Abstract
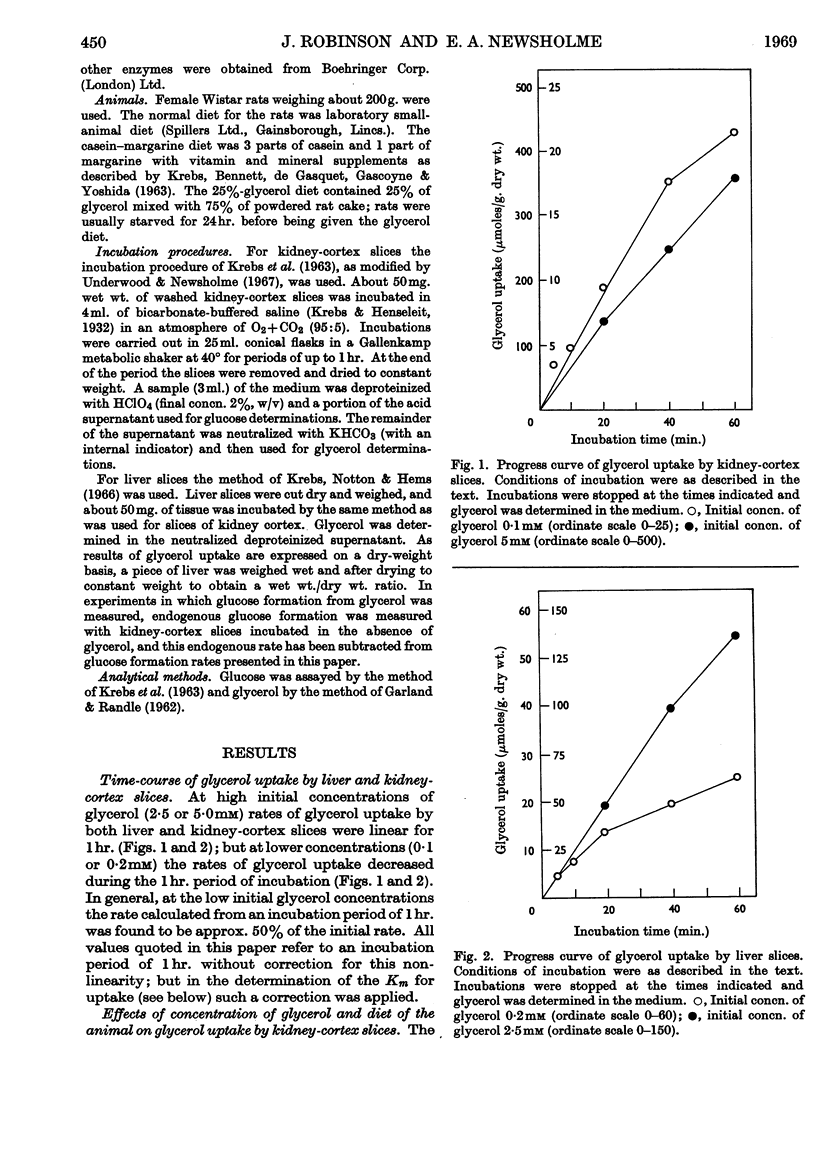
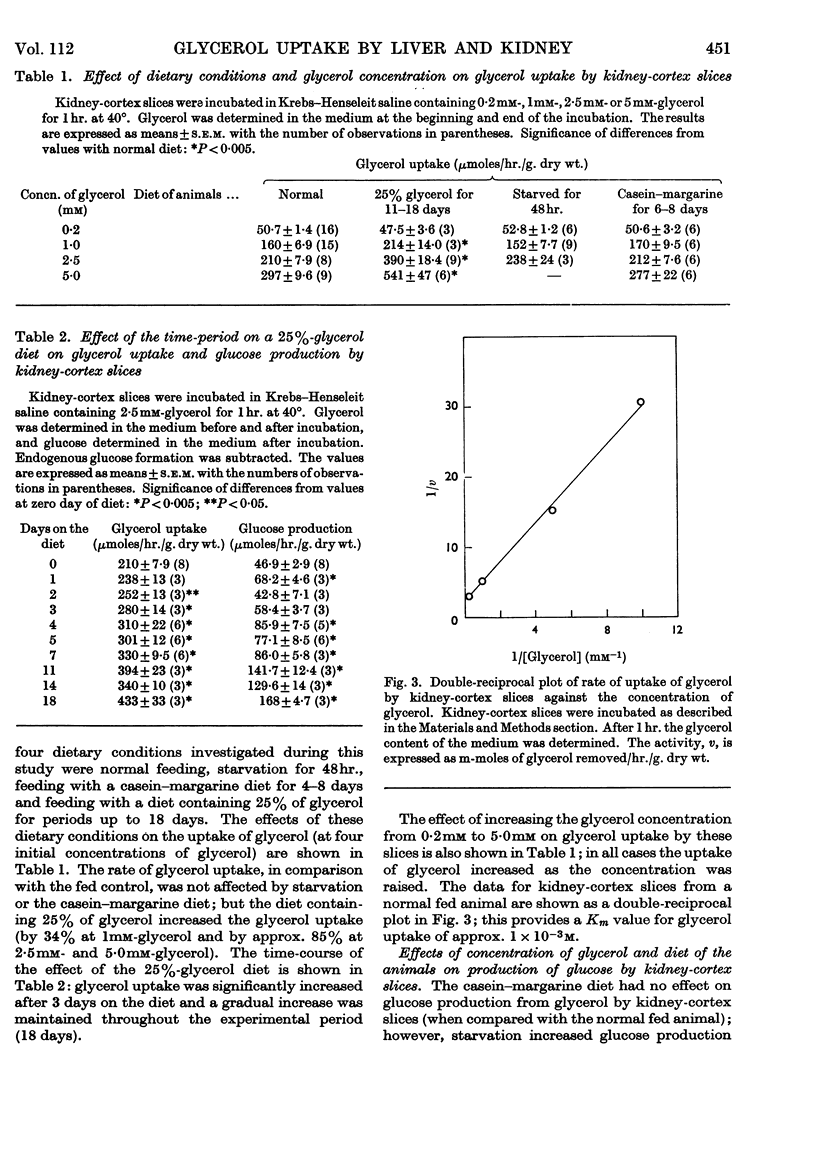
1. Glycerol utilization by rat liver and kidney-cortex slices was studied in an attempt to define factors that might be important in the regulation of glycerol utilization by these tissues in vivo; the formation of glucose from glycerol by kidney-cortex slices was also studied. 2. The rate of glycerol uptake by liver slices was not changed (in comparison with the normal fed control) by starvation (48hr.), feeding with a low-carbohydrate diet (4–8 days) or feeding with a diet containing 25% glycerol (up to 18 days). Similarly, starvation or a low-carbohydrate diet had no effect on uptake by kidney-cortex slices; however, feeding with the glycerol diet increased glycerol uptake by kidney-cortex slices. 3. The rates of glycerol uptake by slices from both tissues were increased on raising the glycerol concentration from 0·2mm to 2·5 or 5·0mm. 4. Starvation increased the conversion of glycerol into glucose by kidney-cortex slices, but there was no effect of the low-carbohydrate diet; the rate of glucose formation was increased by feeding with the 25%-glycerol diet and was proportional to the increase in glycerol uptake. The rate of glucose production by these slices was increased by raising the glycerol concentration in the incubation medium from 0·2mm to 1·0mm, but, except for the slices from animals receiving the 25%-glycerol diet, there was no effect above 1·0mm-glycerol. 5. The significance of plasma glycerol concentration in regulating glycerol uptake by these tissues is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. THE CROONIAN LECTURE, 1963. GLUCONEOGENESIS. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:545–564. doi: 10.1098/rspb.1964.0019. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Notton B. M., Hems R. Gluconeogenesis in mouse-liver slices. Biochem J. 1966 Dec;101(3):607–617. doi: 10.1042/bj1010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Gevers W. Control of glycolysis and gluconeogenesis in liver and kidney cortex. Vitam Horm. 1967;25:1–87. doi: 10.1016/s0083-6729(08)60033-3. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Newsholme E. A. Some properties of hepatic glycerol kinase and their relation to the control of glycerol utilization. Biochem J. 1969 May;112(4):455–464. doi: 10.1042/bj1120455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood A. H., Newsholme E. A. Control of glycolysis and gluconeogenesis in rat kidney cortex slices. Biochem J. 1967 Jul;104(1):300–305. doi: 10.1042/bj1040300. [DOI] [PMC free article] [PubMed] [Google Scholar]


