Abstract
BACKGROUND:
Cardiac allograft vasculopathy (CAV) limits long-term survival after heart transplantation (HT). This study evaluates the relationship between clinically significant cytomegalovirus infection (CS-CMVi) and CAV using cardiac positron emission tomography (PET).
METHODS:
We retrospectively evaluated HT patients from 2005 to 2019 who underwent cardiac PET for CAV evaluation. Multivariable linear and logistic regression models were used to evaluate the association between CS-CMVi and myocardial flow reserve (MFR). Kaplan-Meier and Cox regression analyses were used to assess the relationship between CS-CMV, MFR, and clinical outcomes.
RESULTS:
Thirty-two (31.1%) of 103 HT patients developed CS-CMVi at a median 9 months after HT. Patients with CS-CMVi had a significantly lower MFR at year 1 and 3, driven by reduction in stress myocardial blood flow. Patients with CS-CMVi had a faster rate of decline in MFR compared to those without infection (−0.10 vs −0.06 per year, p < 0.001). CS-CMVi was an independent predictor of abnormal MFR (< 2.0) (odds ratio: 3.8, 95% confidence intervals (CI): 1.4–10.7, p = 0.001) and a lower MFR (β = −0.39, 95% CI: −0.63 to −0.16, p = 0.001) at year 3. In adjusted survival analyses, both abnormal MFR (log-rank p < 0.001; hazard ratio [HR]: 5.7, 95% CI: 4.2–7.2) and CS-CMVi (log-rank p = 0.028; HR: 3.3, 95% CI: 1.8–4.8) were significant predictors of the primary outcome of all-cause mortality, retransplantation, heart failure hospitalization, and acute coronary syndrome.
CONCLUSIONS:
CS-CMVi is an independent predictor of reduced MFR following HT. These findings suggest that CMV infection is an important risk factor in the development and progression of CAV.
Keywords: heart transplant, myocardial flow reserve, microvascular function, cytomegalovirus, cardiac allograft vasculopathy
Cardiac allograft vasculopathy (CAV) limits long-term survival following heart transplantation (HT).1 CAV is characterized by progressive, immune- and nonimmune-mediated intimal hyperplasia and fibrosis of the coronary vasculature that is often asymptomatic until irreversible injury occurs,2 underscoring the need to identify modifiable risk factors. Cytomegalovirus (CMV) is a common opportunistic infection associated with worse outcomes following HT,3,4 and guidelines recommend anti-CMV prophylaxis for at-risk solid organ recipients.4,5 CMV is theorized to contribute to the development of CAV through endothelial injury, inflammation, and enhanced cell-mediated alloreactivity.6 Randomized trials demonstrated that prophylactic treatment of CMV reduces CAV severity.7,8 There are limited recent data on the relationship between CMV and CAV in the setting of contemporary immunosuppression, routine anti-CMV prophylaxis, and earlier use of CAV-directed therapies such as mechanistic target of rapamycin inhibitors (MTI) and statin therapy.9
Observational studies evaluating the relationship between CMV infection and epicardial CAV have had discordant results.10–12 Invasive coronary angiography (ICA) has been the primary modality for the diagnosis of CAV, though it is now understood to have significant limitations. ICA is unable to identify the early intimal thickening and longitudinal lesions commonly seen in CAV.13 Furthermore, ICA is unable to assess early impairments in myocardial blood flow, which may be due to coronary microvascular dysfunction.14 This is a critical limitation, as CAV first manifests with microvasculopathy before progressing to epicardial disease.14 Therefore, ICA may not effectively identify incipient CAV, especially among patients with early lesions or microvascular disease. Importantly, as CMV is theorized to exert its atherogenic effects primarily in the microvasculature,15,16 ICA may not effectively capture the association CMV and CAV.
Positron emission tomography myocardial perfusion imaging (PET MPI) has recently emerged as a powerful alternative diagnostic modality for CAV. PET provides a functional and physiologic assessment of the myocardium and microvasculature through quantification of myocardial flow reserve (MFR), and MFR now has established diagnostic and prognostic value in HT patients.17–20 Identification of microvascular dysfunction via MFR may offer insight into the association between CMV and CAV. The primary aim of this study is to evaluate the relationship between CMV infection and MFR following HT. We hypothesized that CMV infection would be associated with impairment in MFR, driven by lower stress-induced maximal myocardial blood flow.
Materials and methods
Study design and patient selection
This study was approved by the University of Michigan Institutional Review Board and is compliant with the ISHLT ethics statement. We retrospectively evaluated patients 18 and older who underwent a first HT between 2005 and 2019 at the University of Michigan. Inclusion was conditioned on having PET MPI in the third year after HT (defined as 36 ± 12 months). Patients were excluded if they underwent HT at < 18 years of age, underwent multiorgan transplantation or retransplantation, or did not undergo PET MPI. CS-CMVi generally occurs in the first 2 post-transplant years,4,5 and PET MPI in the third year would be likeliest to capture the impact of CS-CMVi on CAV. Follow-up data were collected through 2022.
Post-transplant CAV protocol
All patients are started on aspirin and pravastatin for CAV prevention. Initial immunosuppression generally includes a calcineurin inhibitor, mycophenolate mofetil (MMF), and prednisone. Calcineurin inhibitors may be replaced with an MTI (sirolimus or everolimus) for renal-sparing immunosuppression or the treatment of CAV, if identified. Statin therapy is intensified in all HT patients to the maximally tolerated dose (patient symptoms or laboratory evidence of liver/muscle injury). CAV surveillance is performed by ICA at 12 months following HT, after which time PET MPI is used every 12 to 24 months alongside ICA at the treating cardiologist’s discretion. The protocol for PET MPI and study interpretation at our institution have previously been described.19,21 Briefly, we use a whole-body PET computed tomography scanner (Siemens mCT, Knoxville, TN) to quantify myocardial blood flow at rest (rMBF) and peak hyperemic stress. We use regadenoson as the stress agent and rubidium-82 (Cardiogen-82 [Bracco] or Ruby-FILL [Jubilant Draximage]) as the perfusion tracer. Gated myocardial perfusion images are interpreted by cardiologists trained in the interpretation of nuclear images.22 Global MFR was calculated as the ratio of stress MBF (sMBF) and rMBF for the entire left ventricle using an FDA-approved software tool (Corridor4DM, INVIA, Medical Imaging Solutions, Ann Arbor, MI).23
Post-transplant CMV protocol
Donor and recipient anti-CMV IgG serologic status (D+, donor-positive; D−, donor-negative; R+, recipient-positive; R−, recipient-negative) are determined prior to HT, and patients are categorized as high-risk (D+/R−), intermediate-risk (D+/R+ and D−/R+), and low-risk (D−/R−) for CMV infection. In accordance with current guidelines,4,5 intermediate and high-risk patients receive antiviral prophylaxis with valganciclovir for 3- and 6-months post-transplant, respectively. Low-risk patients receive prophylaxis with acyclovir for 3 months. Donor- or recipient-positive patients are reinitiated on valganciclovir if they receive thymoglobulin treatment or high-dose steroid treatment for acute rejection. HT patients undergo CMV testing via quantitative CMV DNA polymerase chain reaction test if clinically indicated by symptoms and for 3 months after withdrawal of prophylaxis.
Patients with suspected infection are evaluated by a transplant infectious disease physician. For this study, clinically significant CMV infection (CS-CMVi) was defined as confirmation of viral replication in the blood (CMV DNAemia), the presence of either CMV syndrome (fever, malaise, cytopenia) or invasive disease (organ injury), or requirement for antiviral therapy. Persistent CMV DNAemia > 1000 IU/mL in the absence of symptoms is treated if it is determined to be clinically significant. Low-grade CMV DNAemia (< 1000 IU/ml) in asymptomatic patients is generally not treated. CS-CMVi is treated with intravenous ganciclovir or oral valganciclovir for an induction therapy course of at least 21 days. MMF is held during treatment. If there is no decline in CMV viral load within 14 days of treatment, we evaluate for ganciclovir resistance or treatment failure. If response to therapy is noted at 21 days, patients are converted to maintenance therapy for 4 weeks, after which treatment is stopped.
Data collection and variables
We collected PET MPI data at year 1, 3, 5, and 7 with 12-month time windows. PET MPI variables included MFR, rMBF, sMBF, and the presence of ischemia defined as a summed difference score (SDS) > 1. We collected demographic and clinical variables including age, sex, race, CMV serologic status, body mass index (BMI), diabetes mellitus with insulin-requirement, estimated glomerular filtration rate (eGFR) at time of PET MPI, statin therapy, and the incidence of acute cellular rejection (ACR), antibody-mediated rejection (AMR), and de novo donor-specific antibodies (DSA; defined as MFI > 1000). Statin intensity was defined as low, moderate, or high intensity according to ACC/AHA guidelines.24 ACR was defined using the 2004 revised International Society for Heart and Lung Transplantation (ISHLT criteria)25 and included treated biopsy-negative rejection. AMR was defined as either pathologic AMR grade ≥2 or presumed AMR (DSA positivity with left ventricular dysfunction and ACR grade 0–1). Finally, we compared prevalence of epicardial CAV by CS-CMVi status. Epicardial CAV was defined as ISHLT grade 2 or higher by ICA26 or Stanford class 2 or higher by intravascular ultrasound (IVUS).27 Abnormal MFR was defined as a MFR < 2.0, consistent with established thresholds in the general population28 and in HT patients.18–20
Statistical analysis
Descriptive characteristics were summarized with means (standard deviation [SD]) for continuous variables and frequency (percentage) for categorical variables. We compared differences in clinical characteristics, PET MPI parameters, and rates of epicardial CAV by post-transplant CS-CMVi status using Student’s t-test and Fisher’s exact test. We additionally evaluated PET MPI parameters in patients with asymptomatic CMV viremia which was not treated as CS-CMVi.
We generated 3 models to delineate the relationship between CS-CMVi and CAV. First, we constructed a multivariable linear regression model to evaluate the association between CS-CMVi and MFR at year 3. We included variables based on clinical expertise and the published literature, selecting factors with suspected associations with CAV including age, race/ethnicity, sex, obesity (BMI > 30 kg/m2), diabetes mellitus with insulin-requirement, hypertension (systolic blood pressure > 140 mmHg), stage 3+ chronic kidney disease (eGFR < 60 mL/min/1.73 m2 at time of PET MPI), maximally tolerated statin intensity prior to PET MPI, MTI use, and prior ACR. AMR and DSA were excluded due to low number of events. Significant univariable predictors (p < 0.05) were included in the multivariable model in stepwise fashion with an alpha of 0.05 for selection and elimination. β values were calculated to estimate the expected difference in MFR with a history of CS-CMVi. Second, we constructed a multivariable logistic model including all significant univariable predictors from the linear model. We calculated adjusted odds ratios and 95% confidence intervals (CI) to estimate the association between CS-CMVi and an abnormal MFR (MFR < 2.0) at year 3. Third and finally, we constructed linear mixed effects models of serial MFR values (including per-subject random intercepts). Lines of best fit were generated to visualize rates of change in MFR by CS-CMVi status. Addition of a (time) * (CS-CMVi status) interaction was used to determine if the rates of change in MFR over time differed by CS-CMVi status. Finally, Kaplan-Meier and Cox regression analyses were used to assess the association between MFR or CS-CMVi and the primary outcome after the first PET MPI (at 24–36 months). The primary outcome was the time to first event defined as a composite of all-cause mortality, retransplantation, hospitalization for heart failure, or acute coronary syndromes. Patients were followed for clinical outcomes through December 2022. Significance was assigned at an alpha level of 0.05. Analyses were performed in SPSS (version 28.0, IBM Corp., Armonk, NY).
Results
Baseline characteristics
Baseline characteristics for the 103 included patients are reported in Table 1. The cohort was a mean 55 (SD: 11) years of age, and the majority were male (80.6%) and non-Hispanic White (76.7%). Most patients were maintained on an immunosuppression regimen of a calcineurin inhibitor, MMF, and prednisone. Eighty-eight of 103 (85.4%) remained on MMF at 1-year post-transplant. Thirty-four (33%) were high-risk (D+/R−) and 45 (43.7%) were intermediate-risk for CMV infection (Table S1). Compared to patients at low and intermediate-risk, high-risk patients were more likely to develop CS-CMVi (70.6% vs 11.6%). Thirty-eight (36.9%) developed grade 2 or higher ACR at a median of 15 months (interquartile range [IQR], 11–18) after HT. Eighteen of 32 (56.3%) CS-CMVi patients developed ACR, 15 (83.3%) of which occurred after CS-CMVi. A total of 34 (33%) patients were started on an MTI, 24 of which were for the treatment of CAV and 10 for renal-sparing immunosuppression. Twenty-seven of 34 were started on MTI after CS-CMVi occurred. There was no significant difference in MFR in patients who received MTI (2.35 vs 2.51, p = 0.08). All patients received statin therapy, and 44 (42.7%) were escalated to a moderate or high intensity statin in the first 3 years post-HT.
Table 1.
Baseline Characteristics in Patients With and Without Incident CS-CMVi
| Variable | All (N = 103) | CS-CMVi (N = 32) | No CS-CMVi (N = 71) | p-value |
|---|---|---|---|---|
| Age (years), mean (SD) | 54.5 (10.8) | 51.1 (11.2) | 56.0 (10.4) | NS |
| Female sex, % | 20 (19.4%) | 6 (18.8%) | 14 (19.7%) | NS |
| Non-White race, % | 24 (23.3%) | 4 (12.5%) | 20 (28.2%) | < 0.05 |
| Donor age (years), mean (SD) | 33.5 (12.0) | 33.4 (12.1) | 33.6 (12.1) | NS |
| CMV donor/recipient serologic status | ||||
| High risk (D+/R−) | 34 (33.0%) | 24 (75.0%) | 10 (14.1%) | < 0.05 |
| Intermediate risk (D+/R+ or D−/R+) | 45 (43.7%) | 6 (18.8%) | 39 (54.9%) | < 0.05 |
| Low risk (D−/R−) | 24 (23.3%) | 2 (6.3%) | 22 (31.0%) | < 0.05 |
| Baseline eGFR (mL/min/1.73 m2), mean (SD) | 64.8 (19.4) | 67.9 (22.7) | 63.3 (17.7) | NS |
| Baseline BMI (kg/m2), mean (SD) | 27.6 (4.5) | 28.0 (4.1) | 27.3 (4.7) | NS |
| Ischemic cardiomyopathy, % | 29 (28.2%) | 12 (37.5%) | 17 (23.9%) | NS |
| Panel-reactive antibody sensitization > 10%, % | 17 (16.5%) | 5 (15.6%) | 12 (16.9%) | NS |
| Primary immunosuppression, % | ||||
| Calcineurin inhibitor | 103 (100%) | 32 (100%) | 71 (100%) | NS |
| Prednisone | 85 (82.5%) | 27 (84.4%) | 49 (69.0%) | NS |
| Mycophenolate mofetil | 88 (85.4%) | 27 (84.4%) | 61 (85.9%) | NS |
| CNI replaced with MTI | 34 (33.0%) | 13 (40.6%) | 21 (29.6%) | < 0.05 |
| Post-transplant Comorbidities at 1 year, % | ||||
| Hypertension | 64 (62.2%) | 18 (56.3%) | 46 (64.8%) | NS |
| Diabetes mellitus requiring Insulin | 30 (29.1%) | 8 (25.0%) | 22 (31.0%) | NS |
| Stage 3+ CKD (GFR < 60) | 50 (48.5%) | 15 (46.9%) | 35 (49.3%) | NS |
| Post-transplant medical therapy at 1 year, % | ||||
| Aspirin | 89 (86.4%) | 26 (81.3%) | 63 (88.7%) | NS |
| Statin - low intensity | 59 (57.3%) | 19 (59.4%) | 40 (56.3%) | NS |
| Statin - moderate/high intensity | 44 (42.7%) | 13 (40.6%) | 31 (43.7%) | NS |
| ACR (grade 2 or higher), % | 38 (36.9%) | 18 (56.3%) | 20 (28.2%) | < 0.05 |
| AMR (pAMR ≥2), % | 13 (12.6%) | 3 (9.4%) | 5 (7.1%) | NA |
| DSA, % | 14 (13.6%) | 4 (12.5%) | 10 (14.1%) | NS |
Abbreviations: ACR, acute cellular rejection; AMR, antibody-mediated rejection; BMI, body mass index; CAV, cardiac allograft vasculopathy; CS-CMVi, clinically significant cytomegalovirus infection; DSA, de novo donor-specific antibodies; eGFR, estimated glomerular filtration rate; MTI, mTOR inhibitor; NA, not applicable (sample size < 5 patients per group); NS, non-significant.
The table compares baseline differences by CS-CMVi status, reported as mean and standard deviation or frequency and percentage.
CMV infection
A total of 32 (31.1%) patients developed CS-CMVi at a median 9 months (range: 5–17) after HT (Table 2). All infections occurred following completion of post-transplant antiviral prophylaxis. Most patients (75%) with CS-CMVi were at high risk (D+/R−). Patients with CS-CMVi were more likely to be White, be started on an MTI, and have ACR (Table 1). Fourteen of 18 CS-CMVi patients with ACR had the ACR episode after CS-CMVi. Of the 32 patients with infection, all had CMV DNAemia, 18 (56.3%) had CMV syndrome, 10 (31.3%) had CMV invasive disease, and 4 (12.5%) had asymptomatic CMV DNAemia which was treated as a clinical infection. Twenty-seven patients (84.4%) had CMV PCR below the limit of detection of the assay by 6 months after initiation of treatment, and 5 (15.6%) patients had recurrent CS-CMVi requiring re-initiation of treatment.
Table 2.
Characteristics of Post-transplant CS-CMVi
| Variable | Characteristic | CS-CMVi (N = 32) |
|---|---|---|
| Infection timing, % | Postprophylaxis | 32 (100%) |
| Breakthrough on prophylaxis | 0 (0%) | |
| Infection type, % | CMV DNAemia present | 32 (100%) |
| CMV syndrome | 18 (56.3%) | |
| CMV invasive disease | 10 (31.3%) | |
| Enteritis/colitis | 8 | |
| Pancreatitis | 1 | |
| Pneumonitis | 1 | |
| Persistent CMV DNAemia requiring treatment | 4 (12.5%) | |
| Initial treatment, % | Ganciclovir | 21 (65.6%) |
| Valganciclovir | 11 (34.4%) | |
| Treatment outcome, % | Failure | 0 (0%) |
| Viral eradication by 6 months | 27 (84.4%) | |
| Recurrent infection | 5 (15.6%) | |
| Persistent refractory DNAemia | 0 (0%) |
This table describes characteristics of clinically significant cytomegalovirus infection (CS-CMVi), reported as frequency and percentage.
PET MPI and ICA characteristics
All 103 patients underwent PET MPI at year 3 following HT. Sixty patients additionally had PET MPI at year 1, 70 at year 5, and 50 at year 7. All PET MPI studies were well-tolerated with no conduction disorders, hemodynamic complications, or adverse events. A total of 283 PET MPI studies were included in the analysis.
Mean global MFR at year 3 was 2.45 (SD: 0.6) in all patients. We compared differences in PET MPI parameters by CS-CMVi status (Table 3). Patients with CS-CMVi had a significantly lower MFR at year 1 (2.20 vs 2.59), year 3 (2.16 vs 2.58), year 5 (2.15 vs 2.44), and year 7 (1.89 vs 2.24) (Figure 1). The difference in MFR between groups was driven by a lower sMBF after CS-CMVi compared to patients without infection, which was significant at years 3 and 5, and numerically lower at years 1 and 7 (Table 3). There were no differences in rMBF and left-ventricular end-diastolic volume index. Patients with a history of CS-CMVi were 3-times more likely to have an abnormal MFR (MFR < 2.0) at year 3 (43.8% vs 15.5%, p < 0.05) and 1.5-times more likely at year 5 (34.8% vs 23.9%) and year 7 (53.3% vs 38.2%). Patients with CS-CMVi were additionally more likely to have ischemia (SDS > 1) at all time periods. Patients with CS-CMVi also had a lower MFR than the 20 (19.4%) patients who developed asymptomatic low-level CMV viremia which was not treated as a clinically significant infection (2.16 vs 2.49, p < 0.05) and the 51 patients with no post-transplant CMV viremia (2.16 vs 2.64, p < 0.05) (Table S2).
Table 3.
Myocardial Flow Parameters in Patients With and Without Incident CS-CMVi
| Year | PET MPI parameter | CS-CMVi (N = 32) | No CS-CMVi (N = 71) | p-value |
|---|---|---|---|---|
| Year 1, 12–24 months (N = 60 studies) | # PET MPI studies | 14 | 46 | NA |
| Ischemia (SDS > 1), % | 2 (14.3%) | 7 (15.2%) | NS | |
| rMBF (mL/g/min) | 1.36 (0.5) | 1.17 (0.3) | NS | |
| sMBF (mL/g/min) | 2.83 (0.8) | 2.92 (0.8) | NS | |
| MFR | 2.20 (0.6) | 2.59 (0.6) | < 0.05 | |
| MFR < 2.0, % | 6 (18.8%) | 7 (9.9%) | < 0.05 | |
| Year 3, 24–48 months (N = 103 studies) | # PET MPI studies | 32 | 71 | NA |
| Ischemia (SDS > 1), % | 9 (28.1%) | 11 (15.5%) | < 0.05 | |
| rMBF (mL/g/min) | 1.44 (0.6) | 1.33 (0.4) | NS | |
| sMBF (mL/g/min) | 3.16 (0.9) | 3.51 (0.9) | < 0.05 | |
| MFR | 2.16 (0.5) | 2.58 (0.6) | < 0.05 | |
| MFR < 2.0, % | 14 (43.8%) | 11 (15.5%) | < 0.05 | |
| Year 5, 48–72 months (N = 69 studies) | # PET MPI studies | 23 | 46 | NA |
| Ischemia (SDS > 1), % | 8 (34.8%) | 10 (21.7%) | < 0.05 | |
| rMBF (mL/g/min) | 1.20 (0.5) | 1.21 (0.5) | NS | |
| sMBF (mL/g/min) | 2.66 (0.9) | 3.03 (0.8) | < 0.05 | |
| MFR | 2.15 (0.6) | 2.44 (0.7) | < 0.05 | |
| MFR < 2.0, % | 8 (34.8%) | 11 (23.9%) | < 0.05 | |
| Year 7, 72–96 months (N = 49 studies) | # PET MPI studies | 15 | 34 | NA |
| Ischemia (SDS > 1), % | 6 (40%) | 5 (14.7%) | < 0.05 | |
| rMBF (mL/g/min) | 1.24 (0.5) | 1.12 (0.3) | NS | |
| sMBF (mL/g/min) | 2.42 (0.7) | 2.57 (0.7) | NS | |
| MFR | 1.89 (0.7) | 2.24 (0.7) | < 0.05 | |
| MFR < 2.0, % | 8 (53.3%) | 13 (38.2%) | < 0.05 |
Abbreviations: CS-CMVi, clinically significant cytomegalovirus infection; MFR, myocardial flow reserve; rMBF, resting myocardial blood flow; SDS, summed difference score; sMBF, stress myocardial blood flow.
This table compares differences in flow parameters by CS-CMVi status, reported as mean and standard deviation or frequency and percentage.
Figure 1.

Myocardial flow reserve and stress myocardial blood flow in patients with and without incident CS-CMVi, CS-CMVi, clinically significant cytomegalovirus infection; MFR, myocardial flow reserve; sMBF, stress myocardial blood flow.
We evaluated rates of CAV diagnosed by ICA (Table S3). Sixty patients, 21 (35%) of whom developed CS-CMVi, underwent a total of 100 ICAs. IVUS was used in all but 4 procedures. The first ICA was at a median 13 months after HT, and CS-CMVi predated the first ICA procedure in all cases. Among 60 patients with ICA, 56 (93.3%) had no or minimal epicardial CAV by ISHLT criteria; however, 39 (65%) had moderate or greater epicardial CAV by Stanford criteria using IVUS. There were no observed differences in rates of epicardial CAV by ICA or IVUS by CS-CMVi status (Table S3).
Regression analyses of MFR
Multivariable linear and logistic models were used to evaluate the relationship between CS-CMVi and PET MFR at year 3 after HT (Table S4). CS-CMVi was an independent predictor of numerically lower MFR in the multivariable linear model, and a history of CS-CMVi was associated with an MFR that is 0.39 lower at year 3 (β = −0.39, 95% CI: −0.63 to −0.16, p = 0.001) compared to those without a history of CS-CMVi. CS-CMVi was the largest negative predictor of MFR in the multivariable model, with association 2.8-times greater than stage 3+ CKD and obesity, and 1.6-times greater than hypertension (Figure 2). ACR and MTI use were not associated with MFR. CMV high-risk serologic status in the absence of CS-CMVi did not predict MFR (β = −0.31; 95% CI: −1.55 to 0.13). CS-CMVi was additionally a significant predictor of abnormal MFR (< 2.0) at year 3 (adjusted odds ratios: 3.8, 95% CI: 1.4–10.7, p = 0.001) in the multivariable logistic model (Table S5). Finally, patients with CS-CMVi had a faster annual rate of decline (β = −0.10 per year, 95% CI: −0.16 to −0.03) compared to patients without infection (β = −0.06 per year, 95% CI: −0.11 to −0.02) in the mixed effects model (p < 0.001 between groups) (Figure 3).
Figure 2.
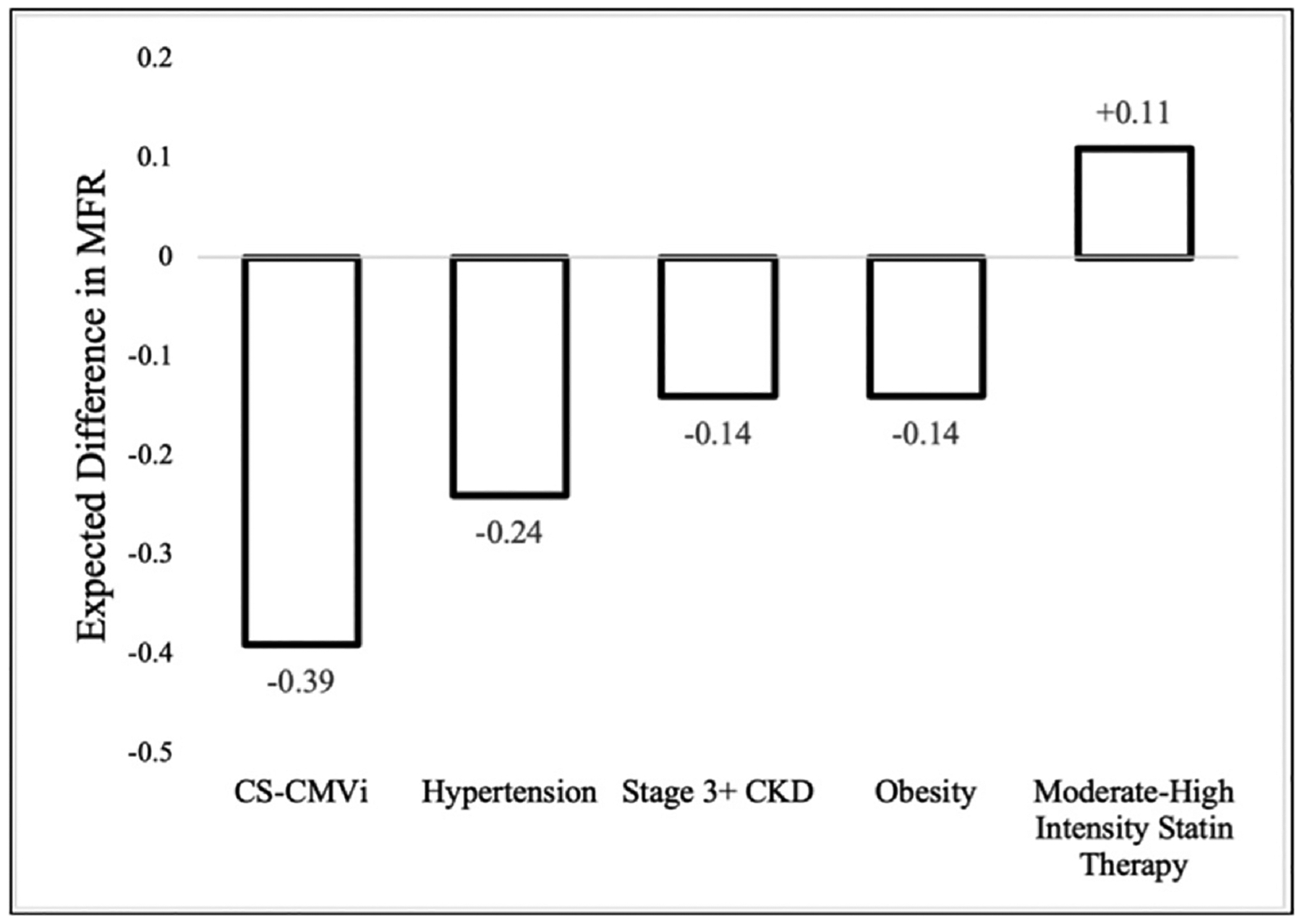
Independent predictors of myocardial flow reserve at 3 years after heart transplantation. The figure shows the expected change in myocardial flow reserve (MFR) with the introduction of a clinical variable in multivariable linear regression analysis. Hypertension was defined as systolic blood pressure > 140 mmHg requiring > 1 anti-hypertensive medications. Stage 3+ chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2. Obesity was defined as BMI > 30 kg/m2. CS-CMVi, clinically significant cytomegalovirus infection.
Figure 3.

Rate of change in myocardial flow reserve in patients with and without incident CS-CMVi. Rate of change was estimated as the adjusted β value (with 95% confidence intervals) in a linear mixed effects model of myocardial flow reserve over time. CS-CMVi, clinically significant cytomegalovirus infection.
Outcome analyses
Twenty-seven of 103 (26.2%) patients experienced the primary outcome at a median 55 (IQR, 46–78) months post-transplant, and 16 of 27 (59.3%) previously had CS-CMVi. Kaplan-Meier analysis demonstrated that patients with abnormal MFR (MFR < 2.0; log-rank p < 0.001) and CS-CMVi (log-rank p = 0.028) had higher risk of the primary outcome of all-cause mortality, retransplantation, hospitalization for heart failure, or acute coronary syndromes (Figure 4). In adjusted Cox regression analyses, both abnormal MFR (< 2.0; hazard ratio [HR]: 5.7, 95% CI: 4.2–7.2, p < 0.001) and CS-CMVi (HR: 3.3, 95% CI: 1.8–4.8, p = 0.008) were significant predictor of the primary outcome (HR: 3.3, 95% CI: 1.8–4.8, p = 0.008) (Table S6). Chronic kidney disease and history of ACR were also significant predictors of the primary outcome.
Figure 4.

Event-free survival after first PET MPI stratified by MFR and CS-CMVi classification in Kaplan-Meier analyses. Analysis began at 36 months post-transplant to reflect incidence of primary outcome after first positron emission tomography study at 24–36 months. Patients are censored at the time of last follow-up. MFR, myocardial flow reserve. CS-CMVi, clinically significant cytomegalovirus infection.
Discussion
CAV is the leading cause of late allograft dysfunction, morbidity, and mortality following HT. CAV is often identified after irreversible allograft fibrosis and injury have already occurred, emphasizing the need to identify potentially modifiable risk factors earlier in the disease course. The primary finding of this study is a significant and clinically relevant association between post-transplant CS-CMVi and impaired MFR following HT. This is the first study to model the relationship between CMV and MFR, and these novel findings suggest that CS-CMVi is an important risk factor for the development of microvascular dysfunction and CAV after HT.
CMV has been theorized for several decades to contribute to the progression of CAV. Grattan et al first demonstrated in 1989 that patients with CMV infection had more frequent allograft rejection and vasculopathy,29 a finding which was replicated in subsequent reports.15,30,31 Lemstrom et al found that antiviral prophylaxis reduced the development of CAV in a rat model.32 In 2 landmark trials, Valantine et al showed that immediate post-transplant prophylaxis with ganciclovir and CMV hyperimmune globulin reduced CAV severity.7,8 The mechanisms by which CMV may propagate CAV are derived from experimental models which suggest that CMV induces endothelial dysfunction through inflammatory cyto-kine release, intimal proliferation, impaired vascular remodeling, nitric oxide deficiency, and vasomotor dysregulation.6,33 CMV is additionally known to cause immune injury via potentiation of alloreactivity and T-cell mediated damage.6 For these reasons, CMV has been suspected to induce CAV, but there is limited clinical data of this association in the contemporary era.
This study finds that CS-CMVi after HT was a significant predictor of abnormal MFR. Specifically, we observed that patients with CS-CMVi develop clinically relevant reduced MFR which was pronounced at the third post-transplant year and worsened over time. MFR is a powerful surrogate for the presence and severity of CAV,17–20 and the observed reduction in MFR after CS-CMVi likely reflects the development of CAV. Notably, we observed differences in MFR after CS-CMVi as early as the first post-transplant year, and this early and sustained reduction in MFR after CS-CMVi represents the early microvascular dysfunction characteristically seen in CAV.2,14 CAV microvasculopathy occurs via endothelial dysfunction and an impaired vasodilatory response to increased myocardial demand,14 consistent with our observed reduction in sMBF after CS-CMVi. Finally, we found that both CS-CMVi and MFR are prognostic for post-transplant adverse events, with an abnormal MFR having particularly high predictive value. This supports that CMV’s relationship with adverse outcomes post-transplant are partially related to the development of CAV, and PET MPI may help identify and stratify patients who at high-risk for adverse outcomes.
These findings suggest several unique characteristics of how CMV may contribute to the development of CAV. First, CS-CMVi was associated with differences in sMBF and MFR as early as 12 to 24 months, suggesting that CMV infection may induce rapid and accelerated CAV. This is informative as CAV is generally a slow process with progressive myocardial ischemia and fibrosis, and the presence of early CAV may portend a comparatively poor prognosis.2 Second, all cases of CS-CMVi in our cohort occurred after completion of prophylaxis, suggesting that current guideline-directed prophylaxis regimens may be inadequate for prevention of CMV-associated CAV. Third, patients with asymptomatic low-level CMV viremia (non-CS-CMVi) had similar myocardial flow parameters as those with no viremia, suggesting that an active viremic syndrome is important or necessary to induce CAV. Finally, we observed substantial differences in MFR despite successful treatment in almost all patients with CS-CMVi with only 5 recurrent infections. This supports the mechanistic hypothesis that CMV is an activator of microvascular sequelae such as increased alloreactivity which persist beyond the acute infective phase.
We observed that CS-CMVi patients were more likely to have grade 2 or higher ACR, with most episodes occurring after CS-CMVi. However, though ACR has an established relationship with development of CAV,1,2 ACR did not predict a lower MFR in our cohort. There are several possible explanations for this finding. First and most likely, grade 2 or higher ACR is aggressively treated which may limit the long-term effect on MFR. Second, these findings were likely impacted by bias related to institutional practices in immunosuppression, rejection surveillance, and rejection treatment. For example, CS-CMVi patients may be more closely monitored for rejection due to higher risk. Third, though observational data have demonstrated a relationship between ACR and epicardial CAV, this relationship is less clear in the modern era of immunosuppression and with noninvasive measures such as MFR. Nevertheless, the observation that ACR did not predict MFR suggests that CS-CMVi propagates CAV through additional mechanisms independent of overt rejection. For example, CMV may induce direct endothelial injury and vasomotor dysfunction through nonimmune mechanisms.6,33 CMV may also increase risk for grade 1 to 2 ACR episodes without overt symptoms, which we may not have captured due to declining use of EMB at our center. CMV is also known to induce subtle, local changes in alloreactivity at the microvascular level which may not present as overt ACR but does impact microvascular function.6 CMV also increases risk for antibody-mediated injury34 which we were limited in investigating due to low sample of overt, biopsy-detected AMR. The mechanisms of CMV’s deleterious impact on MFR require further investigation.
It is notable that patients with CS-CMVi exhibited differences in MFR but no differences in epicardial CAV diagnosed by ICA. This supports and corroborates prior findings that CMV exerts its atherogenic effect at the level of the coronary microvasculature.15,16 However, this finding may also represent the significant limitations of ICA in the detection of early and microvascular CAV.14 Both ICA and IVUS are only able to evaluate epicardial disease, which generally occurs late in CAV progression when microvasculopathy has occurred.35 Furthermore, ICA and IVUS are unable to provide a functional assessment of the coronary microvasculature, which is important as CAV may impair myocardial flow even in the absence of epicardial disease.13,35 In contrast to ICA and IVUS,2 PET MFR is able to identify CAV earlier in patients’ disease trajectories when the disease is primarily microvascular and potentially modifiable. MFR is a sensitive diagnostic parameter for both epicardial and microcirculatory disease,28 and the use of PET MPI may identify CAV earlier, facilitating intensification of risk factor modification and disease-modifying therapies.
This study has several important limitations. First, we cannot establish causation between CMV and abnormal MFR due to the retrospective and observational design of this study. Second, this is a single-center cohort which may introduce bias related to institutional protocols following HT, and our findings may not be broadly generalizable. For example, we had a relatively high incidence of CS-CMV infection which we suspect is due to our use of highly sensitive CMV PCR assays, inclusion of asymptomatic persistent CMV viremia, differing institutional practice patterns, and an era effect related to inclusion of patients receiving HT in the midlate 2000s prior to contemporary CMV management. The management of CS-CMVi differs between centers, though our treatment protocols as described are in accordance with guidelines for CS-CMVi after solid organ transplantation.4,5 Third, we could not compare change in MFR before and after CS-CMVi, as only 2 patients had baseline PET MPI before CS-CMVi occurred. However, the more rapid decline in MFR after 24 months suggests a difference in microvascular disease independent of baseline MFR. Finally, though changes in MFR almost certainly reflect the development of CAV, MFR can be affected by other factors such as left ventricular end-diastolic pressure.
Future studies are necessary to prospectively evaluate the relationship between CMV and the development of CAV, and if CS-CMVi should prompt earlier and more aggressive initiation of CAV-directed therapies. Furthermore, all patients in our cohort developed CS-CMVi after prophylaxis was discontinued, and studies may evaluate whether novel prophylaxis or treatment protocols such as pretransplant CMV vaccination of seronegative recipients,36 preemptive treatment of early asymptomatic CMV viremia,37 and emerging anti-CMV agents such as maribavir and letermovir38,39 reduce incident CAV. It is additionally unknown whether asymptomatic, low-level CMV viremia predisposes to CAV compared to acute clinical infections, especially with the high sensitivity of modern CMV PCR tests. Novel assays that assess CMV cell-mediated immunity may be better suited to predict risk of infection and sequelae.40 Finally, further work is needed to understand the mechanistic basis for CMV’s detrimental effect on the cardiac allograft.
In conclusion, we observed that post-transplant CS-CMVi is associated with a significantly lower sMBF and MFR following HT. These findings suggest that CMV is an important risk factor in the development and progression of CAV after HT.
Supplementary Material
Disclosure statement
J.R.G. receives funding from the NIH (L30HL143700) and receives salary support by an American Heart Association grant (grant number 20SFRN35370008). V.L.M. receives funding from the NIH (U01DK123013; R01AG059729; R01HL136685), a Cardiometabolic Disease Strategically Focused Research Network grant from the American Heart Association, and the Melvyn Rubenfire Professorship in Preventive Cardiology; has stock in General Electric and Cardinal Health; stock options and scientific advisor for Ionetix; research grants from Siemens; paid consultant for INVIA Medical Imaging Solutions. The other authors have no real or perceived conflicts of interest.
$10,000 in funding is provided by an Amplifier Grant Award from the Samuel and Jean Frankel Cardiovascular Center.
Footnotes
CRediT authorship contribution statement
K.T.G.: Study conceptualization, supervision and data collection, formal analysis, visualization, data curation, review and editing of manuscript drafts, and approval of the final draft for submission. D.R.K.: Study conceptualization, supervision and data collection, review and editing of manuscript drafts, and approval of the final draft for submission, project supervision. K.S.G.: Study conceptualization, review and editing of manuscript drafts, and approval of the final draft for submission, project supervision. J.R.G.: Study conceptualization, review and editing of manuscript drafts, and approval of the final draft for submission, project supervision. K.D.A.: Study conceptualization, review and editing of manuscript drafts, and approval of the final draft for submission, project supervision. V.L.M.: Study conceptualization, supervision and data collection, formal analysis, visualization, data curation, review and editing of manuscript drafts, and approval of the final draft for submission. M.C.K.: Study conceptualization, supervision and data collection, formal analysis, visualization, data curation, review and editing of manuscript drafts, and approval of the final draft for submission.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.healun.2023.10.005.
References
- 1.Nikolava AP, Kobashigawa JA. Cardiac allograft vasculopathy: the enduring enemy of cardiac transplantation. Transplantation 2019;103:1338–48. [DOI] [PubMed] [Google Scholar]
- 2.Schmauss and Weis. Cardiac allograft vasculopathy. Circulation 2008;117:2131–43. [DOI] [PubMed] [Google Scholar]
- 3.Heim C, Muller PP, Tandler R, et al. Cytomegalovirus donor seropositivity negative affects survival after heart transplantation. Transplantation 2022;106:1243–52. [DOI] [PubMed] [Google Scholar]
- 4.Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13512. [DOI] [PubMed] [Google Scholar]
- 5.Kotton CN, Kumar D, Caliendo AM, et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ transplantation. Transplantation 2018;102:900–31. [DOI] [PubMed] [Google Scholar]
- 6.Valantine HA. The role of viruses in cardiac allograft vasculopathy. Am J Transplant 2004;4:169–77. [DOI] [PubMed] [Google Scholar]
- 7.Valantine HA, Gao S, Menon SG, et al. Impact of prophylactic immediate posttransplant ganciclovir on development of transplant atherosclerosis. Circulation 1999;10:61–6. [DOI] [PubMed] [Google Scholar]
- 8.Valantine HA, Luikart H, Doyle R, et al. Impact of cytomegalovirus hyperimmune globulin on outcome after cardiothoracic transplantation: a comparative study of combined prophylaxis with CMV hyperimmune globulin plus ganciclovir versus ganciclovir alone. Transplantation 2001;72:1647–52. [DOI] [PubMed] [Google Scholar]
- 9.Golbus JR, Adie S, Yosef M, et al. Statin intensity and risk for cardiovascular events after heart transplantation. ESC Heart Fail 2020;7:2074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgado JF, Reyne AG, Dios S, et al. Influence of cytomegalovirus infection in the development of cardiac allograft vasculopathy after heart transplantation. J Heart Lung Transplant 2015;34:1112–9. [DOI] [PubMed] [Google Scholar]
- 11.Johansson I, Andersson R, Friman V, et al. Cytomegalovirus infection and disease reduce 10-year cardiac allograft vasculopathy-free survival in heart transplant recipients. BMC Infect Dis 2015;15:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klimczak-Tomaniak D, Roest S, Brugts JJ, et al. The association between cytomegalovirus infection and cardiac allograft vasculopathy in the era of antiviral valganciclovir prophylaxis. Transplantation 2022;104:1508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guddeti RR, Matsuo Y, Matsuzawa Y, et al. Clinical implications of intracoronary imaging in cardiac allograft vasculopathy. Circ Cardiovasc Imaging 2015;8:e002636. [DOI] [PubMed] [Google Scholar]
- 14.Chih S, Chong AY, Erthal F, et al. PET assessment of epicardial intimal disease and microvascular dysfunction in cardiac allograft vasculopathy. J Am Coll Cardiol 2018;71:1444–56. [DOI] [PubMed] [Google Scholar]
- 15.Koskinen P, Lemstrom K, Bruggeman C, et al. Acute cytomegalovirus infection induces a subendothelial inflammation (endothelialitis) in the allograft vascular wall. A possible linkage with enhanced allograft arteriosclerosis. Am J Pathol 1994;144:41. [PMC free article] [PubMed] [Google Scholar]
- 16.Petrakopoulou P, Kubrich M, Pehlivanli S, et al. Cytomegalovirus infection in heart transplant recipients is associated with impaired endothelial function. Circulation 2004;110:207–12. [DOI] [PubMed] [Google Scholar]
- 17.Bravo PE, Bergmark BA, Vita T, et al. Diagnostic and prognostic value of myocardial blood flow quantification as non-invasive indicator of cardiac allograft vasculopathy. Eur Heart J 2018;39:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clerkin KJ, Topkara VK, Farr MA, et al. Noninvasive physiologic assessment of cardiac allograft vasculopathy is prognostic for post-transplant events. J Am Coll Cardiol 2022;80:1617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konerman MC, Lazarus JJ, Weinberg RL, et al. Reduced myocardial flow reserve by positron emission tomography predicts cardiovascular events after cardiac transplantation. Circ Heart Fail 2018;11:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller RJ, Manabe O, Tamarappoo B, et al. Comparative prognostic and diagnostic value of myocardial blood flow and myocardial flow reserve after cardiac transplantation. J Nucl Med 2020;61:249–55. [DOI] [PubMed] [Google Scholar]
- 21.Lazarus JJ, Saleh A, Ghannam M, et al. Safety of regadenoson positron emission tomography stress testing in orthotopic heart transplant patients. J Nucl Cardiol 2020;27:943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 23.Murthy VL, Bateman TM, Beanlands RS, et al. Clinical quantification of myocardial blood flow using PET: joint position paper of the SNMMI cardiovascular council and the ASNC. J Nucl Med 2023;59:273–93. [DOI] [PubMed] [Google Scholar]
- 24.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce athero-sclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
- 25.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005;24: 1710–20. [DOI] [PubMed] [Google Scholar]
- 26.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant 2010;29:717–27. [DOI] [PubMed] [Google Scholar]
- 27.St Goar FG, Pinto FJ, Valantine HA, et al. Intracoronary ultrasound in cardiac transplant recipients. In vivo evidence of “angiographically silent” intimal thickening. Circulation 1992;85:979–87. [DOI] [PubMed] [Google Scholar]
- 28.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with non-invasive measures of coronary flow reserve. Circulation 2011;124:2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grattan MT, Moreno-Cabral CE, Starnes VA, et al. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA 1989;261:3561–6. [PubMed] [Google Scholar]
- 30.Everett JP, Hershberger RE, Norman DJ, et al. Prolonged cytomegalovirus infection with viremia is associated with development of cardiac allograft vasculopathy. J Heart Lung Transplant 1992;11:S133. [PubMed] [Google Scholar]
- 31.Wu TC, Hruban RH, Ambinder RF, et al. Demonstration of cytomegalovirus nucleic acids in the coronary arteries of transplanted hearts. Am J Pathol 1992;140:739. [PMC free article] [PubMed] [Google Scholar]
- 32.Lemstrom K, Sihvola R, Bruggeman C, et al. Cytomegalovirus infection–enhanced cardiac allograft vasculopathy is abolished by DHPG prophylaxis in the rat. Circulation 1997;95:2614–6. [DOI] [PubMed] [Google Scholar]
- 33.Weis M, Cookie JP. Cardiac allograft vasculopathy and dysregulation of the NO synthase pathway. Arterioscler Thromb Vasc Biol 2003;23:567. [DOI] [PubMed] [Google Scholar]
- 34.Saldan A, Mengoli C, Sgarabotto D, et al. Human cytomegalovirus and Epstein-Barr virus infections occurring early after transplantation are risk factors for antibody-mediated rejection in heart transplant recipients. Front Immunol 2023;14:1171197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Okada K, Khush K, et al. Coronary endothelial dysfunction and the index of microcirculatory resistance as a marker of subsequent development of cardiac allograft vasculopathy. Circulation 2017;135: 1093–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ljungman P, Bermudez A, Logan AC, et al. A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients. eClinicalMedicine 2021;33:100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh N, Winston DJ, Razonable RR, et al. Effect of preemptive therapy vs antiviral prophylaxis on cytomegalovirus disease in seronegative liver transplant recipients with seropositive donors. A randomized clinical trial. JAMA 2020;323:1378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avery RK, Alain S, Alexander BD, et al. Maribavir for refractory cytomegalovirus infections with or without resistance post-transplant: results from a phase 3 randomized clinical trial. Clin Infect Dis 2022;75:690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golob S, Batra J, DeFilippis EM, et al. Letermovir for cytomegalovirus prophylaxis in high-risk heart transplant recipients. Clin Transplant 2022;36:e14808. [DOI] [PubMed] [Google Scholar]
- 40.Manuel O, Husain S, Kumar D, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis 2013;56:817–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


