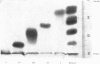
Abstract
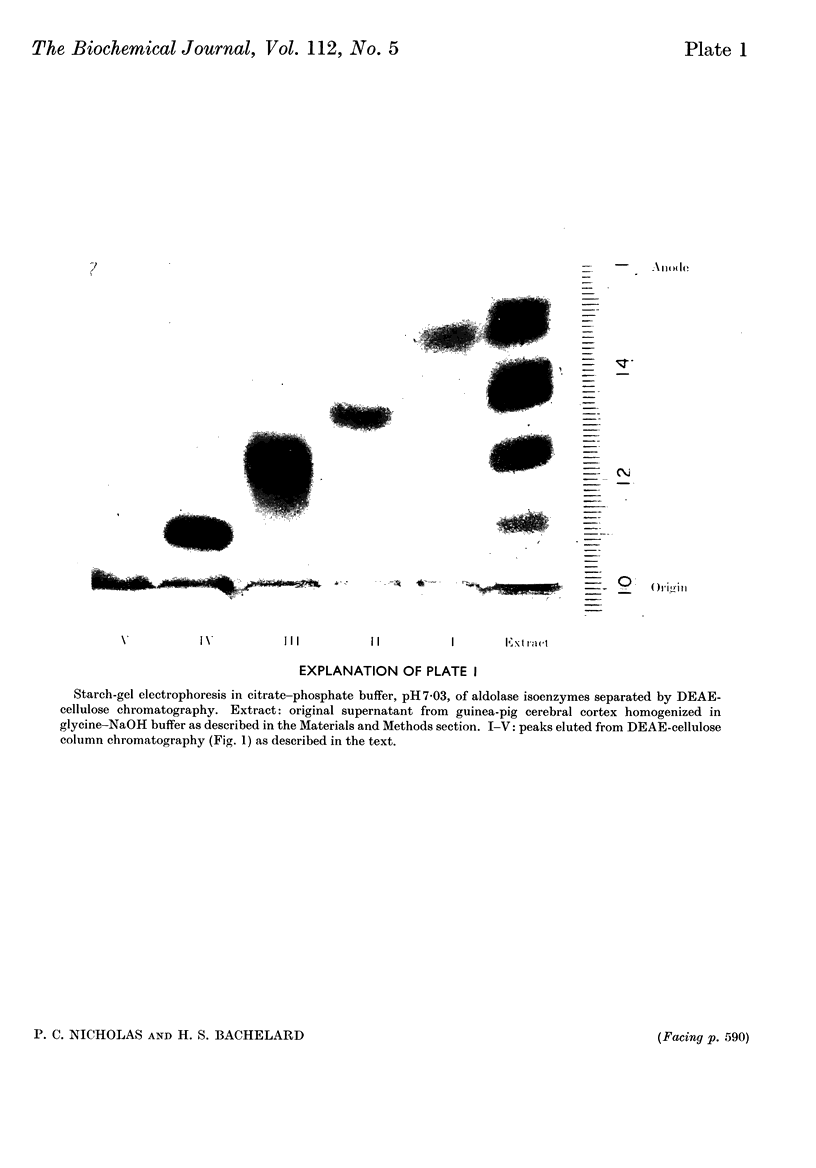
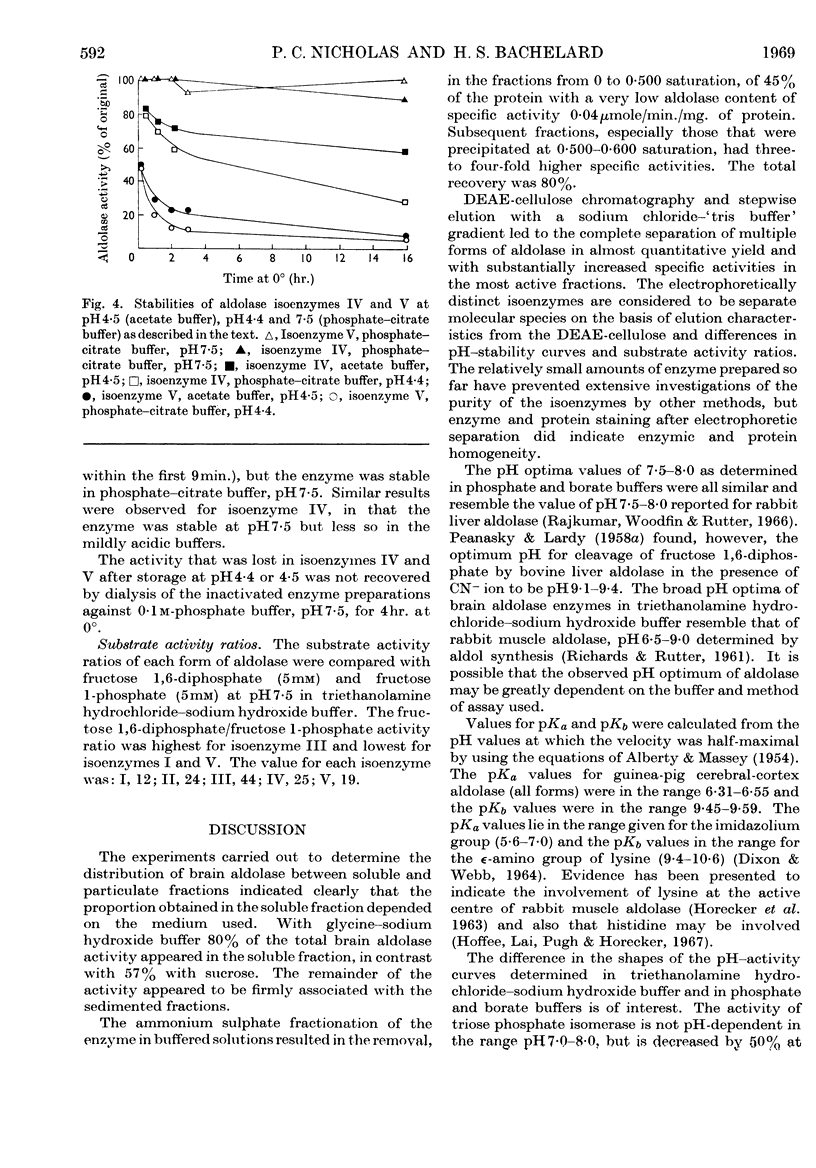
1. Aldolase isoenzymes from guinea-pig cerebral cortex were partially purified and separated by ammonium sulphate fractionation and chromatography on DEAE-cellulose. 2. Each purified isoenzyme was shown to be virtually uncontaminated with other forms by starch-gel electrophoresis. The quantitative distribution of the isoenzymes was: I, 6·2%; II, 5·2%; III, 15·3%; IV, 25·7%; V, 33·3%. 3. The pH optima for the five separated isoenzymes were similar; all were in the range pH7·5–8·0. Values for pKa (6·31–6·55) and pKb (9·45–9·59) were calculated from the data and suggested the involvement of histidine and lysine residues. 4. The stabilities of the isoenzymes were shown to be I=II>III>IV>V at pH4·4 in order of decreasing stability and are discussed in terms of subunit structure. 5. The substrate activity ratios (fructose 1,6-diphosphate/fructose 1-phosphate) were measured and all were in the range 12–44.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTY R. A., MASSEY V. On the interpretation of the pH variation of the maximum initial velocity of an enzyme-catalyzed reaction. Biochim Biophys Acta. 1954 Mar;13(3):347–353. doi: 10.1016/0006-3002(54)90340-6. [DOI] [PubMed] [Google Scholar]
- Anstall H. B., Lapp C., Trujillo J. M. Isozymes of aldolase. Science. 1966 Nov 4;154(3749):657–658. doi: 10.1126/science.154.3749.657. [DOI] [PubMed] [Google Scholar]
- BLOSTEIN R., RUTTER W. J. COMPARATIVE STUDIES OF LIVER AND MUSCLE ALDOLASE. II. IMMUNOCHEMICAL AND CHROMATOGRAPHIC DIFFERENTIATION. J Biol Chem. 1963 Oct;238:3280–3285. [PubMed] [Google Scholar]
- Di Jeso F. Ammonium sulfate concentration conversion nomograph for 0 degrees. J Biol Chem. 1968 Apr 25;243(8):2022–2023. [PubMed] [Google Scholar]
- Ginsburg A., Mehler A. H. Specific anion binding to fructose diphosphate aldolase from rabbit muscle. Biochemistry. 1966 Aug;5(8):2623–2634. doi: 10.1021/bi00872a021. [DOI] [PubMed] [Google Scholar]
- Hartman F. C., Barker R. An exploration of the active site of aldolase using structural analogs of fructose diphosphate. Biochemistry. 1965 Jun;4(6):1068–1075. doi: 10.1021/bi00882a014. [DOI] [PubMed] [Google Scholar]
- Hoffee P., Lai C. Y., Pugh E. L., Horecker B. L. The function of histidine residues in rabbit muscle aldolase. Proc Natl Acad Sci U S A. 1967 Jan;57(1):107–113. doi: 10.1073/pnas.57.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNITZ M. Crystalline inorganic pyrophosphatase isolated from baker's yeast. J Gen Physiol. 1952 Jan;35(3):423–450. doi: 10.1085/jgp.35.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K., Tanford C. The number of polypeptide chains in rabbit muscle aldolase. Biochemistry. 1966 May;5(5):1578–1584. doi: 10.1021/bi00869a018. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PEANASKY R. J., LARDY H. A. Bovine liver aldolase. I. Isolation, crystallization, and some general properties. J Biol Chem. 1958 Aug;233(2):365–370. [PubMed] [Google Scholar]
- PEANASKY R. J., LARDY H. A. Bovine liver aldolase. II. Physical and chemical measurements on the crystalline enzyme. J Biol Chem. 1958 Aug;233(2):371–373. [PubMed] [Google Scholar]
- Penhoet E., Rajkumar T., Rutter W. J. Multiple forms of fructose diphosphate aldolase in mammalian tissues. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1275–1282. doi: 10.1073/pnas.56.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS O. C., RUTTER W. J. Comparative properties of yeast and muscle aldolase. J Biol Chem. 1961 Dec;236:3185–3192. [PubMed] [Google Scholar]
- RUTTER W. J., RICHARDS O. C., WOODFIN B. M. Comparative studies of liver and muscle aldolase. I. Effect of carboxypeptidase on catalytic activity. J Biol Chem. 1961 Dec;236:3193–3197. [PubMed] [Google Scholar]
- Rensing U., Schmid A., Christen P., Leuthardt F. Purification and some properties of aldolase-isoenzymes from bovine brain and muscle. Hoppe Seylers Z Physiol Chem. 1967 Aug;348(8):1001–1004. doi: 10.1515/bchm2.1967.348.1.1001. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOLTER P. D., ADELMAN R. C., WEINHOUSE S. DISTINCTIVE PROPERTIES OF NATIVE AND CARBOXYPEPTIDASE-TREATED ALDOLASES OF RABBIT MUSCLE AND LIVER. J Biol Chem. 1965 Mar;240:1327–1337. [PubMed] [Google Scholar]