Abstract
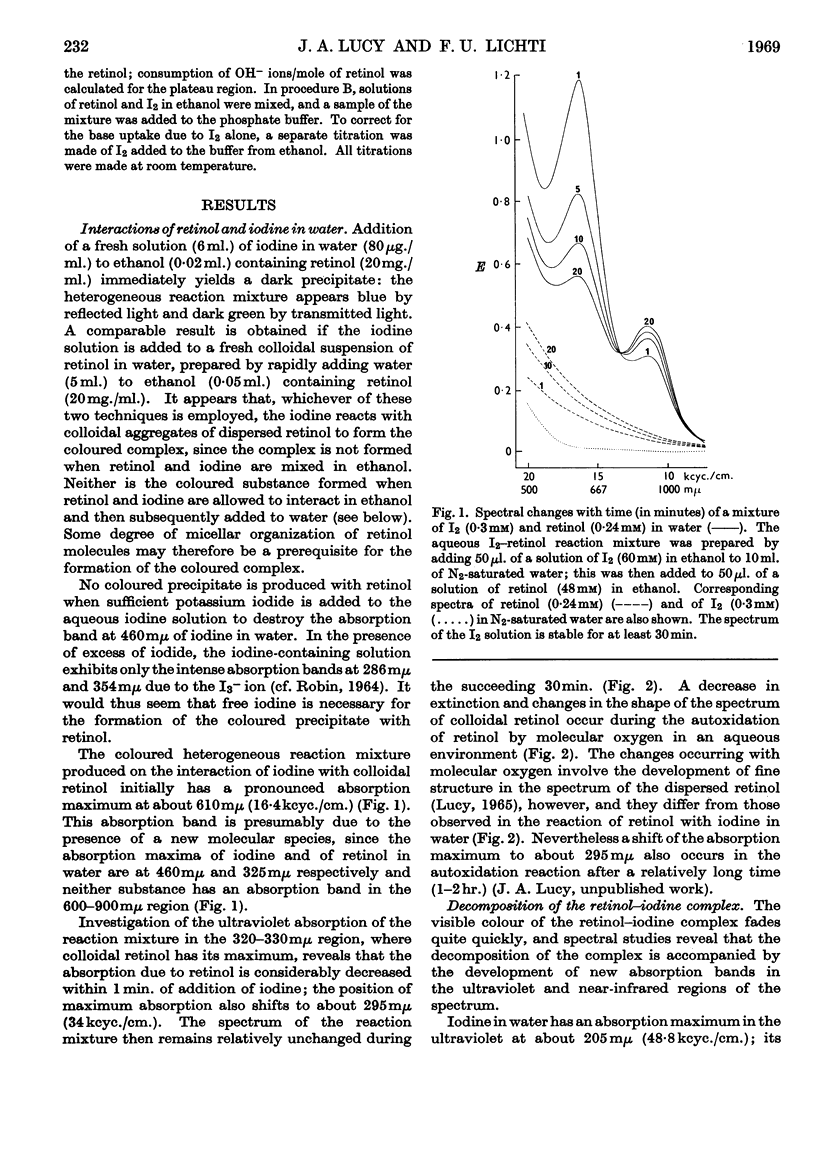
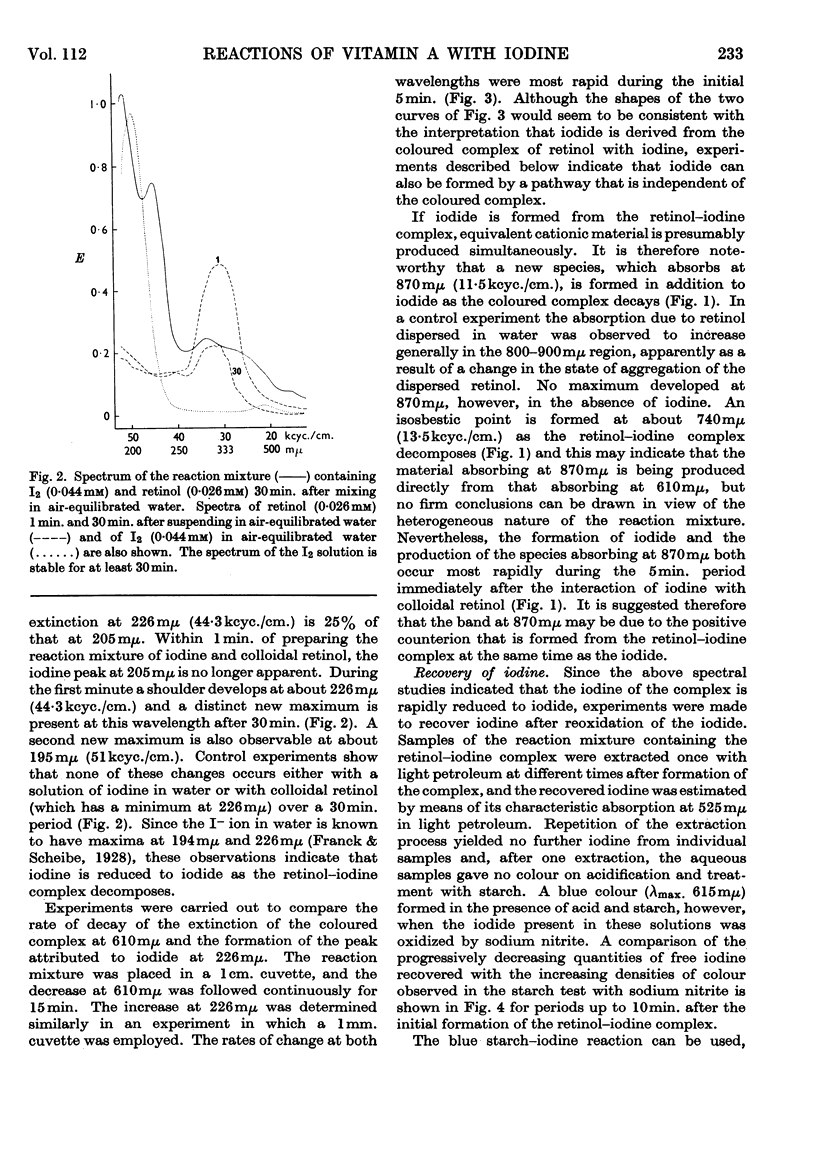
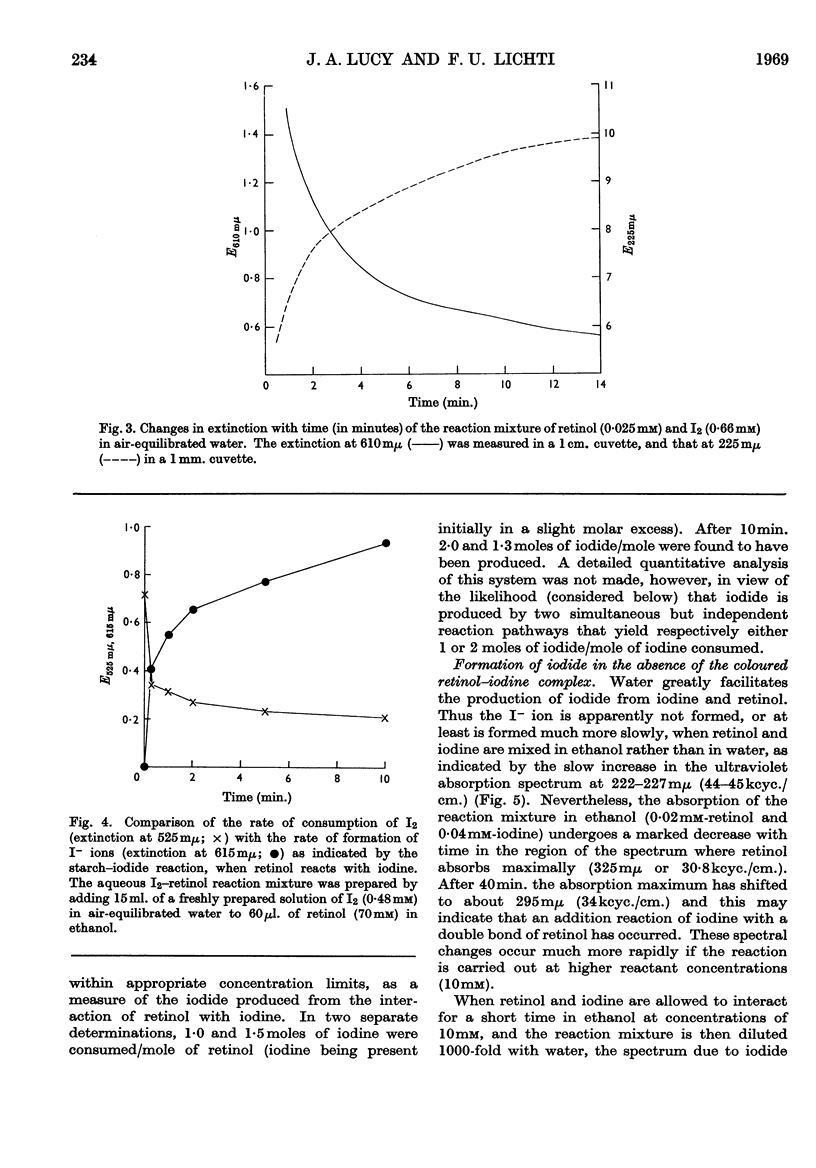
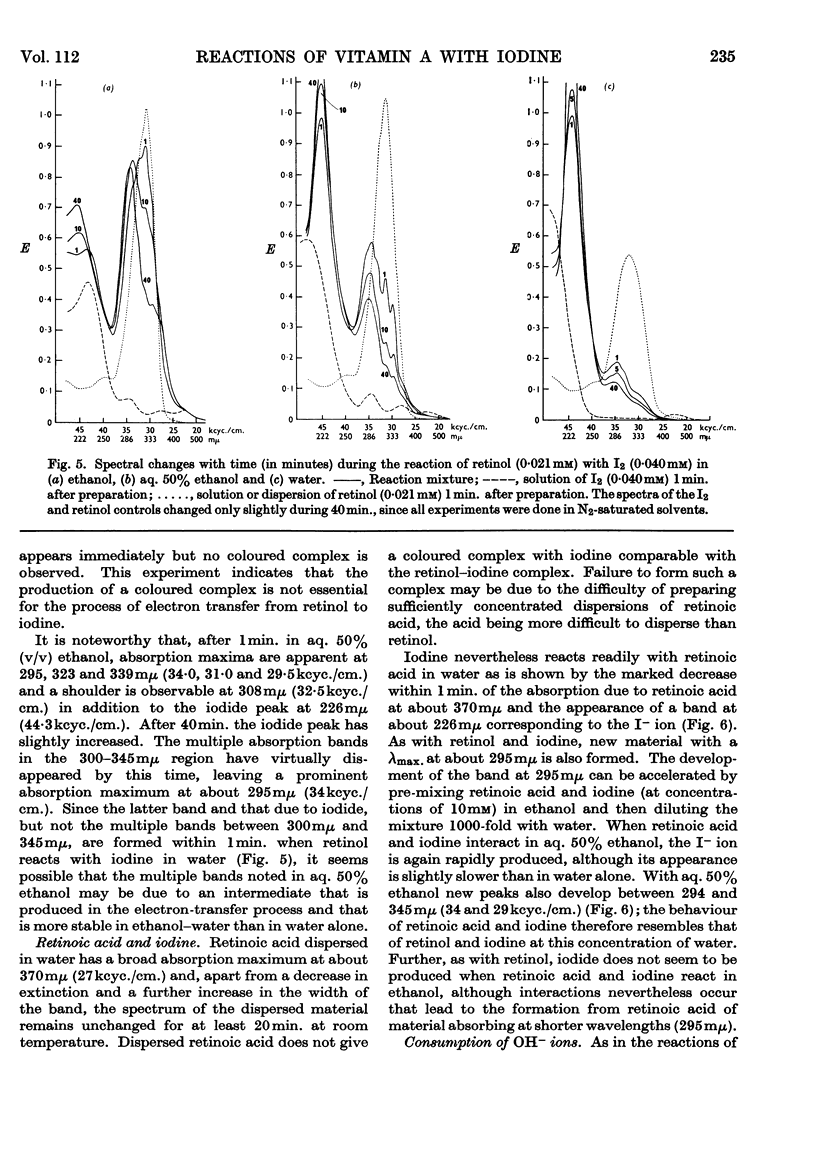
1. The reactions of retinol and retinoic acid with iodine were investigated since knowledge of the chemical reactions of vitamin A with acceptors of electrons may shed light on its biochemical mode of action. 2. Colloidal retinol, but not retinoic acid, reacts with iodine to yield a blue–green complex that rapidly decomposes, giving iodide and an unknown species with λmax. at 870mμ. 3. In addition, both retinol and retinoic acid reduce iodine to iodide by a reaction that does not involve an intermediate coloured complex; this reaction appears to yield unstable carbonium ion derivatives of the vitamin. 4. The presence of water greatly facilitates the production of iodide from vitamin A and iodine. 5. Possible chemical pathways involved in these reactions are discussed. 6. It is suggested that the chemical properties of retinol and retinoic acid that underlie their biochemical behaviour might be apparent only when the molecules are at a lipid–water interface, and that vitamin A might be expected to react with a number of different electron acceptors in vivo.
Full text
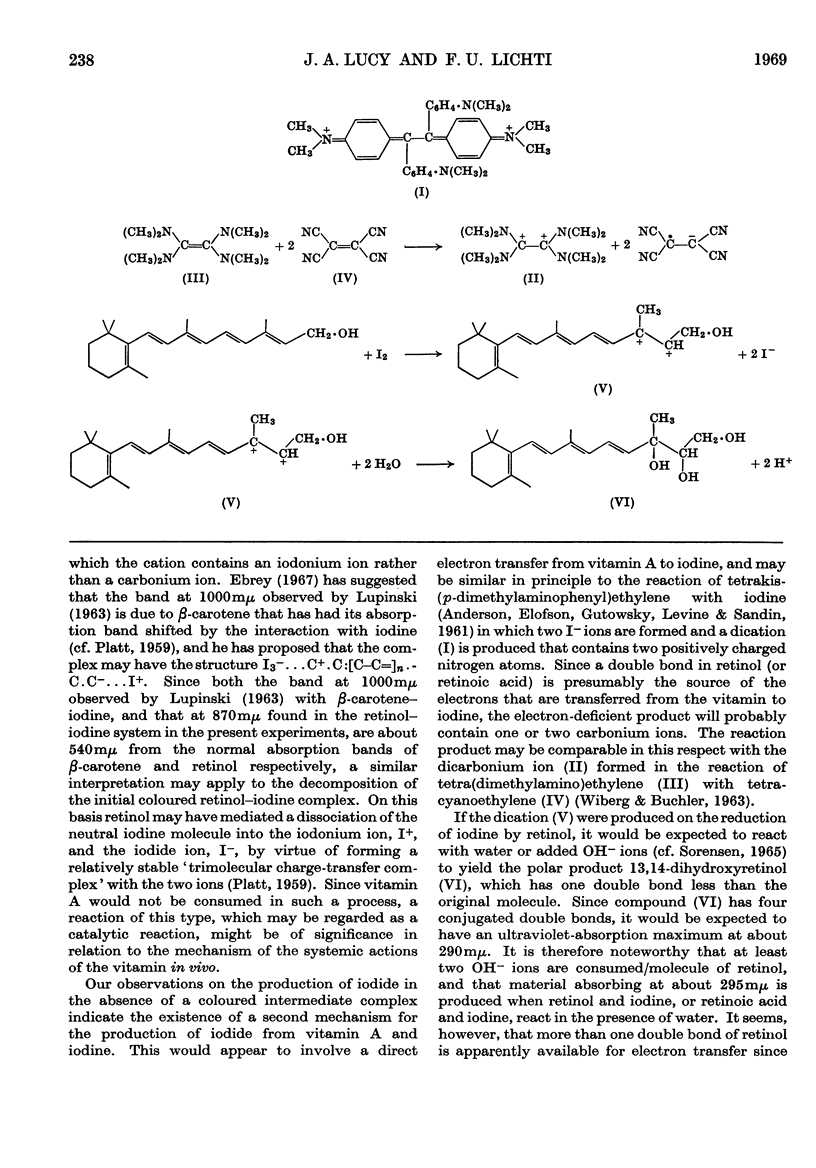
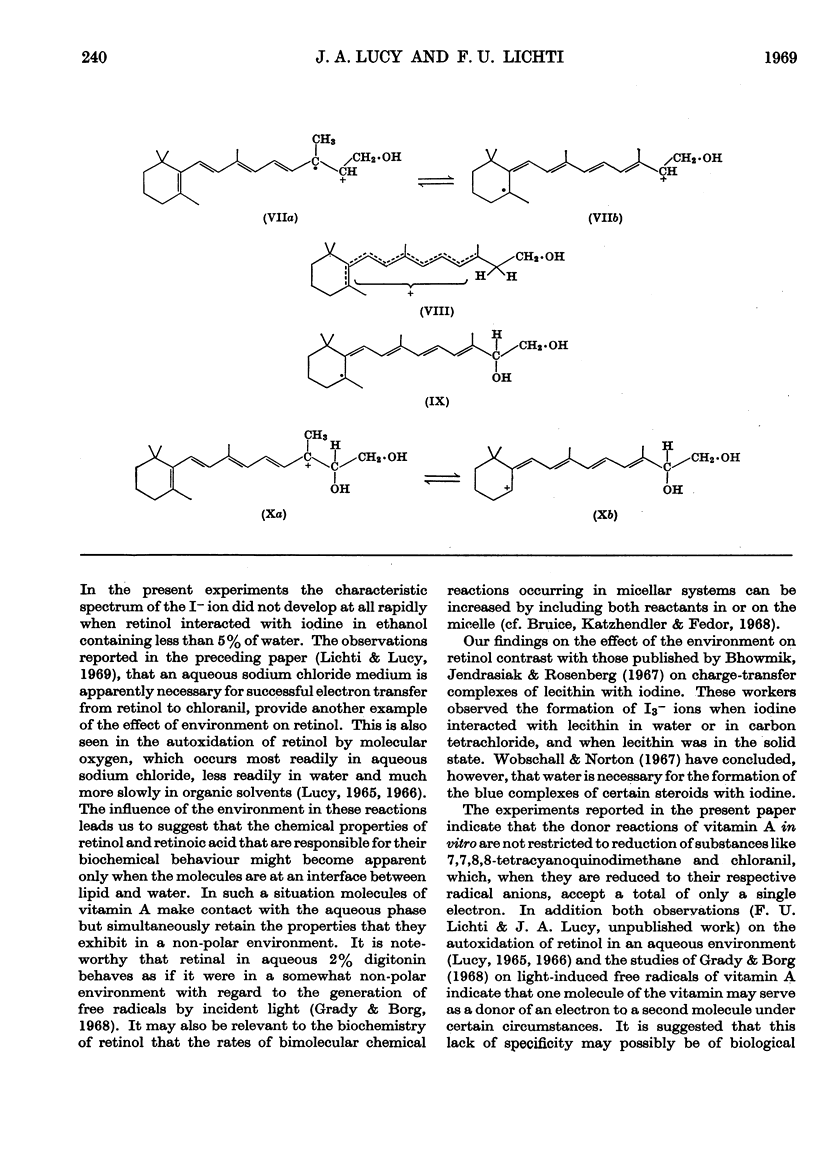
PDF
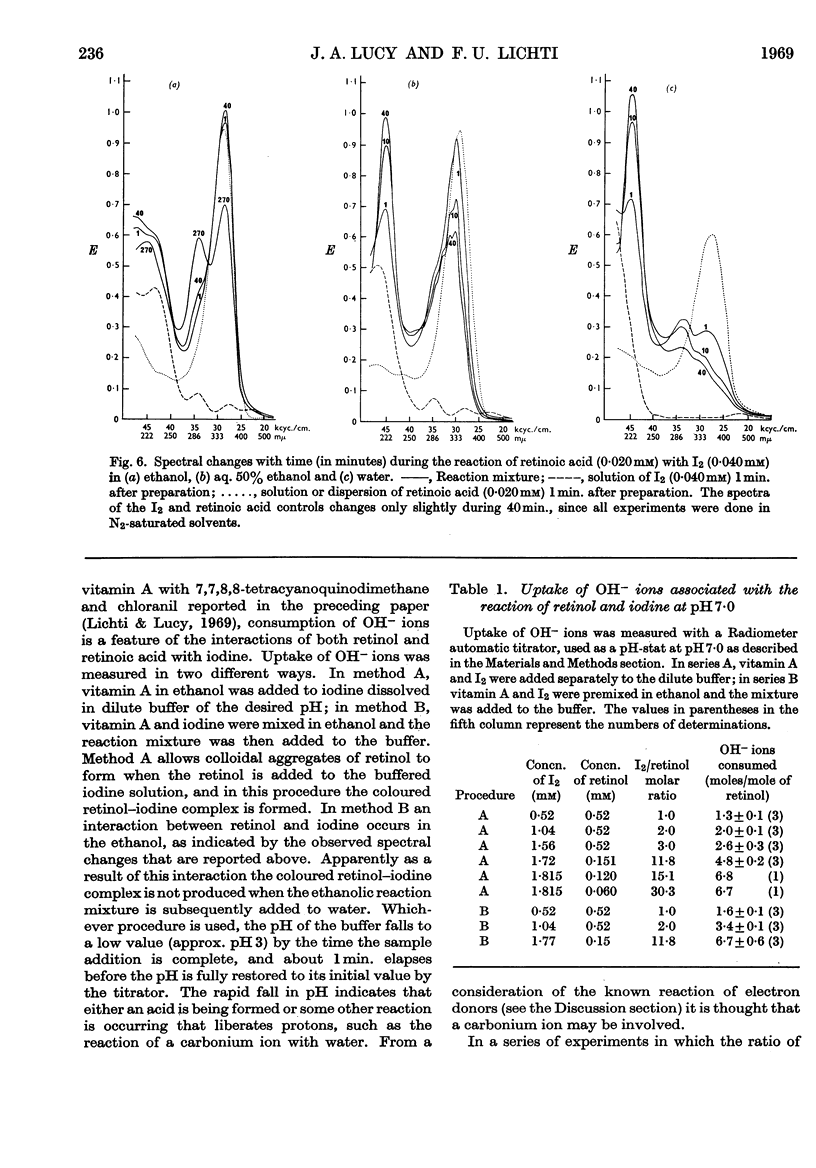



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham A. D., Dingle J. T., Lucy J. A. Studies on the mode of action of excess of vitamin A. 9. Penetration of lipid monolayers by compounds in the vitamin A series. Biochem J. 1964 Jan;90(1):133–140. doi: 10.1042/bj0900133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLE J. T., LUCY J. A. Studies on the mode of action of excess of vitamin A. 5. The effect of vitamin A on the stability of the erythrocyte membrane. Biochem J. 1962 Sep;84:611–621. doi: 10.1042/bj0840611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLE J. T., LUCY J. A. VITAMIN A, CAROTENOIDS AND CELL FUNCTION. Biol Rev Camb Philos Soc. 1965 Aug;40:422–461. doi: 10.1111/j.1469-185x.1965.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G. Charge-transfer complexes of polyenes. J Phys Chem. 1967 May;71(6):1963–1964. doi: 10.1021/j100865a080. [DOI] [PubMed] [Google Scholar]
- Grady F. J., Borg D. C. Light-induced free radicals of retinal, retinol, and rhodopsin. Biochemistry. 1968 Feb;7(2):675–682. doi: 10.1021/bi00842a024. [DOI] [PubMed] [Google Scholar]
- Lichti F. U., Lucy J. A. Reactions of vitamin A with acceptors of electrons. Formation of radical anions from 7,7,8,8-tetracyanoquinodimethane and tetrachloro-1,4-benzoquinone. Biochem J. 1969 Apr;112(2):221–229. doi: 10.1042/bj1120221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. R. Carotene-Donor-Acceptor Complexes in Photosynthesis: The predicted lowering of the excited states of carotenoids may offer a new photosynthetic pathway. Science. 1959 Feb 13;129(3346):372–374. doi: 10.1126/science.129.3346.372. [DOI] [PubMed] [Google Scholar]
- Wobschall D., Norton D. A. Absorption spectra and formation constants of steroid-iodine complexes. Arch Biochem Biophys. 1967 Oct;122(1):85–94. doi: 10.1016/0003-9861(67)90126-9. [DOI] [PubMed] [Google Scholar]


