Abstract
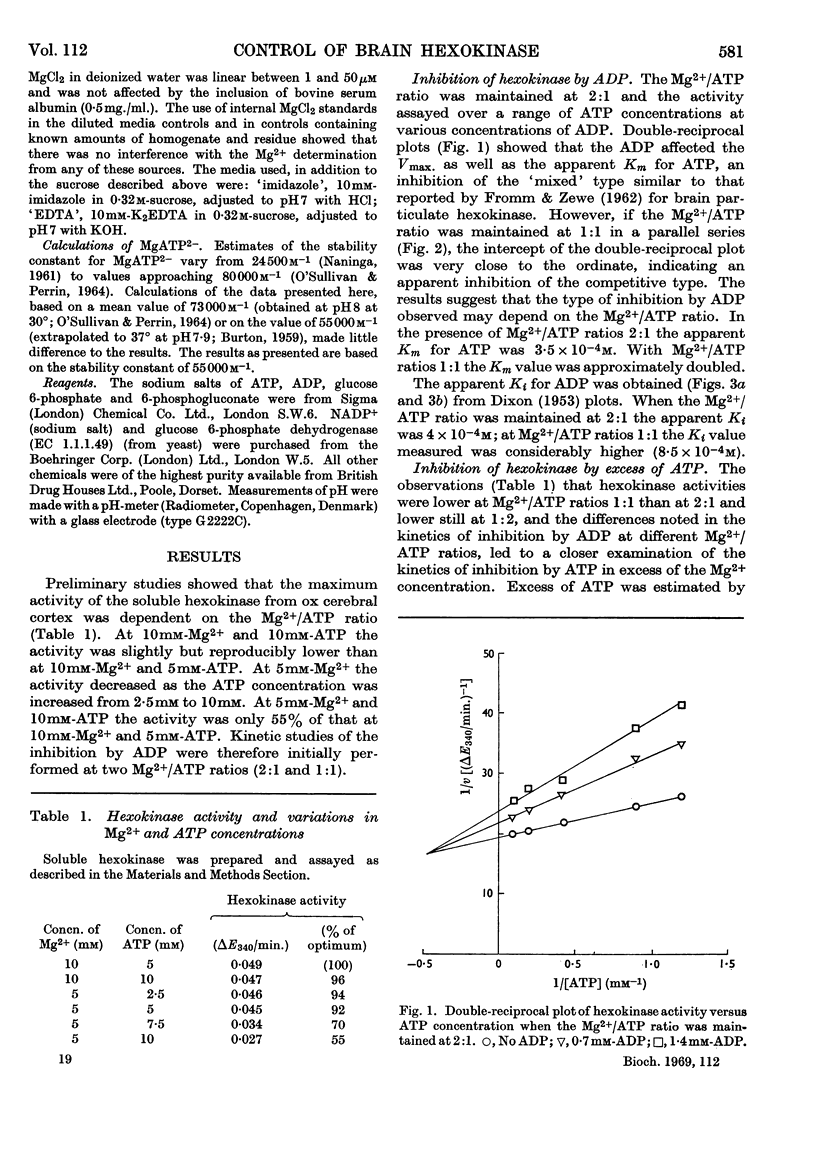
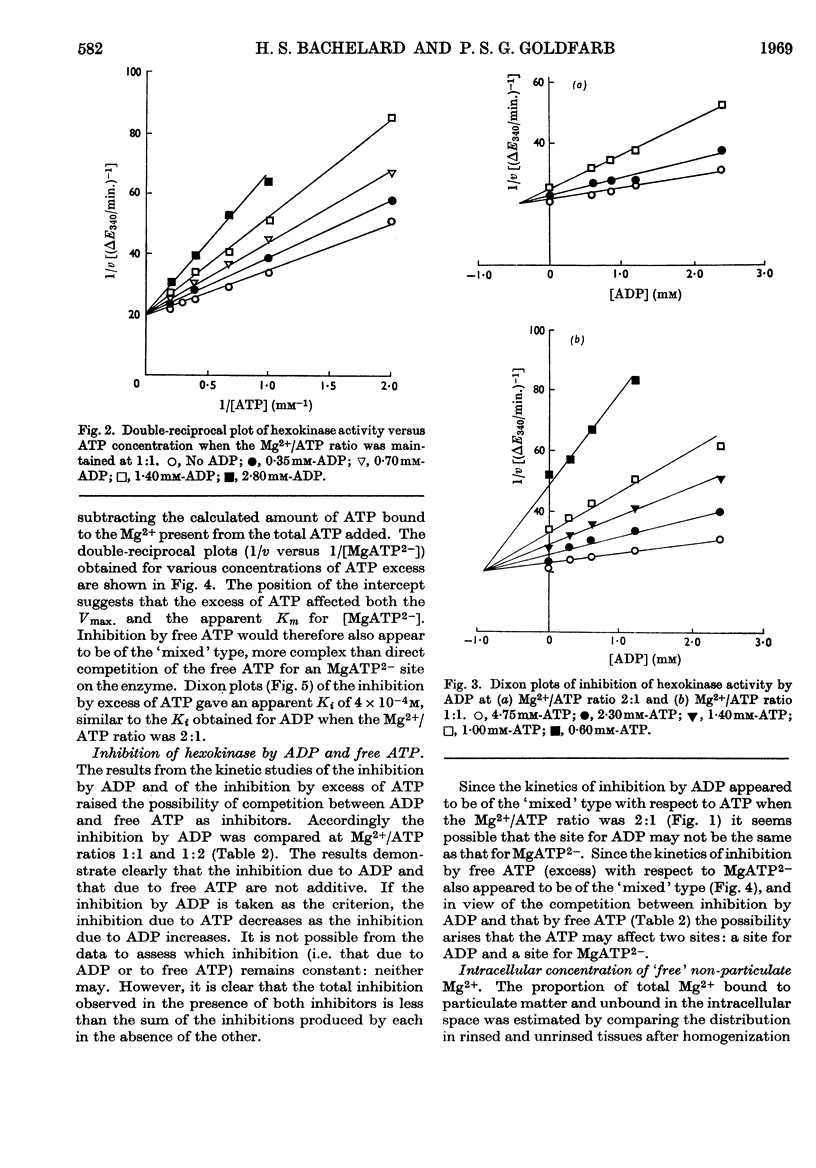
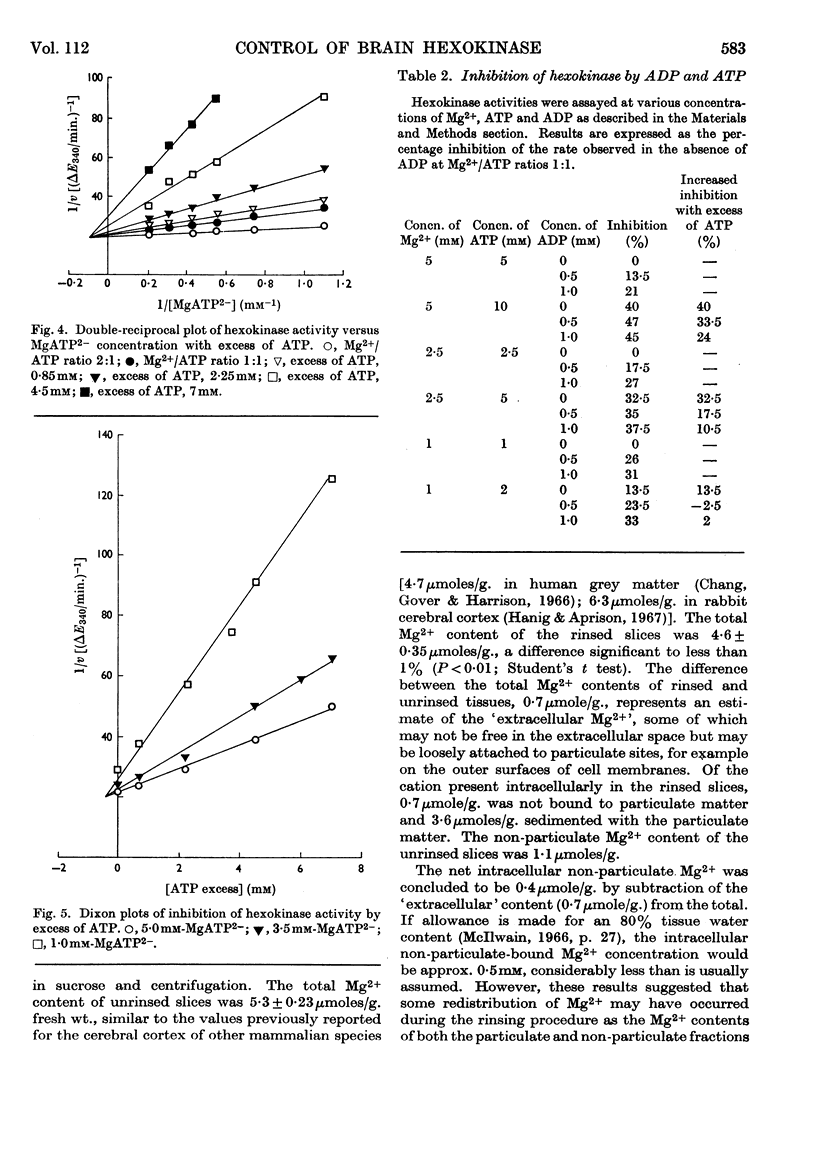
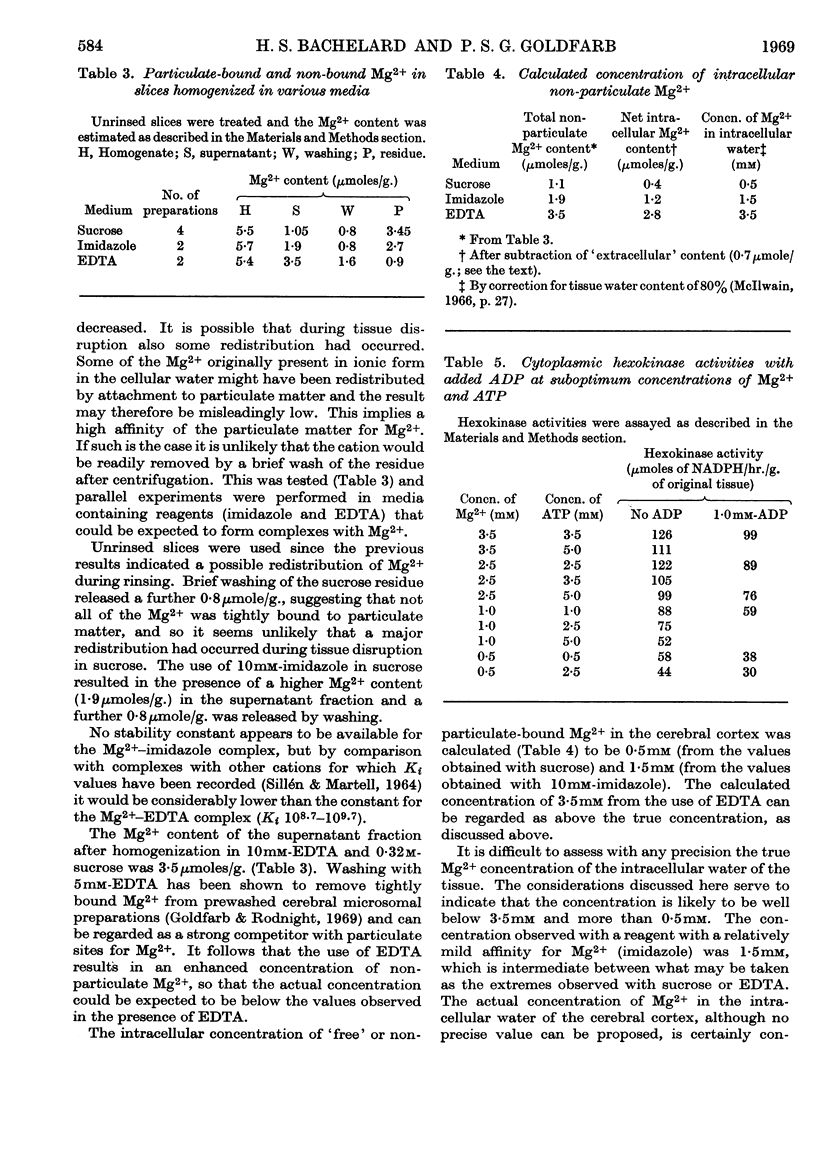
1. The kinetics of inhibition of brain soluble cytoplasmic hexokinase by ADP were examined in relation to variations in the concentrations of Mg2+ and ATP. The type of inhibition observed was dependent on the Mg2+/ATP ratio. 2. ADP at Mg2+/ATP ratios 2:1 exhibited inhibition of the `mixed' type; at Mg2+/ATP ratios 1:1 the inhibition appeared to be competitive with regard to ATP. 3. Inhibition by free ATP was observed when the Mg2+/ATP ratio was less than 1:1. The inhibition was also of the `mixed' type with respect to MgATP2−. 4. The inhibitions due to ADP and to free ATP were not additive. The results suggested that there may be up to four sites in the soluble enzyme: for glucose, glucose 6-phosphate, ADP and MgATP2−. 5. The `free' non-particulate intracellular Mg2+ concentration was measured and concluded to be about 1·5mm. 6. The concentrations in vivo of Mg2+ and ATP likely to be accessible to a cytoplasmic enzyme are suggested to be below those that yield maximum hexokinase rates in vitro. The enzymic rates were measured at relevant suboptimum concentrations of Mg2+ and ATP in the presence of ADP. Calculations that included non-competitive inhibition due to glucose 6-phosphate (56–65% at 0·25mm) resulted in net rates very similar to the measured rates for overall glycolysis. This system may therefore provide a basis for effective control of cerebral hexokinase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcock N. W., MacIntyre I. Methods for estimating magnesium in biological materials. Methods Biochem Anal. 1966;14:1–52. doi: 10.1002/9780470110324.ch1. [DOI] [PubMed] [Google Scholar]
- BACHELARD H. S., CAMPBELL W. J., McILWAIN H. The sodium and other ions of mammalian cerebral tissues, maintained and electrically stimulated in vitro. Biochem J. 1962 Aug;84:225–232. doi: 10.1042/bj0840225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALAZS R., LAGNADO J. R. Glycolytic activity associated with rat brain mitochondria. J Neurochem. 1959 Dec;5:1–17. doi: 10.1111/j.1471-4159.1959.tb13328.x. [DOI] [PubMed] [Google Scholar]
- BENNETT E. L., DRORI J. B., KRECH D., ROSENZWEIG M. R., ABRAHAM S. Hexokinase activity in brain. J Biol Chem. 1962 Jun;237:1758–1763. [PubMed] [Google Scholar]
- BURTON K. Formation constants for the complexes of adenosine di- or tri-phosphate with magnesium or calcium ions. Biochem J. 1959 Feb;71(2):388–395. doi: 10.1042/bj0710388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelard H. S. The subcellular distribution and properties of hexokinases in the guinea-pig cerebral cortex. Biochem J. 1967 Jul;104(1):286–292. doi: 10.1042/bj1040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The association of hexokinase with particulate fractions of brain and other tissue homogenates. J Biol Chem. 1953 Jul;203(1):273–292. [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem. 1954 Oct;210(2):597–606. [PubMed] [Google Scholar]
- Chang T. L., Gover T. A., Harrison W. W. Determination of magnesium and zinc in human brain tissue by atomic absorption spectroscopy. Anal Chim Acta. 1966 Jan;34(1):17–23. doi: 10.1016/s0003-2670(00)88998-9. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROMM H. J., ZEWE V. Kinetic studies of the brain hexokinase reaction. J Biol Chem. 1962 May;237:1661–1667. [PubMed] [Google Scholar]
- Hanig R. C., Aprison M. H. Determination of calcium, copper, iron, magnesium, manganese, potassium, sodium, zinc, and chloride concentrations in several brain areas. Anal Biochem. 1967 Nov;21(2):169–177. doi: 10.1016/0003-2697(67)90178-9. [DOI] [PubMed] [Google Scholar]
- KERLY M., LEABACK D. H. The characteristics of hexokinase from Locusta migratoria muscle. Biochem J. 1957 Oct;67(2):245–250. doi: 10.1042/bj0670245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V. THE RELATIONSHIPS BETWEEN SUBSTRATES AND ENZYMES OF GLYCOLYSIS IN BRAIN. J Biol Chem. 1964 Jan;239:31–42. [PubMed] [Google Scholar]
- NANNINGA L. B. The association constant of the complexes of adenosine triphosphate with magnesium, calcium, strontium, and barium ions. Biochim Biophys Acta. 1961 Dec 9;54:330–338. doi: 10.1016/0006-3002(61)90373-0. [DOI] [PubMed] [Google Scholar]
- NYMAN M., WHITTAKER V. P. The distribution of adenosine triphosphate in subcellular fractions of brain tissue. Biochem J. 1963 May;87:248–255. doi: 10.1042/bj0870248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Rolleston F. S., Taylor K. Factors affecting the glucose 6-phosphate inhibition of hexokinase from cerebral cortex tissue of the guinea pig. Biochem J. 1968 Jan;106(1):193–201. doi: 10.1042/bj1060193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. The inhibition of brain hexokinase by adenosinediphosphate and sulfhydryl reagents. J Biol Chem. 1954 Feb;206(2):925–936. [PubMed] [Google Scholar]
- Uyeda K., Racker E. Regulatory mechanisms in carbohydrate metabolism. VII. Hexokinase and phosphofructokinase. J Biol Chem. 1965 Dec;240(12):4682–4688. [PubMed] [Google Scholar]
- WEIL-MALHERBE H., BONE A. D. Studies on hexokinase. 1. The hexokinase activity of rat-brain extracts. Biochem J. 1951 Aug;49(3):339–347. doi: 10.1042/bj0490339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIS J. B. Determination of lead in urine by atomic absorption spectroscopy. Nature. 1961 Jul 22;191:381–382. doi: 10.1038/191381a0. [DOI] [PubMed] [Google Scholar]


