Abstract
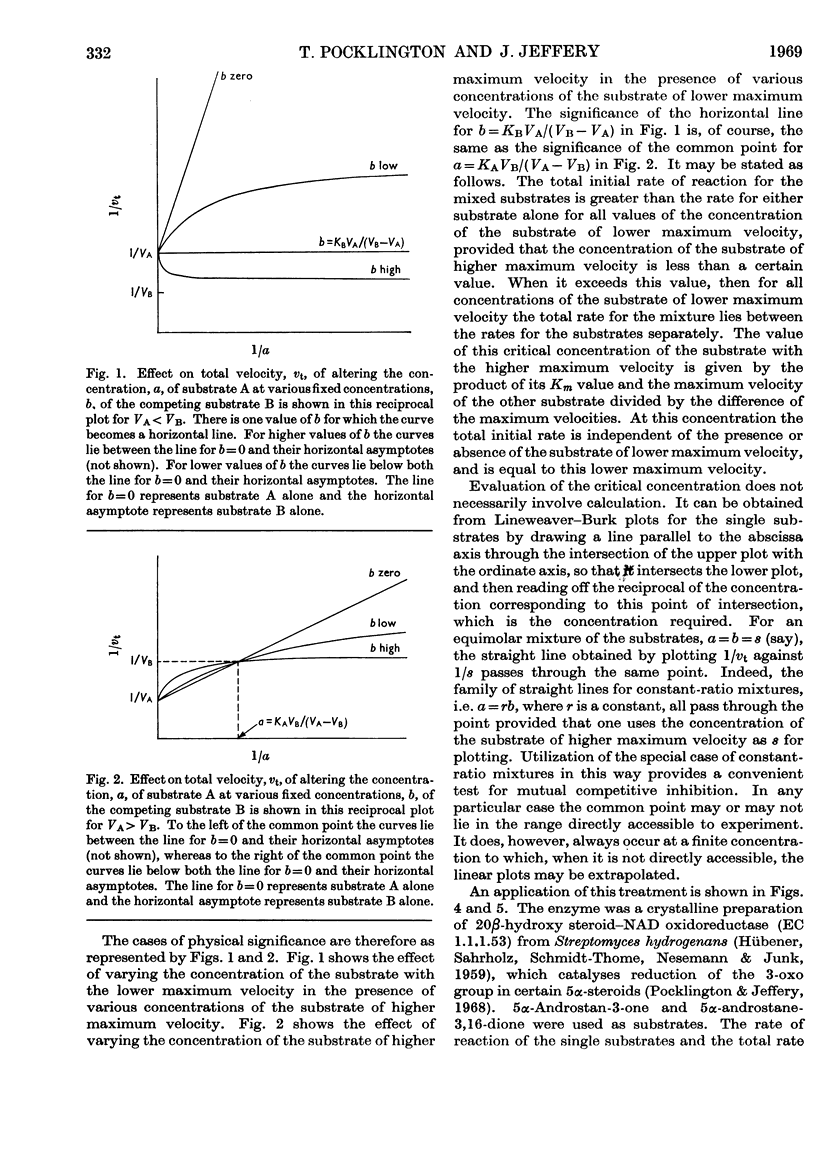
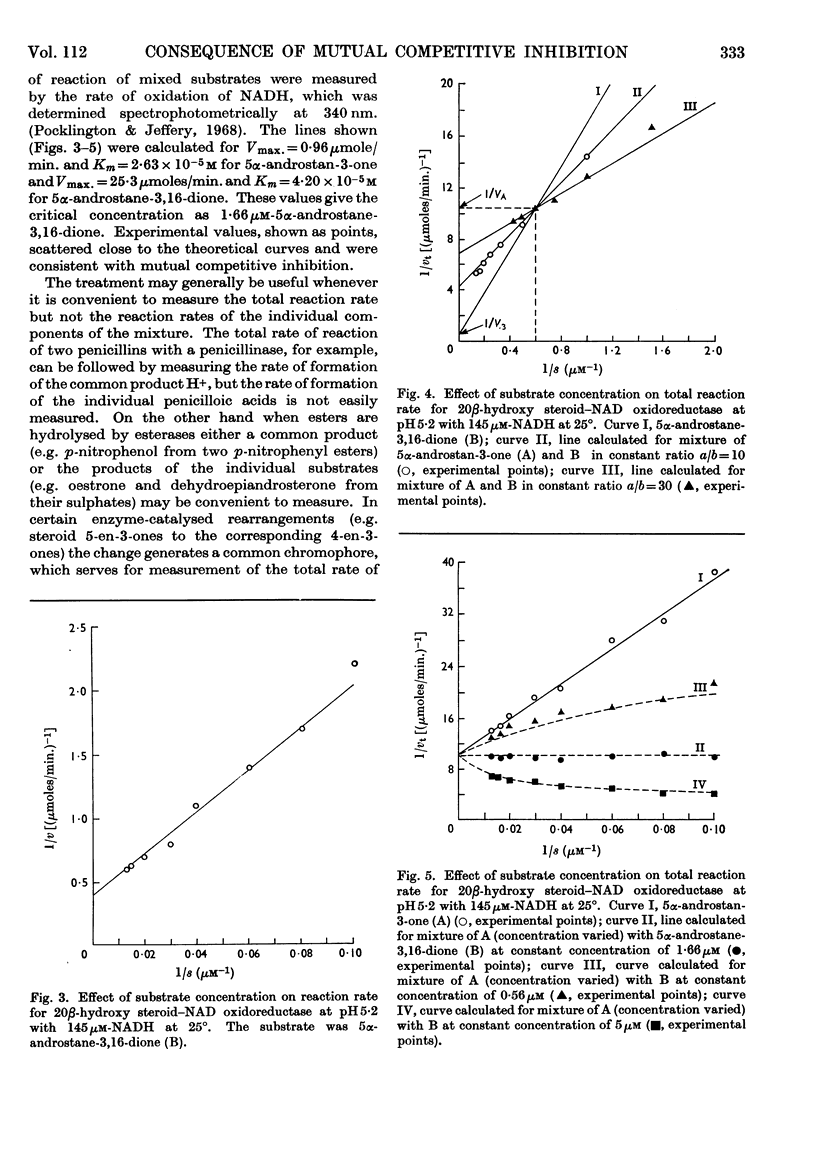
1. If two compounds are substrates for a single enzyme, and do not form any ternary complex with the enzyme or combine directly with each other, then the total initial rate of reaction for a mixture of the two compounds may be greater than the rate for either compound alone, or may lie between the rates for the compounds alone. It is the concentration of the compound with the higher maximum velocity that determines which applies, and there is one concentration of the compound of higher maximum velocity at which the total rate of reaction is independent of the presence or absence of the substrate of lower maximum velocity. The values concerned are derived. 2. An example is given of 5α-androstan-3-one and 5α-androstane-3,16-dione as substrates competing for a hydroxy steroid–NAD oxidoreductase (EC 1.1.1.53).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HAKALA M. T., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. II. Variation of kinetic and equilibrium constants with temperature. J Biol Chem. 1956 Jul;221(1):191–209. [PubMed] [Google Scholar]
- HUEBENER H. J., SAHRHOLZ F. G., SCHMIDT-THOME J., NESEMANN G., JUNK R. [20 beta-Hydroxysteroid dehydrogenase, a new crystalline enzyme]. Biochim Biophys Acta. 1959 Sep;35:270–272. doi: 10.1016/0006-3002(59)90366-x. [DOI] [PubMed] [Google Scholar]
- Pocklington T., Jeffery J. 3 alpha-hydroxysteroid: NAD oxidoreductase activity in crystalline preparations of 20 beta-hydroxysteroid: NAD oxidoreductase. Eur J Biochem. 1968 Dec;7(1):63–67. doi: 10.1111/j.1432-1033.1968.tb19574.x. [DOI] [PubMed] [Google Scholar]


