Abstract
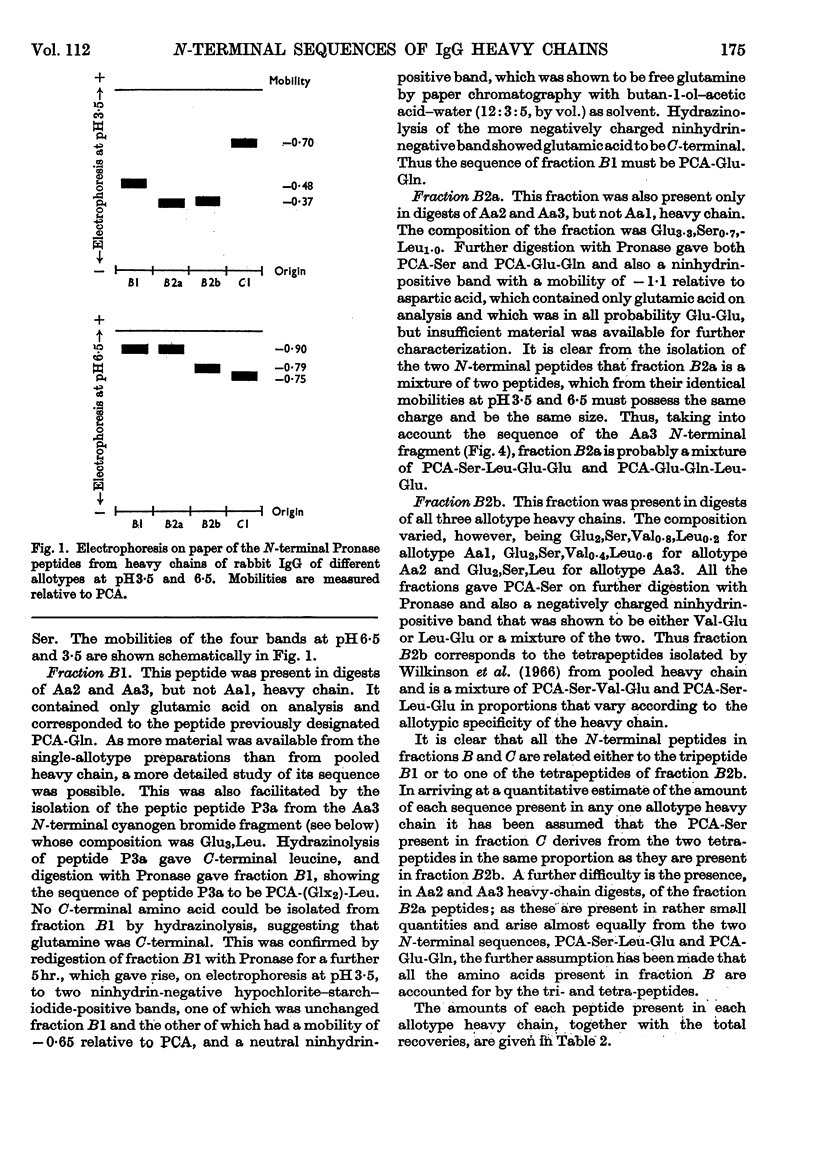
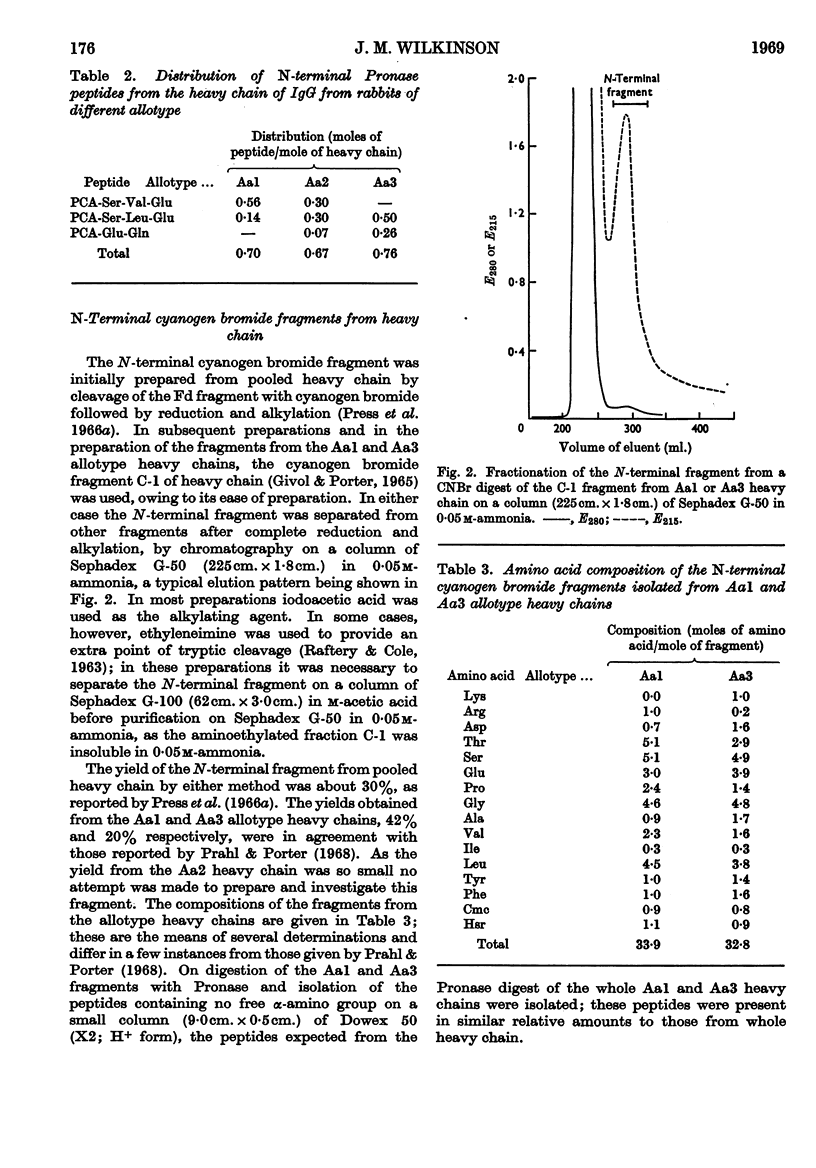
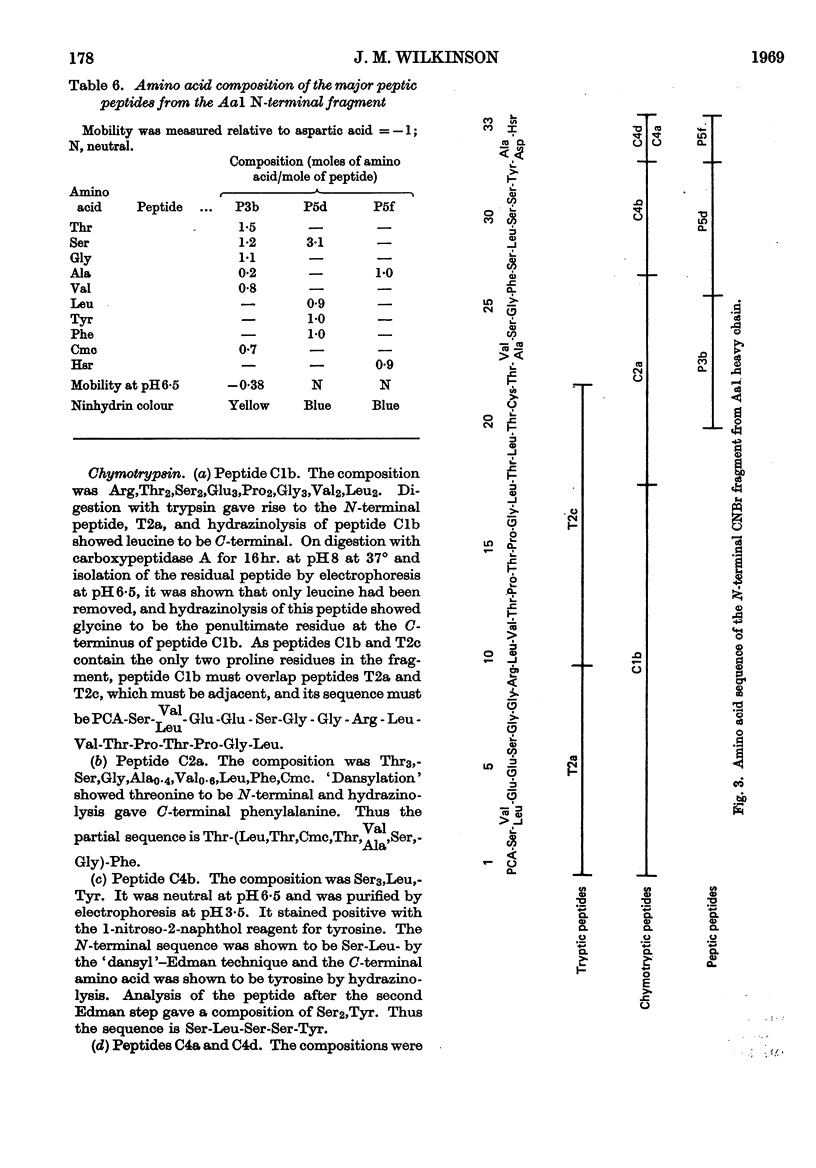
The sequences of the N-terminal peptides prepared by Pronase digestion of the heavy chain of rabbit immunoglobulin G of allotype Aa1, Aa2 and Aa3 were determined and were shown to be related to the allotype. An N-terminal fragment of about 34 residues was also prepared from the allotype heavy chains, by cleavage with cyanogen bromide; the yield varied with the allotype. The sequences of the cyanogen bromide fragments from the Aa1 and Aa3 heavy chains contain allotype-related variations similar to those found in the N-terminal Pronase peptides, and these sequences are thought to be representative of the whole heavy-chain populations. There is about 60% homology between the two sequences, and superimposed on the differences between them there are a number of positions within each sequence at which at least two amino acids are present.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Alescio Zonta L., Cioli D., Carbonara A. Allelic antigenic factor Inv(a) of the light chains of human immunoglobulins: chemical basis. Science. 1966 Jun 10;152(3728):1517–1519. doi: 10.1126/science.152.3728.1517. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Harris J. I., Hartley B. S., Leberman R. Reversible blocking of peptide amino groups by maleic anhydride. Biochem J. 1967 Jun;103(3):78P–79P. [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Milstein C. Structure and biological properties of immunoglobulins. Adv Immunol. 1967;7:1–89. doi: 10.1016/s0065-2776(08)60126-1. [DOI] [PubMed] [Google Scholar]
- DRAY S., DUBISKI S., KELUS A., LENNOX E. S., OUDIN J. A notation for allotypy. Nature. 1962 Aug 25;195:785–786. doi: 10.1038/195785a0. [DOI] [PubMed] [Google Scholar]
- DRAY S., YOUNG G. O., NISONOFF A. DISTRIBUTION OF ALLOTYPIC SPECIFICITIES AMONG RABBIT GAMMA-GLOBULIN MOLECULES GENETICALLY DEFINED AT TWO LOCI. Nature. 1963 Jul 6;199:52–55. doi: 10.1038/199052a0. [DOI] [PubMed] [Google Scholar]
- FEINSTEIN A. CHARACTER AND ALLOTYPY OF AN IMMUNE GLOBULIN IN RABBIT COLOSTRUM. Nature. 1963 Sep 21;199:1197–1199. doi: 10.1038/1991197b0. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- FRANKLIN T. J., GODFREY A. RESISTANCE OF ESCHERICHIA COLI TO TETRACYCLINES. Biochem J. 1965 Jan;94:54–60. doi: 10.1042/bj0940054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Disulphide bridges of immunoglobin G-1 heavy chains. Nature. 1967 Dec 2;216(5118):939–941. doi: 10.1038/216939b0. [DOI] [PubMed] [Google Scholar]
- HABER E. RECOVERY OF ANTIGENIC SPECIFICITY AFTER DENATURATION AND COMPLETE REDUCTION OF DISULFIDES IN A PAPAIN FRAGMENT OF ANTIBODY. Proc Natl Acad Sci U S A. 1964 Oct;52:1099–1106. doi: 10.1073/pnas.52.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilschmann N., Craig L. C. Amino acid sequence studies with Bence-Jones proteins. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1403–1409. doi: 10.1073/pnas.53.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D., Horecker B. L. Thin-layer chromatographic separation of DNS-amino acids. Anal Biochem. 1966 Mar;14(3):429–433. doi: 10.1016/0003-2697(66)90285-5. [DOI] [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F., HARTLEY B. S., SHAW D. C. The amino acid sequence around the reactive serine residue of some proteolytic enzymes. Biochem J. 1960 Oct;77:149–163. doi: 10.1042/bj0770149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Torrigiani G., Amante L., Kelus A. S., Cebra J. J. Identical allotypic markers of heavy polypeptide chains present in different immunoglobulin classes. Immunology. 1968 Mar;14(3):445–451. [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Press E. M. Cyanogen bromide cleavage and partial sequence of the heavy chain of a pathological immunoglobulin G. Biochem J. 1967 Aug;104(2):616–626. doi: 10.1042/bj1040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinck J. R., Milstein C. Disulphide bridges of a human immunoglobulin G protein. Nature. 1967 Dec 2;216(5118):941–942. doi: 10.1038/216941a0. [DOI] [PubMed] [Google Scholar]
- Porter R. R. The structure of the heavy chain of immunoglobulin and its relevance to the nature of the antibody-combining site. The Second CIBA Medal Lecture. Biochem J. 1967 Nov;105(2):417–426. doi: 10.1042/bj1050417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl J. W., Porter R. R. Allotype-related sequence variation of the heavy chain of rabbit immunoglobulin G. Biochem J. 1968 May;107(6):753–763. doi: 10.1042/bj1070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl J. W. The C-terminal sequences of the heavy chains of human immunoglobulin G myeloma proteins of differing isotopes and allotypes. Biochem J. 1967 Dec;105(3):1019–1028. doi: 10.1042/bj1051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press E. M., Givol D., Piggot P. J., Porter R. R., Wilkinson J. M. The chemical structure of the heavy chains of rabbit and human immunoglobulin G (IgG). Proc R Soc Lond B Biol Sci. 1966 Nov 22;166(1003):150–158. doi: 10.1098/rspb.1966.0090. [DOI] [PubMed] [Google Scholar]
- Press E. M., Piggot P. J., Porter R. R. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. doi: 10.1042/bj0990356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAFTERY M. A., COLE R. D. Tryptic cleavage at cysteinyl peptide bonds. Biochem Biophys Res Commun. 1963 Mar 25;10:467–472. doi: 10.1016/0006-291x(63)90381-4. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Dray S., Nisonoff A. Differences in amino acid composition of rabbit gamma-G-immunoglobulin light polypeptide chains controlled by allelic genes. Immunochemistry. 1965 Jun;2(2):155–167. doi: 10.1016/0019-2791(65)90017-0. [DOI] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. Halogenation of tyrosine during acid hydrolysis. Biochim Biophys Acta. 1963 May 14;71:468–471. doi: 10.1016/0006-3002(63)91108-9. [DOI] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Stemke G. W. A study of soluble complexes and uncombined material in antigen-antibody reactions involving allotypic specificities of purified rabbit gamma-globulin. Immunochemistry. 1965 Dec;2(4):359–377. doi: 10.1016/0019-2791(65)90036-4. [DOI] [PubMed] [Google Scholar]
- TODD C. W. Allotypy in rabbit 19S protein. Biochem Biophys Res Commun. 1963 May 3;11:170–175. doi: 10.1016/0006-291x(63)90329-2. [DOI] [PubMed] [Google Scholar]
- Thorpe N. O., Deutsch H. F. Studies on papain produced subunits of human gamma-G-globulins. II. Structures of peptides related to the genetic Gm activity of gamma-G-globulin Fc-fragments. Immunochemistry. 1966 Jul;3(4):329–337. [PubMed] [Google Scholar]
- WHITNEY P. L., TANFORD C. RECOVERY OF SPECIFIC ACTIVITY AFTER COMPLETE UNFOLDING AND REDUCTION OF AN ANTIBODY FRAGMENT. Proc Natl Acad Sci U S A. 1965 Mar;53:524–532. doi: 10.1073/pnas.53.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. M., Press E. M., Porter R. R. The N-terminal sequence of the heavy chain of rabbit immunoglobulin IgG. Biochem J. 1966 Aug;100(2):303–308. doi: 10.1042/bj1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]


