Abstract
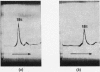
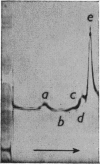
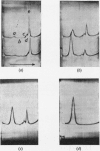
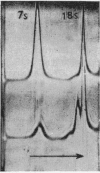
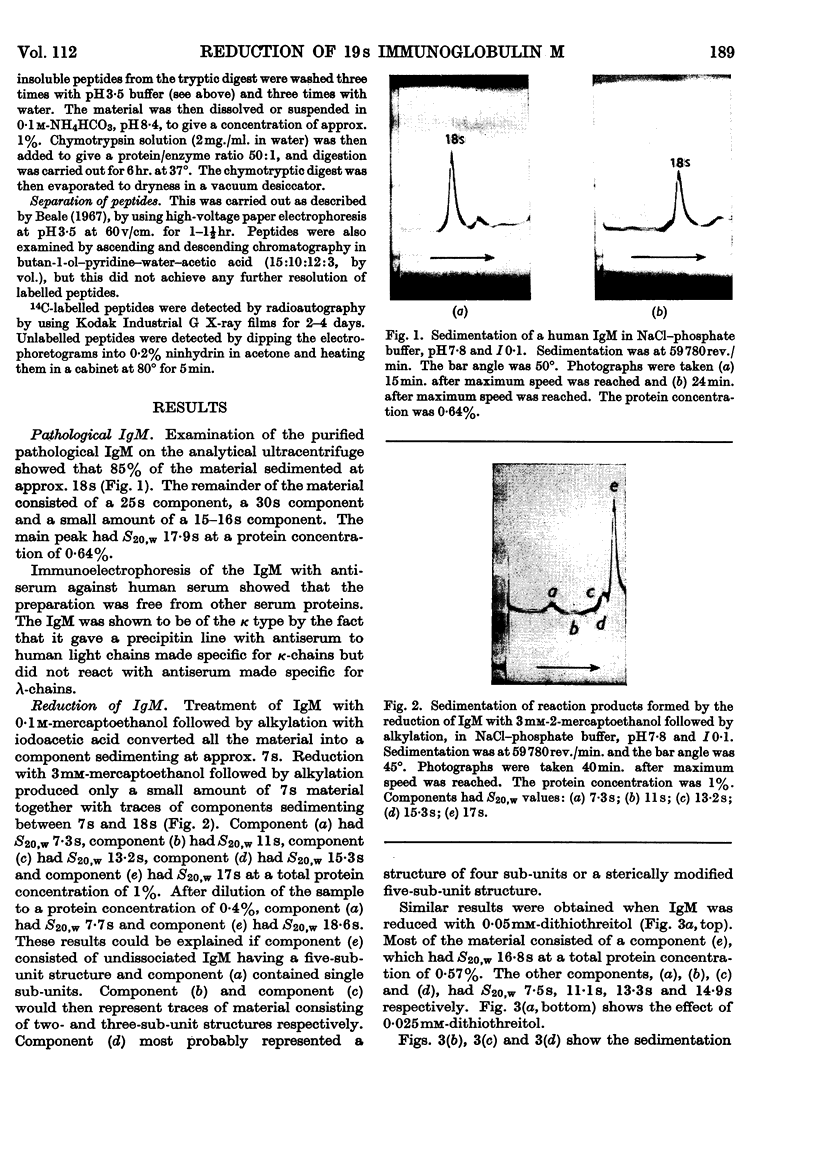
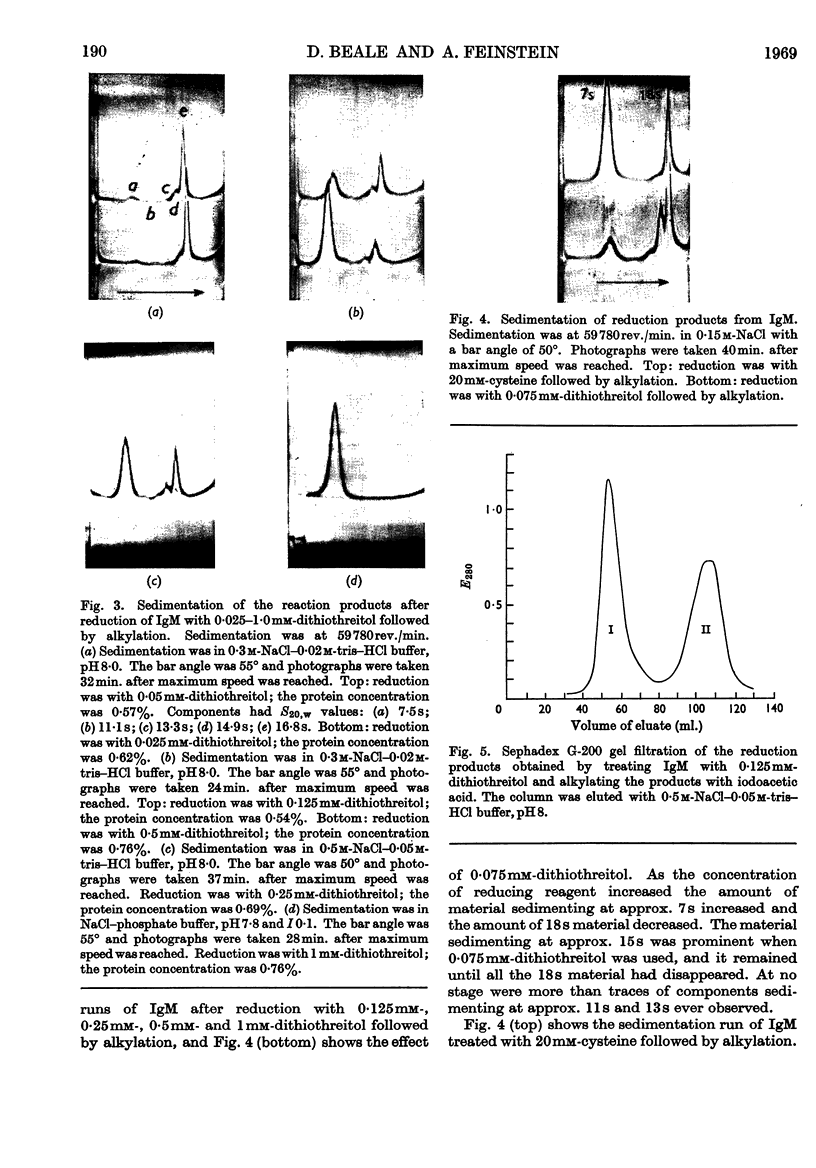
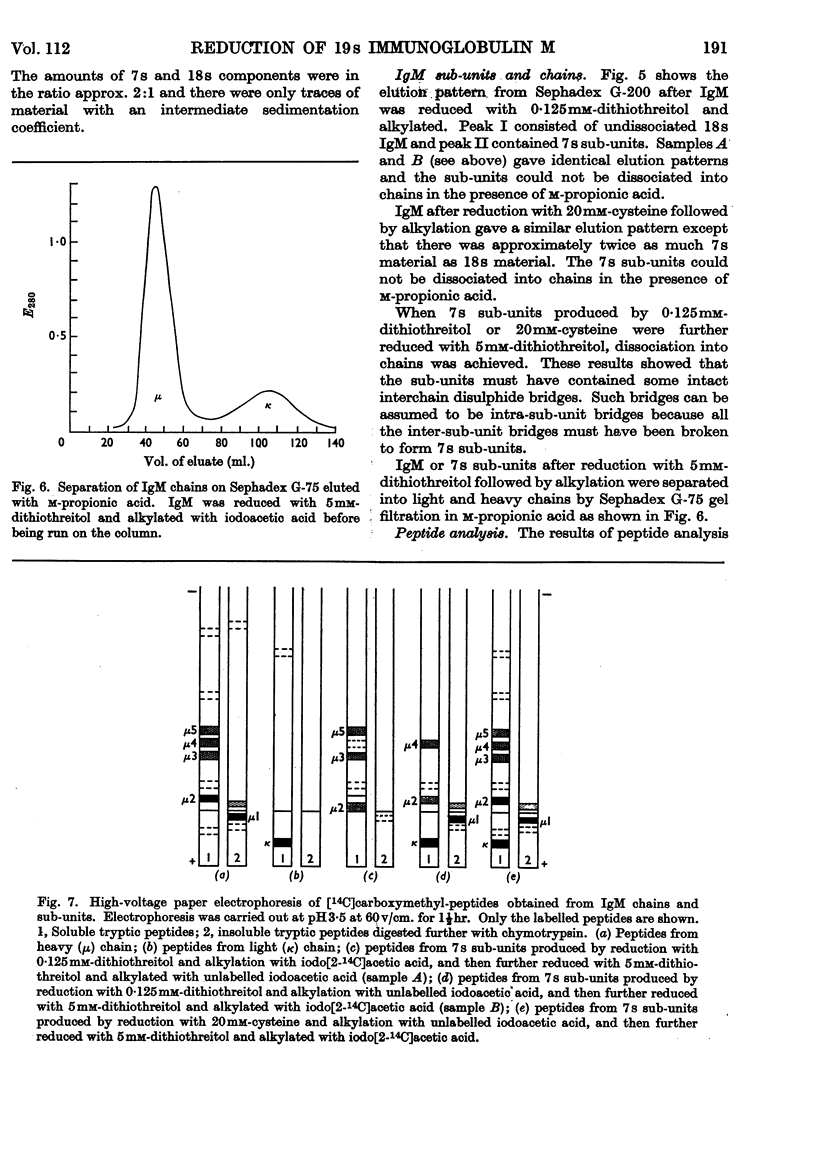
1. Reduction of a 19s immunoglobulin M with 3mm-mercaptoethanol or 0·05–0·5mm-dithiothreitol followed by alkylation gave sedimentation patterns indicating products compatible with structures consisting of one, two, three, four and five 7s sub-units. This supports the concept of a five-sub-unit structure for immunoglobulin M. 2. Reduction with 0·125mm-dithiothreitol or 20mm-cysteine produced 7s sub-units that could not be dissociated into chains in m-propionic acid. 3. By labelling (with iodo[2-14C]acetic acid) the thiol groups liberated during reduction with 0·125mm-dithiothreitol, it was possible to identify the tryptic peptides involved in the disulphide bridges that link the 7s sub-units together (inter-sub-unit bridges). 4. By further reducing and labelling (with iodo[2-14C]acetic acid) the 7s sub-units produced by 0·125mm-dithiothreitol, it was possible to identify tryptic peptides derived from intra-sub-unit bridges. 5. Sub-units produced by reduction with 20mm-cysteine proved to be unsuitable for distinguishing between inter-sub-unit bridges and intra-sub-unit bridges. 6. The possible arrangement of the interchain disulphide bridges was deduced.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale D. A partial amino acid sequence for sheep haemoblogin A. Biochem J. 1967 Apr;103(1):129–140. doi: 10.1042/bj1030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Bloth B., Svehag S. E. The ultrastructure of normal and pathological IgM immunoglobulins. J Exp Med. 1968 Mar 1;127(3):399–410. doi: 10.1084/jem.127.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. G. Hemagglutinating 7S subunits of 19S cold agglutinins. Science. 1967 Aug 25;157(3791):933–935. doi: 10.1126/science.157.3791.933. [DOI] [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Dissociation of human serum macroglobulins. Science. 1957 Mar 29;125(3248):600–601. doi: 10.1126/science.125.3248.600. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- KOENIG V. L. Partial specific volumes for some porcine and bovine plasma protein fractions. Arch Biochem. 1950 Feb;25(2):241–245. [PubMed] [Google Scholar]
- Lamm M. E., Small P. A., Jr Polypeptide chain structure of rabbit immunoglobulins. II. gamma-M-immunoglobulin. Biochemistry. 1966 Jan;5(1):267–276. doi: 10.1021/bi00865a035. [DOI] [PubMed] [Google Scholar]
- MILLER F., METZGER H. CHARACTERIZATION OF A HUMAN MACROGLOBULIN. I. THE MOLECULAR WEIGHT OF ITS SUBUNIT. J Biol Chem. 1965 Aug;240:3325–3333. [PubMed] [Google Scholar]
- Miller F., Metzger H. Characterization of a human macroglobulin. II. Distribution of the disulfide bonds. J Biol Chem. 1965 Dec;240(12):4740–4745. [PubMed] [Google Scholar]
- PIERCE A. E., FEINSTEIN A. BIOPHYSICAL AND IMMUNOLOGICAL STUDIES ON BOVINE IMMUNE GLOBULINS WITH EVIDENCE FOR SELECTIVE TRANSPORT WITHIN THE MAMMARY GLAND FROM MATERNAL PLASMA TO COLOSTRUM. Immunology. 1965 Jan;8:106–123. [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Deutsch H. F. Dissociation, reaggregation, and subunit structure studies of some human gamma-M-globulins. J Biol Chem. 1967 Jun 10;242(11):2725–2738. [PubMed] [Google Scholar]