and b). Experiment sets were conducted by aerating both systems separately to reach the saturated DO levels while keeping the operating conditions of the ASP system constant (hydraulic retention time of 8 h). The mixed liquor suspended solids (MLSS) concentration was about 3000 mg/l, and there was a variation only in the airflow rate: 0.5 LPM (low flow rate), 2.5 LPM (medium flow rate), and 5 LPM (high flow rate).
Fig. 2.
Schematic representation of experimental setup for the activated sludge process.
3. Results and discussion
3.1. Bubble characterization results
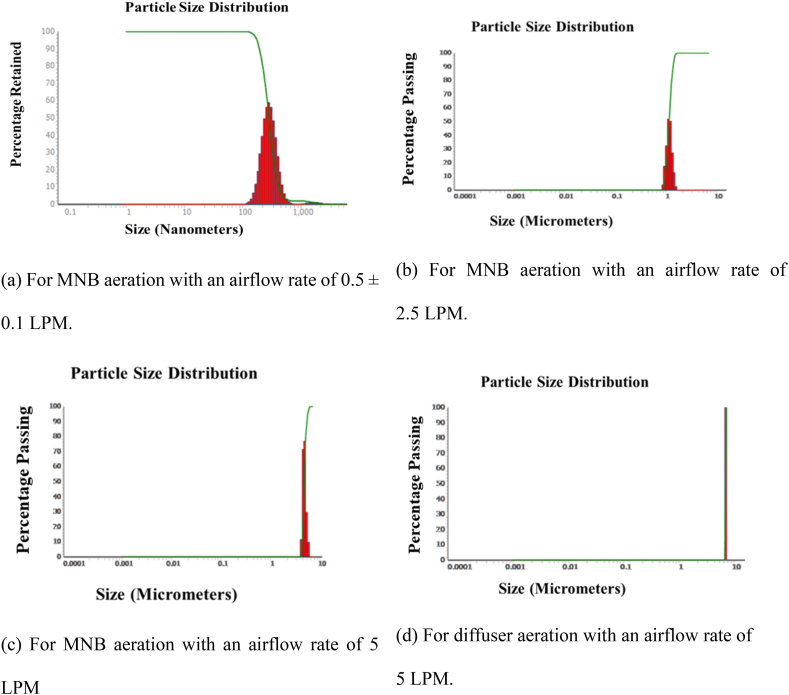
The study analyzed the mean size and distribution of bubbles generated using two aeration methods, employing a NANOTRAC Wave II Particle Analyzer. Fig. 3a–c illustrate the particle size distribution curves for various flow rates (0.5 LPM, 2.5 LPM, and 5 LPM) of the clean water experiments. The results indicated that MNB aeration effectively generated MNBs in clean and processed water conditions. The most favorable outcomes regarding MNB concentration and mean sizes were observed at a flow rate of 0.5 LPM. As the flow rate increased, so did the mean size of the bubbles, while the distribution intensity decreased. This suggests that insufficient time may be available for gas molecules to dissolve into the liquid phase at high flow rates, reducing the gas concentration necessary for bubble formation despite the increased gas flow rate.
Fig. 3.
Bubble size distribution of the aerated clean water samples at different airflow rates for MNB and diffuser aeration.
The results presented in Fig. 3a and b highlight the critical influence of airflow rate on the size distribution and mean size of micro-nano bubbles (MNBs). At an airflow rate of 0.5 LPM, the MNBs generated were predominantly in the 100–1000 nm range, although the generation was not entirely uniform. The mean size of approximately 1000 nm suggests that this flow rate is optimal for generating smaller MNBs, which are crucial for maximizing surface area and enhancing mass transfer efficiency. This observation aligns with prior research, indicating that smaller bubble sizes significantly improve oxygen transfer by increasing the interfacial area for gas-liquid interactions.
As the airflow rate increased to 2.5 LPM, a notable narrowing in the size distribution and an increase in the mean bubble size were observed. This trend indicates that higher flow rates likely promote coalescence or other physical effects that lead to the formation of larger MNBs. The narrowing distribution curve suggests a more uniform generation process at this rate, potentially due to a stabilization of the bubble generation mechanism. However, the increase in size may reduce the effectiveness of MNBs in certain applications, as larger bubbles typically exhibit lower mass transfer efficiencies due to reduced interfacial area.
At 5 LPM, the mean particle size further increased, and the distribution curve narrowed even more. The observed size increase and narrowing may also result from the limitations of the Microtrac nanoparticle analyzer, which cannot detect larger bubbles. This indicates that at higher flow rates, a transition occurs where the generation of larger bubbles dominates, potentially leading to a decrease in MNB production efficiency. Larger bubbles are less effective for mass transfer and may hinder the intended application, emphasizing the importance of optimizing flow rates for specific operational goals.
These findings reinforce the importance of carefully controlling airflow rates in MNB systems to achieve the desired bubble size distribution. While lower flow rates favor smaller MNB generation, higher rates may compromise performance by producing larger bubbles. This trade-off highlights the need for balancing flow rates to optimize mass transfer processes for applications such as wastewater treatment, chemical processing, and biomedical engineering.
Bubble sizes depend on temperature, air flow rate, and air-to-water ratio, affecting bubble dispersion in water. An air-to-liquid flow rate ratio of 0.04–0.05 was optimal, influencing the pressure in the vessel, with approximately 0.4–0.45 MPa being optimal for the best results. This pressure was achieved at a gas flow rate of 0.5 LPM. An increase in the air-to-water ratio led to reduced vessel pressure (0.3–0.4 MPa), affecting MNB generation. Thus, an optimal air-to-liquid flow rate ratio (0.04–0.05) and pressure (0.4–0.45 MPa) were identified as effective for producing favorable results in MNB generation, which directly correlated with efficient MNB generation. This optimal pressure range was achieved at a gas flow rate of 0.5 LPM, suggesting that precise control of operational parameters is essential for maximizing MNB production.
The average size of the bubbles fluctuated over time but consistently fell below the range of 6000–7000 nm. The highest concentration was around 10^9 for an airflow rate of 0.5 L per minute (LPM), while for higher gas flow rates of 2.5 LPM and 5 LPM, the concentration was around 10^7 bubbles/per ml.
Temperature fluctuations also played a crucial role in bubble size dynamics, with even minor temperature increases (1–2 °C per minute) potentially causing bubble breakage or coalescence. This phenomenon can alter the production of MNBs, particularly in systems with inefficient thermal regulation. In the current study, the use of ice packs and a water bath within the water volume used resulted in a manageable temperature rise. This highlights the necessity of integrating thermal management strategies into MNB generation systems to maintain optimal operating conditions.
The diffuser aeration method employed in this study yielded distinct outcomes compared to MNB aeration, particularly regarding particle size distribution. Analysis of the samples revealed no detectable concentration of MNBs within the size range of less than 2000 nm. However, few particles were detected in the 6000 nm range (Fig. 3d) for a gas flow rate of 5 LPM. In the case of a gas flow rate of 0.5 LPM, demonstrating optimal MNB concentration with MNB generation, diffuser aeration resulted in inadequate aeration in the water. Insufficient air bubbles were produced through the diffuser pores, likely due to the low-pressure difference achieved at the openings. Nonetheless, an increase in the gas flow rate led to improved bubble formation; however, upon analysis with the particle analyzer, the concentration of MNBs fell below the detectable limit. This could be attributed to the bubbles formed being larger than the MNB range, estimated to be greater than 10000 nm. Consequently, diffuser aeration under the conditions of this study did not generate a sufficient quantity of MNBs. Therefore, a comparative study of these two aeration methods is warranted to elucidate the dynamics parameters of MNB and MB (>10000 nm) aeration in water and wastewater.
The findings demonstrate that bubble size and generation efficiency in micro-nano bubble (MNB) systems are significantly influenced by the air-to-liquid flow rate ratio, pressure, temperature, and gas flow rate.
3.2. Comparison of DO profiles
The comparison of DO profiles between the two aeration methods—MNB aeration and diffuser aeration—provides valuable insights into their respective efficiencies in oxygen transfer. As observed, the DO concentration gradually increased over time before stabilizing at the equilibrium concentration, denoted as Cs in Equation (1). This trend is typical in aeration systems, where oxygen transfer continues until saturation is reached.
MNB aeration was notably more effective in rapidly increasing the DO concentration, achieving higher saturation levels than diffuser aeration. The rapid rise in DO concentration can be attributed to the smaller size of MNBs, which provides a larger surface area for oxygen exchange between the gas and liquid phases. This is consistent with prior studies that have highlighted the superior mass transfer capabilities of micro-nano bubbles in enhancing oxygen solubility in water.
At the optimal airflow rate of 0.5 LPM for MNB aeration, the DO concentration reached its maximum peak, demonstrating that controlled airflow rates are crucial for maximizing oxygen transfer efficiency. This finding underscores the importance of fine-tuning operational parameters to achieve optimal performance in MNB systems. In contrast, diffuser aeration showed a more gradual increase in DO concentration, with higher airflow rates resulting in incremental increases in oxygen levels. This suggests that while diffuser aeration can increase DO levels, it does so at a slower rate and may not achieve the same peak saturation as MNB aeration under comparable conditions.
The DO profiles of MNB aeration in clean water, as shown in Fig. 4a, further confirm the efficiency of MNBs in enhancing oxygen transfer, with the concentration stabilizing at a higher level in less time compared to traditional diffuser aeration. This indicates that MNB technology could offer significant advantages for applications that require rapid and efficient oxygenation, such as wastewater treatment and chemical processing, where high DO levels are critical for microbial activity and reaction rates.
Fig. 4.
Experimental DO profile for MNB and diffuser aeration.
Overall, these results highlight the potential of MNB aeration to outperform traditional aeration methods in terms of both speed and maximum DO concentration, reinforcing the suitability of MNBs for environments where oxygen transfer is a limiting factor.
In Fig. 4a, for MNB aeration in clean water, the plots depict the DO levels throughout the aeration. Specifically, aeration took 12, 15, and 14 min to reach saturated DO levels using airflow rates of 0.5, 2.5, and 5 LPM, respectively, with the highest saturation of 13.1 mg/l achieved by MNB at 0.5 LPM, suggesting that an airflow rate beyond a threshold did not increase saturation levels. This high saturation at a low airflow rate could be attributed to the high concentration of MNBs with smaller mean diameters generated than those produced at higher flow rates, as seen from the results obtained in Section 3.1. These smaller bubble sizes resulted in a higher surface area-to-volume ratio, facilitating rapid oxygen dissolution in the water. Conversely, even at a high air supply rate of 5 LPM, diffuser aeration recorded a significantly lower maximum DO level than MNB aeration. In addition, diffuser aeration required more time to reach saturated DO levels, taking about 20 min for airflow rates of 2.5 and 5 LPM, as shown in Fig. 4b. In contrast, the maximum DO level was recorded by diffuser aeration at a high air supply rate of 5 LPM.
However, the saturation level achieved was significantly below that achieved by the MNB aeration. The DO saturation achieved for 2.5 LPM and 5 LPM showed a slight difference of about 0.2 mg/l even though the air supply was doubled. The experiments of diffuser aeration with an airflow rate of 0.5 LPM only showed a slight increase in DO from the initial value, and it was not plotted in the graph. The DO profile for wastewater experiments for different airflow rates is shown in Fig. 4c and d for MNB and diffuser aeration, respectively. Like clean water, the DO profile showed the same DO increase pattern, but the saturation levels achieved were lower than the clean water. This result could be because of the hindrance to the DO solubility caused by the suspended particles and microbial activity, which used oxygen for exogenous and endogenous respiration. Another important observation was that the temperature rise was fast in the case of MNB aeration, increasing more when the airflow rate increased. However, the temperature rise in diffuser aeration was not significant.
The observed DO saturation concentrations of the diffuser aeration were consistently lower than the MNB aeration DO saturation concentrations across all respective flow rates. However, it was plausible that the samples did not reach saturation levels by the end of the testing period in diffuser aeration, indicating that the aeration was inefficient in the quick mass transfer of DO into water.
In summary, the results demonstrate MNB aeration rapidly increases DO levels, achieving higher saturation and faster stabilization, especially at the optimal airflow rate of 0.5 LPM. In contrast, diffuser aeration shows a more gradual increase in DO, with higher airflow rates leading to only incremental improvements in oxygen transfer. These findings highlight the efficiency of MNB aeration for applications requiring quick and effective oxygenation, such as wastewater treatment and chemical processing, where maximizing DO concentration is crucial for optimal performance.
3.3. Determination of volumetric oxygen transfer coefficients
Various methods have been discussed in the literature to estimate the KLa, including chemical, Na2SO4 oxidation, CO2 absorption, and physical methods [40]. The dynamic desorption and absorption method, categorized as a physical method, is considered more reliable, as it only necessitates measuring oxygen concentration in the liquid over time. The dynamic absorption technique involves eliminating oxygen from the liquid phase, achieved through methods such as bubbling nitrogen or introducing Na2SO4 until the oxygen concentration reaches near zero. In this study, the kLa was determined using the dynamic method. This can help estimate oxygen transfer rates under different conditions, facilitating the optimal design of full-scale aeration systems and the implementation of cost-saving practices in large-scale wastewater treatment plants. The variation in DO levels in the water from the start to the end of the experiment until saturation DO is reached, forming the basis of kLa determination.
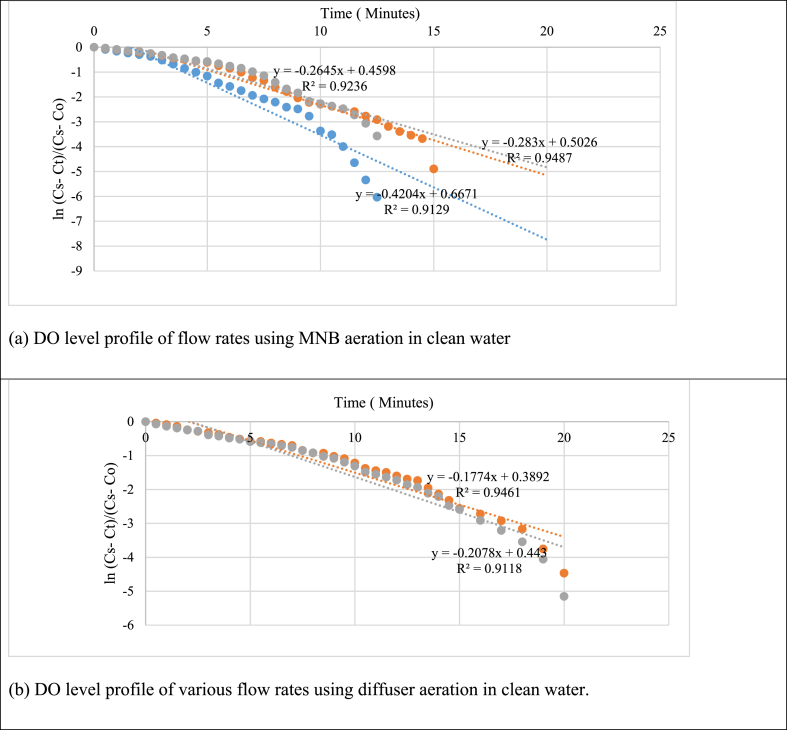
The initial time-varying and saturation DO concentrations, as in Equation (1), were obtained for all experiment runs. Then, by plotting Ln Cs–Ct/Cs–C0 versus the time plot, several curve fittings were used to capture the relationship between ln [(Cs–Ct)/(Cs–C0)] and time. The curve-fitting process involved iterative steps to accurately capture the relationship between DO saturation and time. From the fitted curve, the slope of the linear portion of the plot provides the value of kLa. This slope represents the oxygen transfer rate from the gas phase to the liquid phase per unit volume per unit of time. The kLa values obtained for the experiments were analyzed to compare the oxygen transfer efficiency between MNB aeration and diffuser aeration at different gas flow rates in clean water and wastewater.
The results illustrated in Fig. 5a–d clearly demonstrate MNB aeration consistently achieves higher kLa values compared to diffuser aeration across all tested flow rates. Specifically, in clean water experiments, the kLa values for MNB aeration at 0.5 LPM, 2.5 LPM, and 5 LPM were 0.4204 min−1, 0.283 min−1, and 0.264 min−1, respectively, MNB showing a slight decrease with increasing airflow. This decline may reflect diminishing efficiency in gas-liquid mass transfer as bubble generation becomes less effective at higher airflow rates, potentially due to coalescence or turbulence. Conversely, in diffuser aeration, the kLa increased with higher air flow rates, with values of 0.177 min−1 and 0.2078 min−1 observed for 2.5 LPM and 5 LPM, respectively. This behavior aligns with the conventional understanding that larger airflow enhances oxygen delivery in diffuser systems but remains less efficient than MNB aeration due to larger bubble sizes and shorter residence times.
Fig. 5.
Plot of ln (Cs–Ct)/(Cs–Co) vs. time for various flow rates to determine kLa values.
The trends were similar in wastewater experiments, although kLa values were lower overall due to the presence of suspended particles and microbial activity, which inhibit oxygen transfer. MNB aeration maintained its superiority under these challenging conditions, likely due to the bubbles' ability to penetrate into biofilms and overcome the resistance posed by particulate matter. These findings underscore the potential of MNB aeration as a more effective alternative for applications requiring high oxygen transfer efficiency, especially in complex fluids like wastewater. The data also suggest that optimizing flow rates could further enhance the performance of MNB aeration systems while maintaining energy efficiency.
The experiments revealed that MNB aeration consistently provided higher kLa values than diffuser aeration, even at lower airflow rates. The findings suggest that MNB aeration provides significant advantages in terms of oxygen transfer efficiency due to its unique properties, which enhance solubility and improve the rate of oxygen transfer. This could make MNB aeration a valuable approach for optimizing oxygenation in water treatment processes, offering potential cost savings and improved performance over traditional diffuser aeration methods. The consistent higher kLa values for MNB aeration underscore its potential as a promising technology for enhancing oxygen transfer in various applications, including wastewater treatment and other industrial processes where efficient oxygenation is critical.
3.4. Estimation of SOTR and SOTE
The calculation and analysis of SOTR and SOTE provided crucial insights into the efficiency and performance of both aeration systems employed in this study. These parameters serve as key indicators of the oxygen transfer capabilities of aeration methods, allowing evaluation of their effectiveness in meeting DO requirements for various applications. By quantifying the rate at which oxygen is transferred from the gas phase to the liquid phase and assessing the efficiency with which this transfer occurs, SOTR and SOTE offer a more comprehensive understanding of the aeration process. Moreover, the analysis of these metrics helps in identifying the optimal operational conditions for maximizing oxygen transfer, such as optimal gas flow rates, air-to-water ratios, and system configurations. Additionally, the findings suggest that SOTR and SOTE are invaluable tools for scaling up aeration systems in industrial settings, offering a practical approach to optimizing system design and operational strategies for enhanced performance and cost-effectiveness.
SOTR measures the oxygenation capacity of an aeration system, indicating the amount of oxygen that can be supplied to the water per unit of time. Conversely, SOTE quantifies the efficiency of the aeration system in transferring oxygen from the gas phase to the liquid phase and is expressed as a percentage. The parameters considered to calculate SOTR and SOTE included the DO concentration in the water, the water flow rate, and the oxygen transfer rate. Equations (Equation 8), (Equation 9) show the formulas used for these calculations.
In our experiments, conducted with clean water and wastewater using MNB aeration and diffuser aeration at different air flow rates (0.5 LPM, 2.5 LPM, and 5 LPM), SOTR and SOTE values were determined for the experimental volume of 25 L. The results revealed varying SOTR and SOTE values across different aeration methods and airflow rates. In clean water experiments, MNB aeration consistently demonstrated higher SOTR and SOTE values than diffuser aeration, indicating superior oxygen transfer efficiency. Similarly, in wastewater experiments, MNB aeration exhibited better performance in SOTR and SOTE, albeit at slightly lower levels compared to clean water, due to the presence of suspended particles and microbial activity. The values for SOTR and SOTE for each aeration method and airflow rate are summarized in Table 1, highlighting the significant improvements in oxygen transfer efficiency achieved with MNB aeration. These findings further support the potential of MNB technology for applications where maximizing oxygen transfer is critical, such as in wastewater treatment, aquaculture, and other industrial processes. The results underscore the importance of optimizing aeration methods and operational conditions to achieve the most efficient oxygen transfer, and they highlight MNB aeration as a promising solution for enhancing the performance of aeration systems across different water types.
Table 1.
Estimated SOTR and SOTE values for various experimental conditions.
| Aeration Method | Air Flowrate (LPM) | kLa hour−1 | kLa 20 h-1 | SOTR SOTE % mg/hr | |
|---|---|---|---|---|---|
| MNB aeration, Clean water | 0.5 | 25.23 | 18.67 | 139.78 | 54.33 |
| MNB aeration, Clean water | 2.5 | 16.98 | 12.57 | 75.70 | 11.77 |
| MNB aeration, Clean water | 5 | 15.87 | 11.75 | 67.44 | 5.24 |
| MNB aeration, Wastewater | 0.5 | 24.35 | 18.03 | 109.59 | 42.60 |
| MNB aeration, Wastewater | 2.5 | 14.07 | 10.42 | 51.59 | 8.02 |
| MNB aeration, Wastewater | 5 | 13.25 | 9.85 | 49.12 | 3.81 |
| Diffuser aeration, Clean water | 0.5 | 1.56 | 1.15 | 0.455 | 0.176 |
| Diffuser aeration, Clean water | 2.5 | 12.48 | 9.23 | 45.71 | 7.10 |
| Diffuser aeration, Clean water | 5 | 13.12 | 9.71 | 45.79 | 3.56 |
| Diffuser aeration, Wastewater | 0.5 | 3.72 | 2.75 | 1.083 | 0.42 |
| Diffuser aeration, Wastewater | 2.5 | 10.38 | 7.68 | 33.75 | 5.24 |
| Diffuser aeration, Wastewater | 5 | 12.36 | 9.14 | 37.54 | 2.91 |
In clean water, MNB aeration consistently demonstrated superior oxygen transfer rates than diffuser aeration, with the highest SOTR recorded at 139.78 mg/h for MNB aeration at a flow rate of 0.5 LPM. Conversely, diffuser aeration yielded substantially lower SOTR values, with a minimum value of about 1 mg/h observed at the same flow rate. At the same time, the maximum was obtained for a high airflow rate, with a value of about 45 mg/l. Moreover, the SOTE values for MNB aeration remained notably higher than those for diffuser aeration across all flow rates, indicating the enhanced efficiency of MNB in oxygen transfer. This further underscores the superior efficiency of MNB aeration in facilitating oxygen transfer, likely due to the smaller bubble sizes and increased surface area of MNBs, which enhance gas-liquid interaction and oxygen dissolution. In wastewater, similar trends were observed, with MNB aeration consistently outperforming diffuser aeration in terms of both SOTR and SOTE. For example, in wastewater, MNB aeration at 0.5 LPM achieved an SOTR of 109.59 mg/h, while diffuser aeration at the same flow rate resulted in an SOTR of only 1.083 mg/h. Thus, the results obtained from the experiments on MNB and diffuser aeration in both clean water and wastewater reveal significant variations in oxygen transfer rates and oxygen transfer efficiencies across different air flow rates.
While evaluating performance of various aeration systems it can be observed that MNB aeration achieves the highest oxygen transfer efficiency of about 30–50 % due to its small bubble size and prolonged residence time. Fine and coarse bubble diffusers, surface aerators, and venturi systems usually exhibit moderate to low SOTE (5–20 %) values with varying strengths in mixing or scalability.
3.5. Estimation of OUR, α-factor and β-factor
In this study, wastewater samples enriched with MLSS were used to determine the OUR and compare its values between MNB and diffuser aeration systems. The experiments involved aerating MLSS rich wastewater under controlled conditions and monitoring the changes in DO levels after stopping the aeration. The change in DO levels was measured in a biochemical oxygen demand sample bottle under closed conditions, which allowed for the estimation of the OUR using Equation (10). By plotting the change in DO levels over time and determining the slope of this curve, the OUR was calculated, providing a quantitative measure of the oxygen consumption rate in the wastewater sample.
The OUR values obtained for both MNB and diffuser aeration under different experimental conditions are summarized in Table 2. The comparison of OUR values between the two aeration methods reveals how effectively each system supports the oxygen consumption process in the presence of MLSS. MNB aeration demonstrated higher OUR values compared to diffuser aeration, reflecting its superior performance in transferring oxygen into the liquid phase, even under the challenging conditions of wastewater with high microbial activity. This suggests that MNB aeration provides more efficient oxygenation, potentially promoting better biological activity and enhancing the treatment of wastewater.
Table 2.
Values of OUR and α- and β-factors.
| Aeration Method | Air Flowrate (LPM) | OUR (mg/s) | Maximum DO Achieved (mg/l) |
Alpha Factor | Beta Factor | |
|---|---|---|---|---|---|---|
| Clean water | Wastewater process | |||||
| MNB aeration, Wastewater | 0.5 | 0.0042 | 13.12 | 12.46 | 0.965 | 0.949 |
| MNB aeration, Wastewater | 2.5 | 0.0035 | 11.3 | 9.5 | 0.83 | 0.84 |
| MNB aeration, Wastewater | 5 | 0.002 | 11.3 | 9.4 | 0.834 | 0.831 |
| Diffuser aeration, Wastewater | 0.5 | – | ||||
| Diffuser aeration, Wastewater | 2.5 | 0.0028 | 9.1 | 8.2 | 0.894 | 0.90 |
| Diffuser aeration, Wastewater | 5 | 0.0031 | 9.3 | 8.43 | 0.947 | 0.906 |
These findings highlight the importance of monitoring OUR as an indicator of system performance and oxygen consumption. The higher OUR values associated with MNB aeration suggest that this method can support more efficient biochemical processes in wastewater treatment. Furthermore, the results emphasize the potential of MNB aeration in optimizing wastewater treatment systems, particularly in enhancing the aerobic degradation of organic matter and improving overall treatment efficiency.
Table 2 presents the calculated beta values for various samples, determined by comparing the average saturation values obtained from the wastewater and clean water experiments. For MNB and diffuser aeration, maximum beta values reached up to 0.949 and 0.906, respectively, influenced by flow rate and the aeration method employed to obtain saturation values. Interestingly, wastewater characteristics did not significantly impact the average values while using the MNB aeration method at 0.5 LPM gas flow rate, averaging about 0.949 (Table 2). However, the diffuser aeration showed a lower values, which can be attributed to the high TSS and low turbulence of the water. Consequently, MNB aeration yielded saturation levels as close as clean water.
Our investigation observed a direct correlation between the MNB aeration temperature and OUR of the activated sludge. The particle size distribution of the MNBs in our study showed that the lower the bubble sizes, the better the results of DO transfer in activated sludge treatment processes. Furthermore, our findings indicate that OUR serves as an indicator of biomass activity. However, gaining insights into the operation of a treatment plant necessitates measuring the oxygen uptake rate continuously over a long duration, ranging from one to 4 h. This extended observation period allowed a comprehensive understanding of the process.
4. Conclusions
This study investigated the effectiveness of MNB aeration systems in enhancing volumetric mass transfer coefficients and optimizing oxygen transfer efficiency in water treatment and other applications. The findings revealed that MNBs, due to their smaller size, higher surface area, and stability, significantly outperformed traditional microbubble systems, offering superior performance in clean water and process conditions.
-
1.
Optimal Air-to-Water Flow Rate Ratio: The study identified that the MNB system performed optimally at an air-to-water flow rate ratio of 0.04–0.06. Higher gas-to-liquid flow rate ratios led to reduced MNB production and larger bubble formation, negatively impacting mass transfer efficiency.
-
2.
Effect of MNB Concentration: An increase in MNB concentration correlated directly with higher volumetric mass transfer. This observation aligns with prior literature emphasizing the role of smaller bubble sizes in enhancing oxygen transfer efficiency.
-
3.
Superiority of MNBs over Microbubbles: Compared to traditional microbubble systems, MNB technology exhibited significantly superior performance in enhancing oxygen mass transfer, both in clean water and process conditions. This is attributed to MNBs' unique characteristics, such as smaller size, larger surface area, and stability in the liquid phase.
-
4.
Improved Oxygen Transfer Efficiency: The findings highlighted that the larger population of micro- and nano-sized bubbles provided by MNBs significantly improves oxygen dissolution rates, making them ideal for applications requiring efficient oxygen transfer.
-
5.
Implications for Industrial Applications: The study underscores the potential of MNB aeration technology in critical industries like wastewater treatment, chemical processing, and biomedical applications. Enhanced oxygenation efficiency makes MNBs particularly suitable for processes involving microbial growth, biological systems, or oxygen-dependent chemical reactions.
-
6.
Future Research Directions: The study suggests further research into optimizing MNB generation, scaling up the technology for industrial applications, and exploring its potential in diverse sectors. These efforts could drive significant advancements in process efficiencies and cost-effectiveness.
In conclusion, MNB aeration systems demonstrate remarkable potential as an effective and efficient solution for improving volumetric mass transfer coefficients and oxygen transfer rates across various applications.
Based on the study, several recommendations can be made to enhance the application of MNB technology. Optimizing MNB generation parameters, such as air-to-water flow rate ratios, is crucial for maintaining smaller bubble sizes and maximizing mass transfer efficiency. Scaling up the technology for industrial applications and diversifying its use in areas like aquaculture, chemical manufacturing, and bioprocessing could unlock significant potential. Additionally, improving system design to better control bubble size and stability, along with analyzing energy efficiency and environmental impact, will ensure sustainable and cost-effective implementation. Finally, long-term performance evaluations are necessary to establish the durability and reliability of MNB systems under continuous operation.
CRediT authorship contribution statement
Mohamed Ibrahim kizhisseri: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Data curation, Conceptualization. Marwa Sakr: Writing – review & editing, Methodology, Data curation. Munjed Maraqa: Writing – review & editing, Visualization, Supervision, Methodology, Conceptualization. Mohamed M. Mohamed: Writing – review & editing, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Funding
This work was funded by Abu Dhabi Department of Education and Knowledge (ADEK) through ADEK Award for Research Excellence (AARE) funding program (award # AARE19-047) and the National Water and Energy Center at United Arab Emirates University, UAE (Grants No. 21N226 and 12R176).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Metcalf and Eddy, Wastewater Engineering . McGraw-Hill; New York: 2004. Treatment and Reuse, Metcalf and Eddy. 2004) vol. s. [Google Scholar]
- 2.Agarwal A., Ng W.J., Liu Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere. 2011;84(9):1175–1180. doi: 10.1016/j.chemosphere.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 3.Campo G., Miggiano A., Panepinto D., Zanetti M. Enhancing the energy efficiency of wastewater treatment plants through the optimization of the aeration systems. Energies. Mar. 2023;16(6):2819. doi: 10.3390/en16062819. [DOI] [Google Scholar]
- 4.Gu Y., Li Y., Yuan F., Yang Q. Optimization and control strategies of aeration in WWTPs: a review. J. Clean. Prod. Sep. 2023;418 doi: 10.1016/j.jclepro.2023.138008. [DOI] [Google Scholar]
- 5.Haris S., Qiu X., Klammler H., Mohamed M.M.A. The use of micro-nano bubbles in groundwater remediation: a comprehensive review. Groundwater for Sustainable Development. Oct. 2020;11 doi: 10.1016/j.gsd.2020.100463. [DOI] [Google Scholar]
- 6.Levitsky I., Tavor D., Gitis V. Micro and nanobubbles in water and wastewater treatment: a state-of-the-art review. J. Water Process Eng. Jun. 2022;47 doi: 10.1016/j.jwpe.2022.102688. [DOI] [Google Scholar]
- 7.Sakr M., et al. A critical review of the recent developments in micro–nano bubbles applications for domestic and industrial wastewater treatment. Alex. Eng. J. Aug. 2022;61(8):6591–6612. doi: 10.1016/j.aej.2021.11.041. [DOI] [Google Scholar]
- 8.Cruz F.C., Marouchos A., Bilton A.M. Experimental characterization of an oxygen transfer model of a fine pore diffuser aerator. Aquacult. Eng. Aug. 2022;98 doi: 10.1016/j.aquaeng.2022.102259. [DOI] [Google Scholar]
- 9.Temesgen T., Bui T.T., Han M., Kim T., Park H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: a review. Adv. Colloid Interface Sci. 2017;246:40–51. doi: 10.1016/j.cis.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Campbell K.A. 2020. “Physical and Biological Factors Affecting Oxygen Transfer in the Activated Sludge Wastewater Treatment Process,”. [Google Scholar]
- 11.Li H., Hu L., Song D., Lin F. Characteristics of micro‐nano bubbles and potential application in groundwater bioremediation. Water Environ. Res. Sep. 2014;86(9):844–851. doi: 10.2175/106143014X14062131177953. [DOI] [PubMed] [Google Scholar]
- 12.Najafpour G.D., Lin L.C. 2002. “Oxygen Transfer Rate in an Aerated Tank for Pharmaceutical Wastewater Treatment,”. [Google Scholar]
- 13.Zhang M., Qiu L., Liu G. Basic characteristics and application of micro-nano bubbles in water treatment. IOP Conf. Ser. Earth Environ. Sci. Jun. 2020;510(4) doi: 10.1088/1755-1315/510/4/042050. [DOI] [Google Scholar]
- 14.Alisawi H.A.O. Performance of wastewater treatment during variable temperature. Appl. Water Sci. Apr. 2020;10(4):89. doi: 10.1007/s13201-020-1171-x. [DOI] [Google Scholar]
- 15.Bunkin N.F., Yurchenko S.O., Suyazov N.V., Shkirin A.V. Structure of the nanobubble clusters of dissolved air in liquid media. J. Biol. Phys. Jan. 2012;38(1):121–152. doi: 10.1007/s10867-011-9242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma H., Nirmalkar N. Enhanced gas-liquid mass transfer coefficient by bulk nanobubbles in water. Mater. Today: Proc. 2022;57:1838–1841. doi: 10.1016/j.matpr.2022.01.029. [DOI] [Google Scholar]
- 17.Terasaka K., Hirabayashi A., Nishino T., Fujioka S., Kobayashi D. Development of microbubble aerator for waste water treatment using aerobic activated sludge. Chem. Eng. Sci. Jul. 2011;66(14):3172–3179. doi: 10.1016/j.ces.2011.02.043. [DOI] [Google Scholar]
- 18.Ulatowski K., Sobieszuk P., Mróz A., Ciach T. Stability of nanobubbles generated in water using porous membrane system. Chemical Engineering and Processing - Process Intensification. Feb. 2019;136:62–71. doi: 10.1016/j.cep.2018.12.010. [DOI] [Google Scholar]
- 19.Ushikubo F.Y., et al. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. A Physicochem. Eng. Asp. May 2010;361(1–3):31–37. doi: 10.1016/j.colsurfa.2010.03.005. [DOI] [Google Scholar]
- 20.Azevedo A., Etchepare R., Calgaroto S., Rubio J. Aqueous dispersions of nanobubbles: generation, properties and features. Miner. Eng. 2016;94:29–37. [Google Scholar]
- 21.A. T et al., “Oxygen Transfer of Microbubble Clouds in Aqueous Solutions- Application to Wastewater”.
- 22.Zimmerman W.B., Tesar V., Bandulasena S.B., C.H H. Recent Patents on Engineering. 2007. Microbubble generation. [Google Scholar]
- 23.Alkhalidi A.A.T., Amano R.S. Factors affecting fine bubble creation and bubble size for activated sludge. Water Environ. J. Mar. 2015;29(1):105–113. doi: 10.1111/wej.12083. [DOI] [Google Scholar]
- 24.Ashley K., Mavinic D., Hall K. Bench-scale study of oxygen transfer in coarse bubble diffused aeration. Water Res. Oct. 1992;26(10):1289–1295. doi: 10.1016/0043-1354(92)90123-L. [DOI] [Google Scholar]
- 25.Fan H., Qi Lu. Promotion and inhibition of oxygen transfer under fine bubble aeration by activated sludge. Water Environ. J. 2014;28(3) doi: 10.1111/wej.12061. [Online]. Available: [DOI] [Google Scholar]
- 26.He Z., Petiraksakul A., Meesapya W. Oxygen-transfer measurement in clean water. 2003;13(1) [Google Scholar]
- 27.Dold P., Fairlamb M. ESTIMATING OXYGEN TRANSFER KLa, SOTE AND AIR FLOW REQUIREMENTS IN FINE BUBBLE DIFFUSED AIR SYSTEMS. proc water environ fed. Jan. 2001;2001(13):780–791. doi: 10.2175/193864701790864070. [DOI] [Google Scholar]
- 28.Dyagelev M.Y., Isakov V.G., Grakhova E.V. α-factor experimental determination of aeration system in aeration tanks. IOP Conf. Ser. Mater. Sci. Eng. Dec. 2019;687(6) doi: 10.1088/1757-899X/687/6/066071. [DOI] [Google Scholar]
- 29.Moutafchieva D., Popova D., Dimitrova M., Tchaoushev S. 2013. Experimental Determination of the Volumetric Mass Transfer Coefficient. [Google Scholar]
- 30.Stenstrom M.K., Kido W., Shanks R.F., Mulkerin M. Estimating oxygen transfer capacity of a full-scale pure oxygen activated sludge plant. 1989;61(2) [Google Scholar]
- 31.Perez-Garibay R., Martínez-Ramos E., Rubio J. Gas dispersion measurements in microbubble flotation systems. Miner. Eng. 2012;26:34–40. [Google Scholar]
- 32.Parmar R. Terminal rise velocity, size distribution and stability of microbubble suspension. Asia Pac. J. Chem. Eng. 2015;10:450–465. 10, pp. 450–465. [Google Scholar]
- 33.Kim M.S., Han M., Lee J.W., Lee J.W., Kwak D.H. Effect of nanobubbles for improvement of water quality in freshwater: flotation model simulation. Sep. Purif. Technol. 2020;241 doi: 10.1016/j.seppur.2020.116731. [Online]. Available: [DOI] [Google Scholar]
- 34.Zhou S., Liu M., Chen B., Sun L., Lu H. Microbubble- and nanobubble-aeration for upgrading conventional activated sludge process: a review. Bioresour. Technol. Oct. 2022;362 doi: 10.1016/j.biortech.2022.127826. [DOI] [PubMed] [Google Scholar]
- 35.Lewis W.K., Whitman W.C. Principles of gas absorption. Ind. Eng. Chem. 1924;16(3):12–15. [Google Scholar]
- 36.Higbie R. The rate of absorption of a pure gas into a still liquid during short periods of exposure. Inst Chem Eng. 1935;35:36–60. [Google Scholar]
- 37.ASCE . American Society of Civil Engineers; Reston, VA., no. ASCE/EWRI 2-06: 2007. Measurement of oxygen transfer in clean water; pp. 1–11. [Google Scholar]
- 38.ASCE . American Society of Civil Engineers; 2018. Standard guidelines for in-process oxygen transfer testing; pp. 2–24. ASCE/EWRI-18-18. [Google Scholar]
- 39.Boyd C.E. 1998. Pond Water Aeration Systems. [Google Scholar]
- 40.Garcia-Ochoa F., Gomez E. Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol. Adv. Mar. 2009;27(2):153–176. doi: 10.1016/j.biotechadv.2008.10.006. [DOI] [PubMed] [Google Scholar]