Abstract
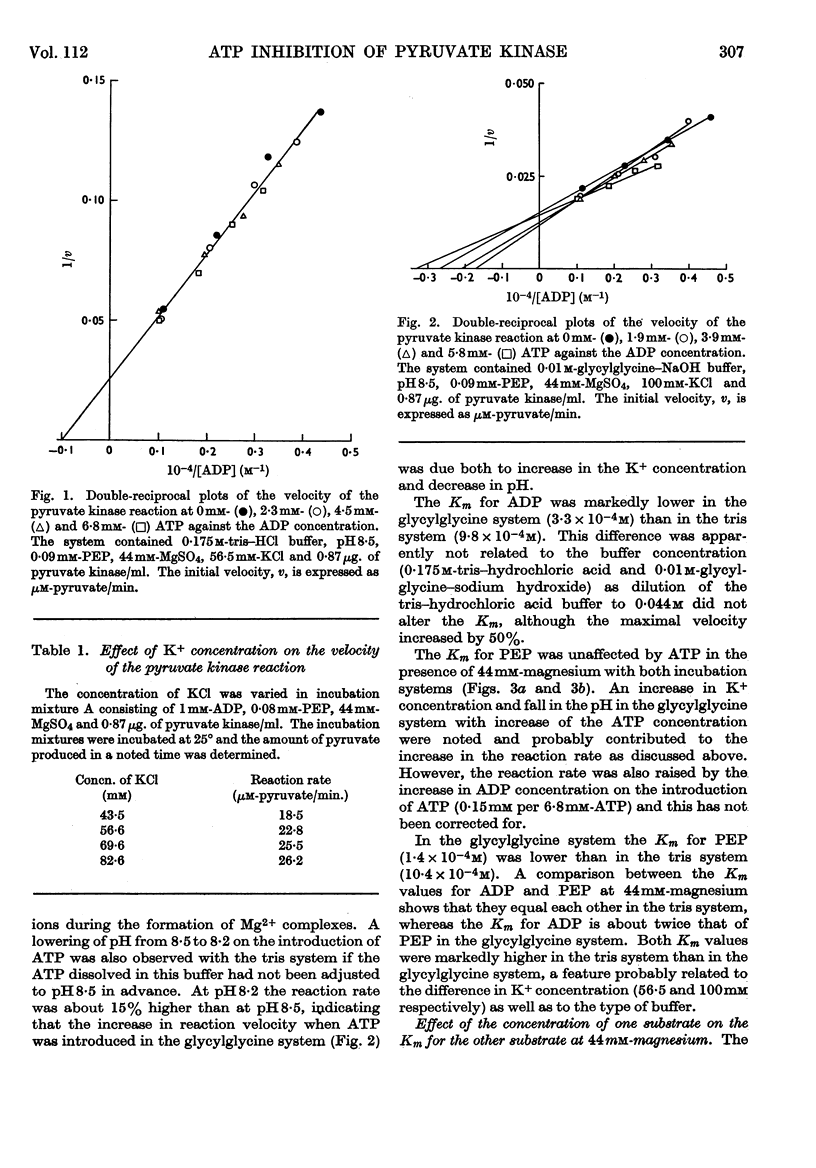
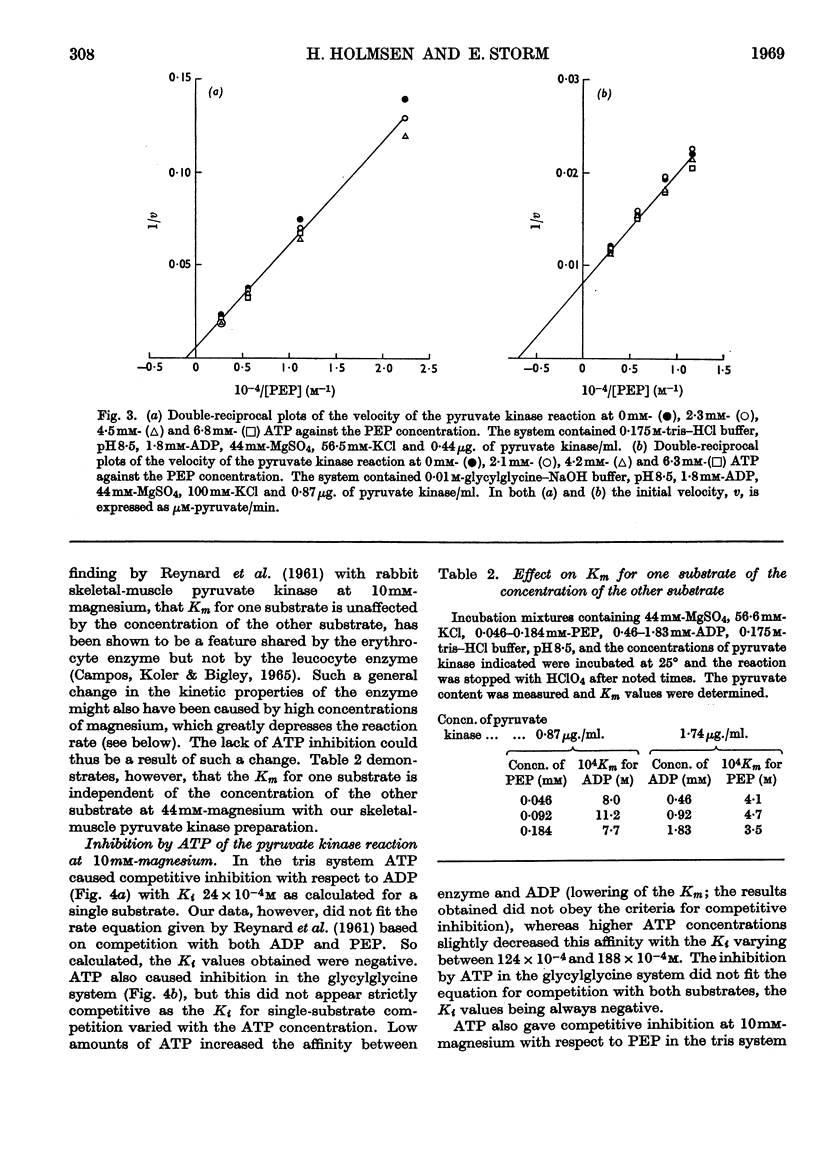
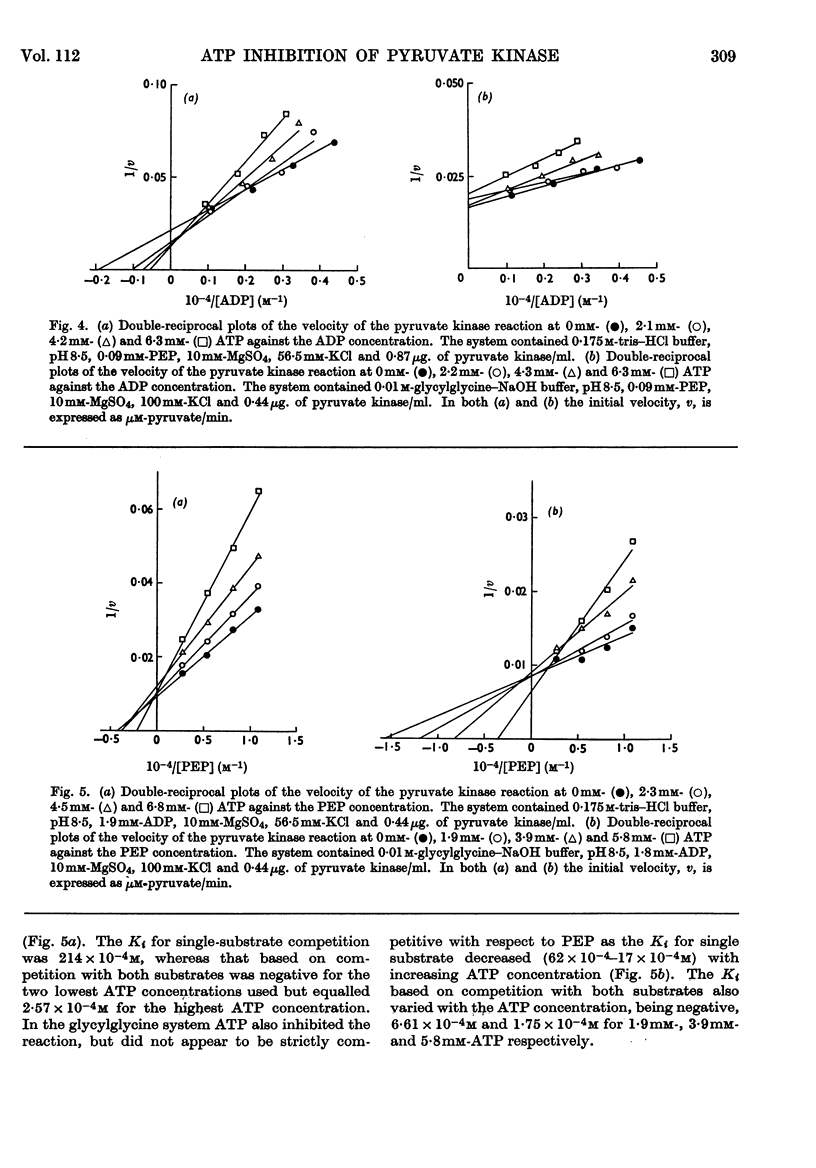
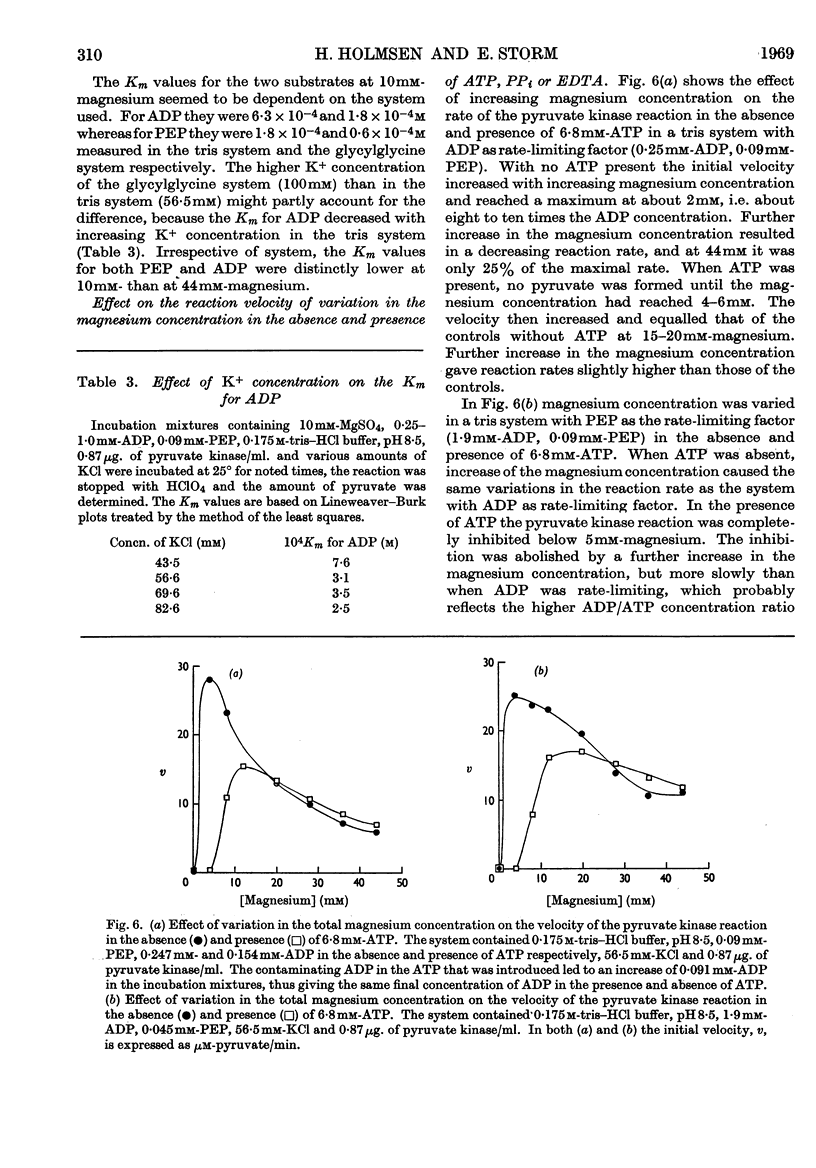
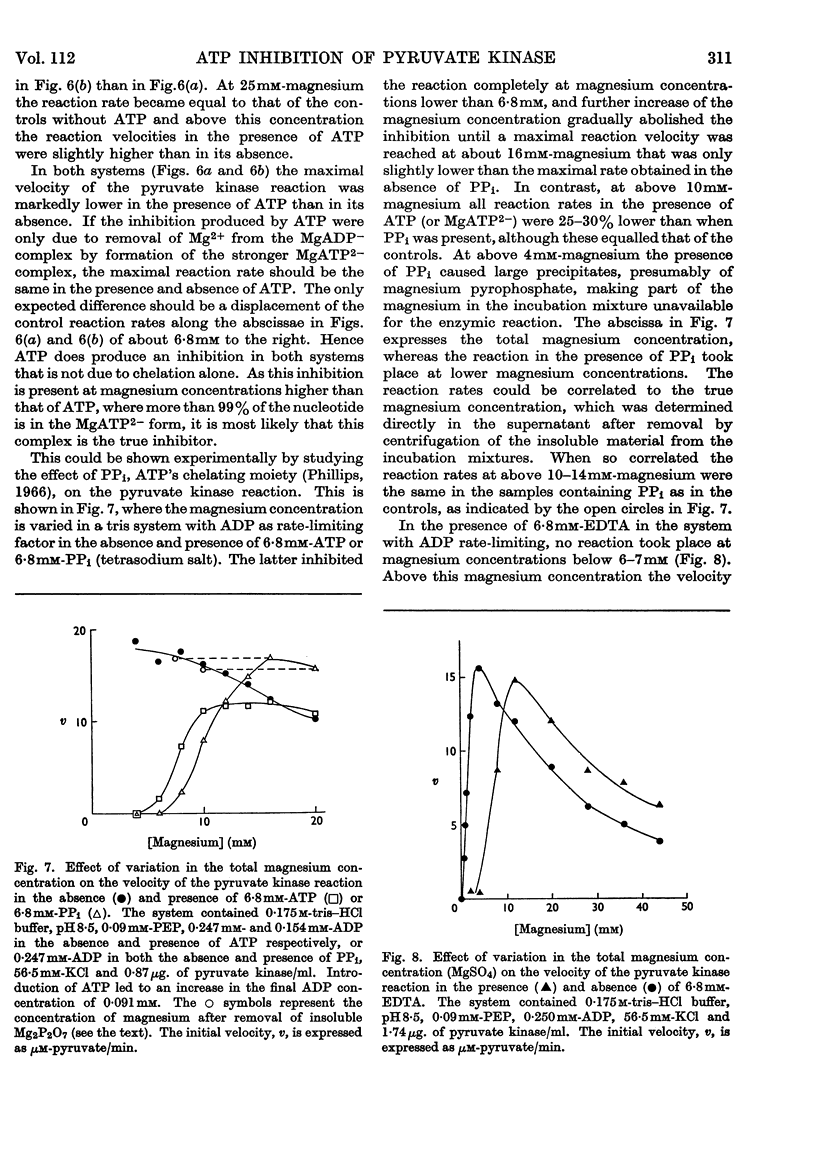
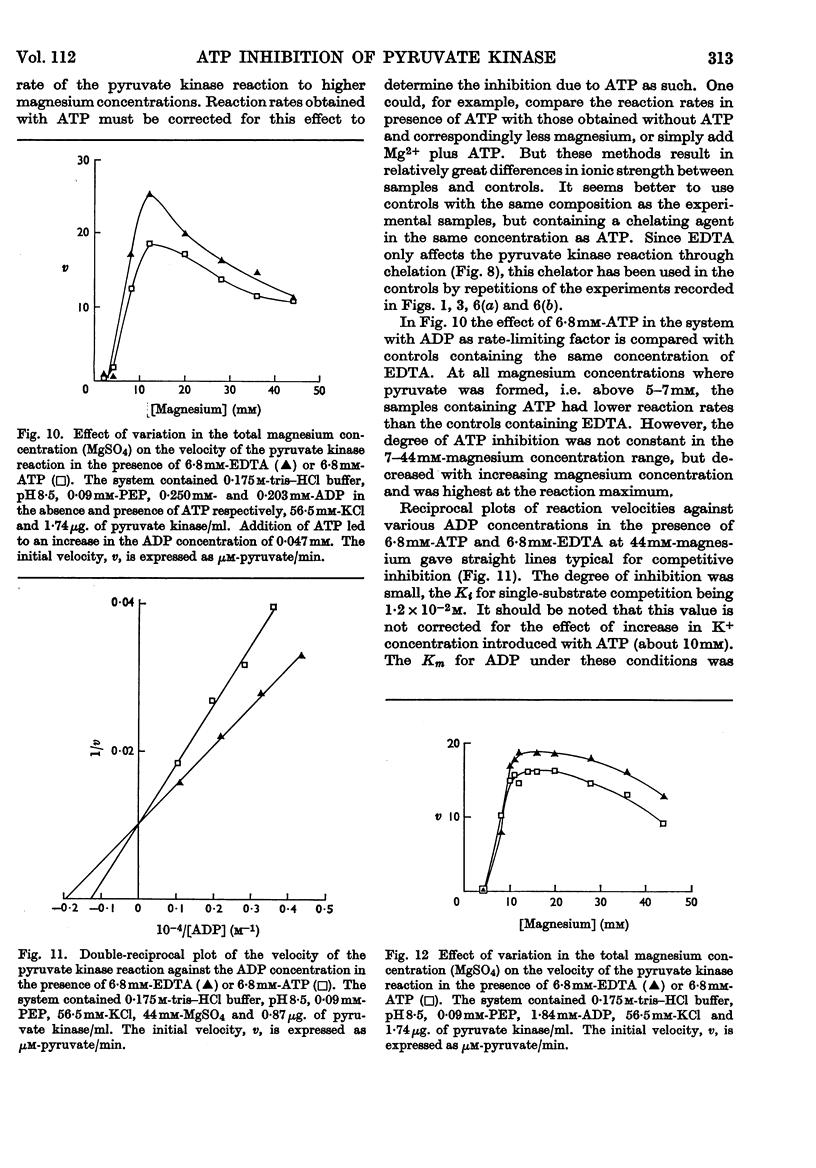
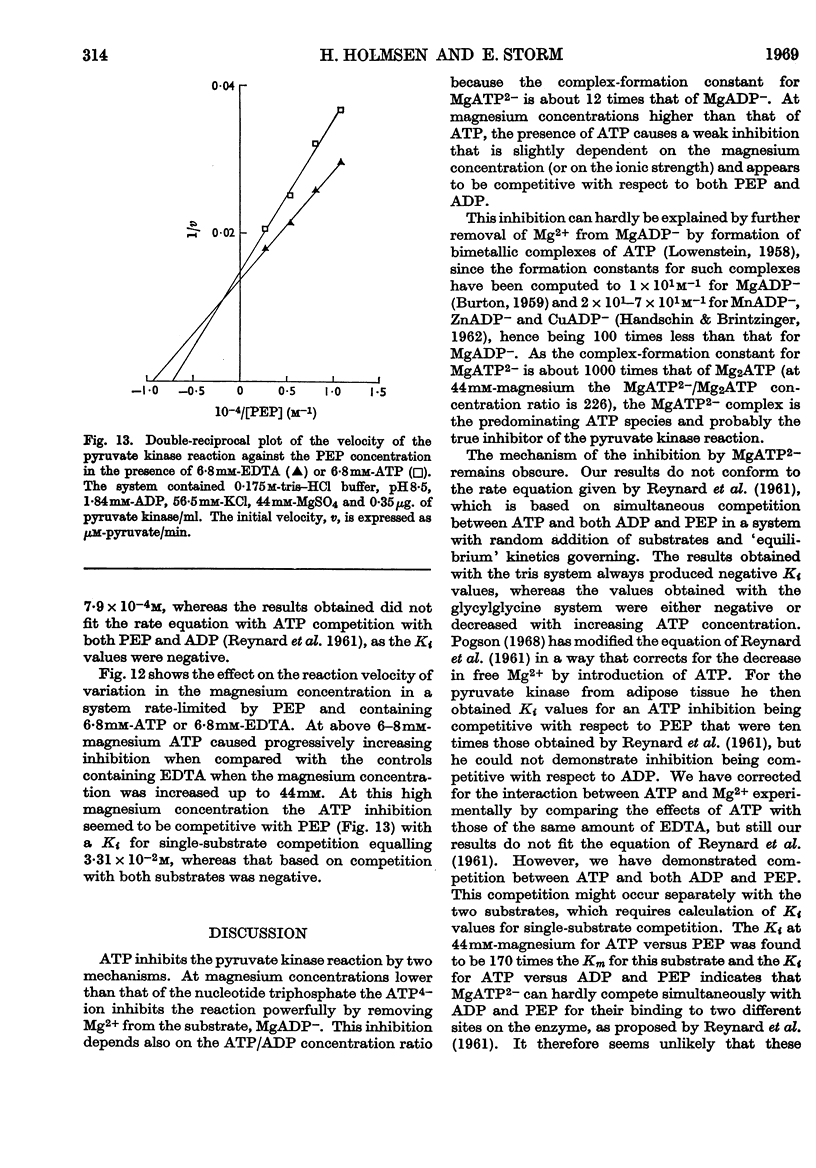
1. The effects of ATP, PPi and EDTA on the skeletal-muscle pyruvate kinase reaction at various concentrations of magnesium (where `magnesium' refers to total Mg2+, both free and in the form of complexes) were investigated. The reaction rate was determined as the amount of pyruvate formed in a recorded time of incubation. 2. At 44mm-magnesium the Km values for ADP and phosphoenolpyruvate were unaltered by the presence of ATP up to 6·8mm in systems buffered with either tris–hydrochloric acid or glycylglycine–sodium hydroxide, but the Km values were different in these systems. The Km for one substrate was independent of the concentration of the second substrate. 3. At 10mm-magnesium in the tris–hydrochloric acid system ATP inhibited the reaction competitively with respect to ADP and phosphoenolpyruvate. In the glycylglycine–sodium hydroxide system the inhibition appeared to be non-competitive. At 10mm-magnesium the Km values were lower than at 44mm-magnesium and dependent on the system used. 4. In the tris–hydrochloric acid system the reaction rate rose with increasing magnesium concentration up to a maximum at a concentration 10–20 times that of ADP. Further increase inhibited the reaction and at 44mm-magnesium the rate was 25–50% of its maximum. This inhibition paralleled that produced by increasing trimethylammonium chloride concentrations and was not due to a specific effect of the Mg2+ ion. 5. In the presence of 6·8mm-ATP no reaction occurred below 4–6mm-magnesium, and further increase apparently abolished the inhibition as the reaction rate increased and became equal to those obtained in the absence of ATP at 10–25mm-magnesium. Further increase in magnesium concentration gave reaction rates that were slightly higher in the presence of ATP than in its absence. The maximal rate in the presence of ATP was distinctly lower than in its absence. When 6·8mm-PPi or 6·8mm-EDTA was present the variations in reaction rate with rising magnesium concentration were similar to that obtained in the presence of ATP below 6–8mm-magnesium but further increase in the magnesium concentration resulted in an increase in the rate up to a maximum comparable with that of the control. The effect of pure chelation was thus a displacement of the reaction maximum to higher magnesium concentrations without changing the maximal rate. When correction had been made for this effect, ATP gave inhibition at 44mm-magnesium that was competitive with respect to ADP (Ki 2·1×10−2m). This degree of inhibition is far less than was reported earlier and its importance for the mechanism of the pyruvate kinase reaction is discussed.
Full text
PDF



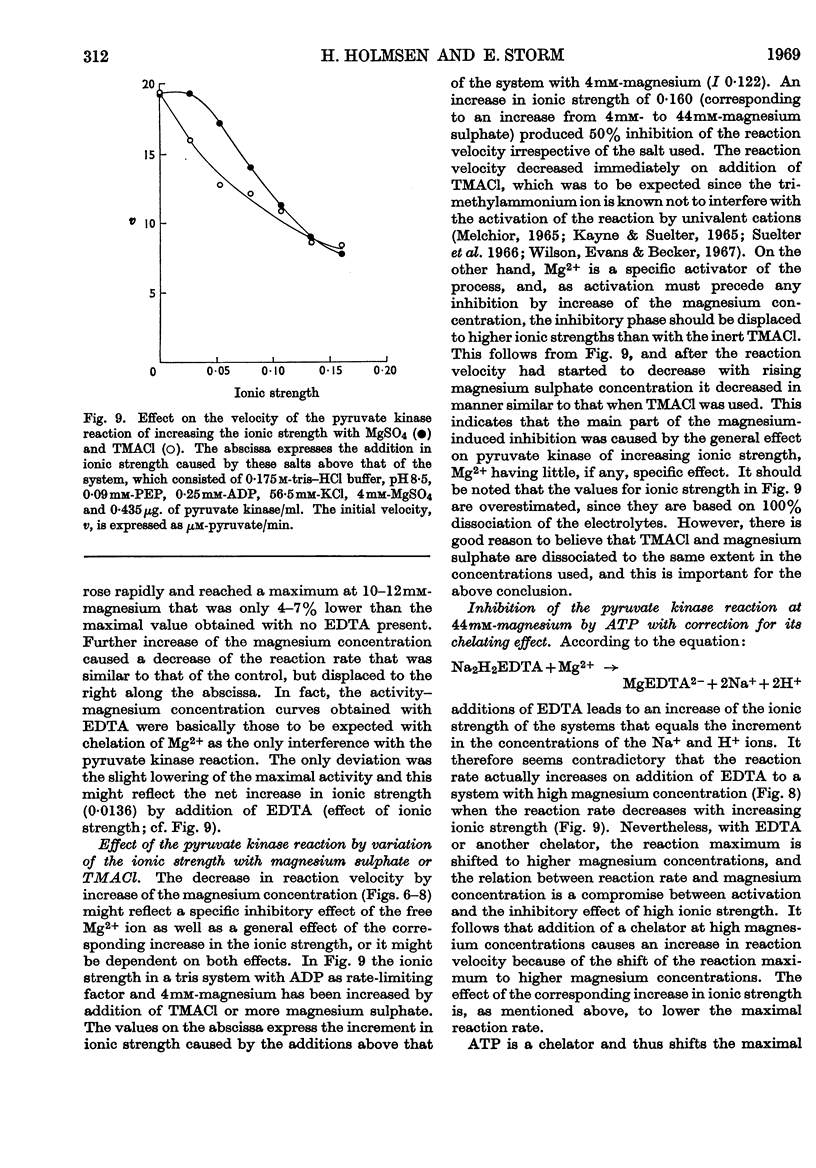


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. Formation constants for the complexes of adenosine di- or tri-phosphate with magnesium or calcium ions. Biochem J. 1959 Feb;71(2):388–395. doi: 10.1042/bj0710388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J. O., Koler R. D., Bigley R. H. Kinetic differences between human red cell and leucocyte pyruvate kinase. Nature. 1965 Oct 9;208(5006):194–195. doi: 10.1038/208194a0. [DOI] [PubMed] [Google Scholar]
- Hess B., Haeckel R., Brand K. FDP-activation of yeast pyruvate kinase. Biochem Biophys Res Commun. 1966 Sep 22;24(6):824–831. doi: 10.1016/0006-291x(66)90322-6. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Stormorken H., Goote T. M. Kinetic studies on the breakdown of adenosine diphosphate in human plasma. Scand J Clin Lab Invest. 1965;17(Suppl):138+–138+. [PubMed] [Google Scholar]
- KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem. 1953 Feb;200(2):669–682. [PubMed] [Google Scholar]
- KAYNE F. J., SUELTER C. H. EFFECTS OF TEMPERATURE, SUBSTRATE, AND ACTIVATING CATIONS ON THE CONFORMATIONS OF PYRUVATE KINASE IN AQUEOUS SOLUTIONS. J Am Chem Soc. 1965 Feb 20;87:897–900. doi: 10.1021/ja01082a035. [DOI] [PubMed] [Google Scholar]
- Kerson L. A., Garfinkel D., Mildvan A. S. Computer simulation studies of mammalian pyruvate kinase. J Biol Chem. 1967 May 10;242(9):2124–2133. [PubMed] [Google Scholar]
- LOWENSTEIN J. M. Transphosphorylations catalysed by bivalent metal ions. Biochem J. 1958 Oct;70(2):222–230. doi: 10.1042/bj0700222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V. THE RELATIONSHIPS BETWEEN SUBSTRATES AND ENZYMES OF GLYCOLYSIS IN BRAIN. J Biol Chem. 1964 Jan;239:31–42. [PubMed] [Google Scholar]
- McQUATE J. T., UTTER M. F. Equilibrium and kinetic studies of the pyruvic kinase reaction. J Biol Chem. 1959 Aug;234(8):2151–2157. [PubMed] [Google Scholar]
- Melchior J. B. The role of metal ions in the pyruvic kinase reaction. Biochemistry. 1965 Aug;4(8):1518–1525. doi: 10.1021/bi00884a009. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Cohn M. Kinetic and magnetic resonance studies of the pyruvate kinase reaction. II. Complexes of enzyme, metal, and substrates. J Biol Chem. 1966 Mar 10;241(5):1178–1193. [PubMed] [Google Scholar]
- Pogson C. I. Adipose-tissue pyruvate kinase. Properties and interconversion of two active forms. Biochem J. 1968 Nov;110(1):67–77. doi: 10.1042/bj1100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNARD A. M., HASS L. F., JACOBSEN D. D., BOYER P. D. The correlation of reaction kinetics and substrate binding with the mechanism of pyruvate kinase. J Biol Chem. 1961 Aug;236:2277–2283. [PubMed] [Google Scholar]
- Sorger G. J., Ford R. E., Evans H. J. Effects of univalent cations on the immunoelectrophoretic behavior of pyruvic kinase. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1614–1621. doi: 10.1073/pnas.54.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suelter C. H., Singleton R., Jr, Kayne F. J., Arrington S., Glass J., Mildvan A. S. Stuies on the interaction of substrate and monovalent and divalent cations with pyruvate kinase. Biochemistry. 1966 Jan;5(1):131–139. doi: 10.1021/bi00865a017. [DOI] [PubMed] [Google Scholar]
- Wilson R. H., Evans H. J., Becker R. R. The effect of univalent cation salts on the stability and on certain physical properties of pyruvate kinase. J Biol Chem. 1967 Sep 10;242(17):3825–3832. [PubMed] [Google Scholar]


