Abstract
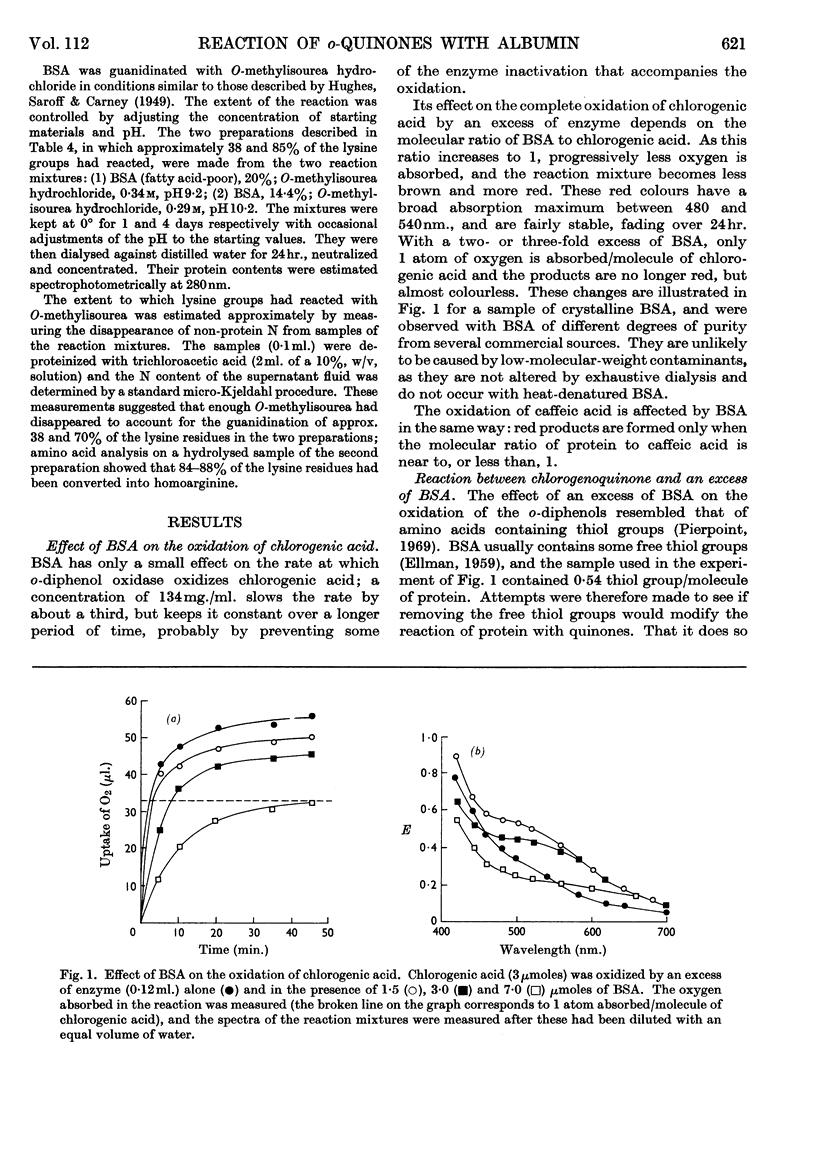
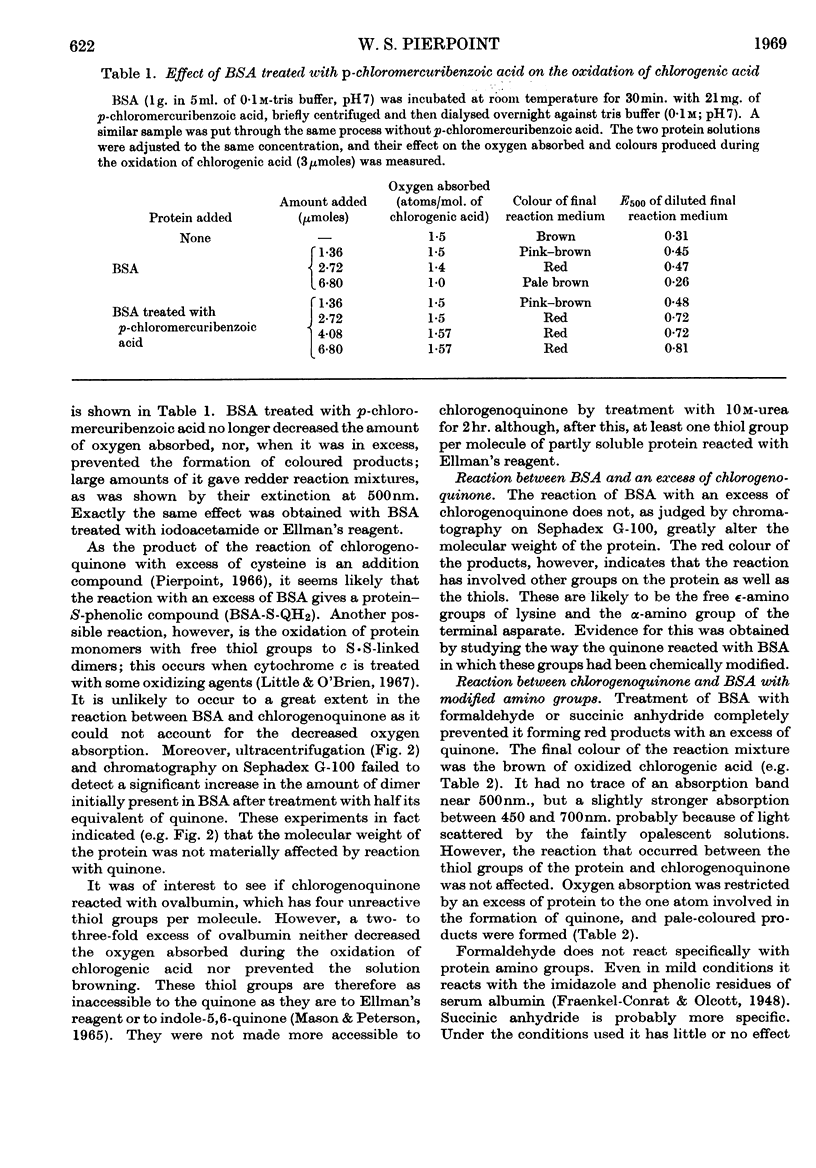
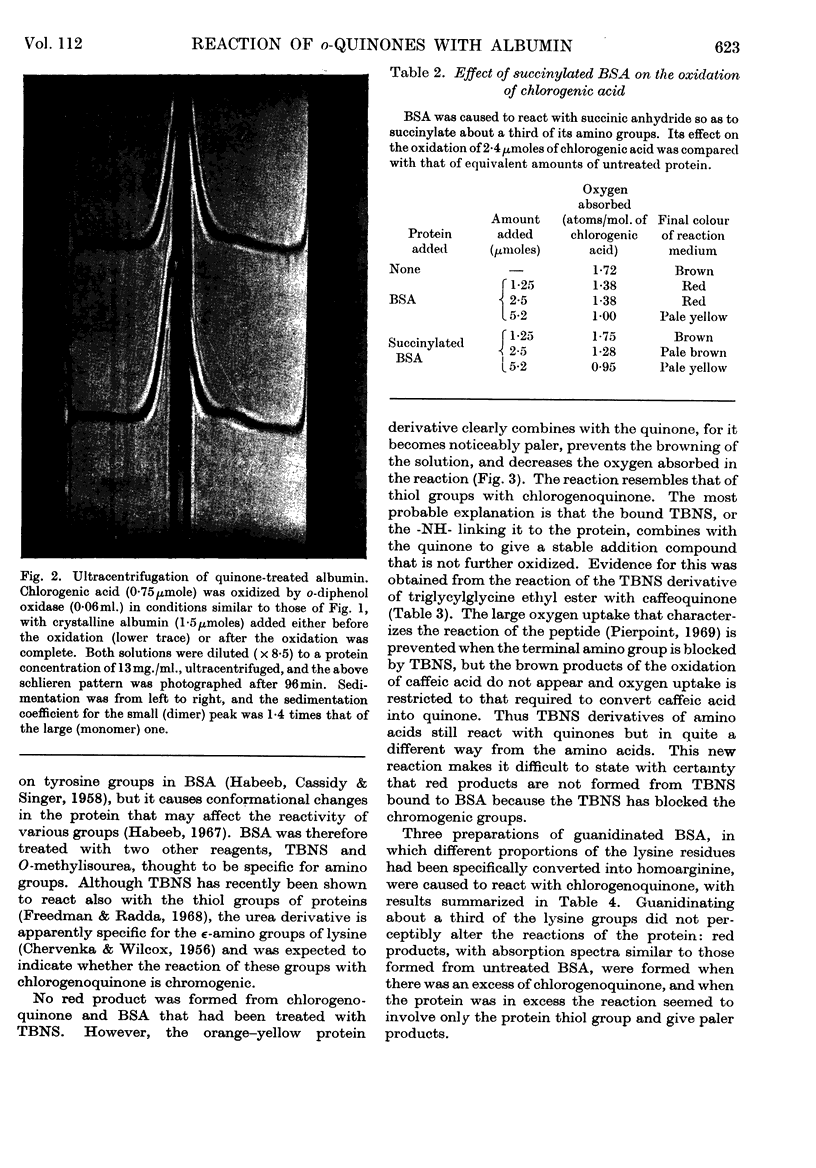
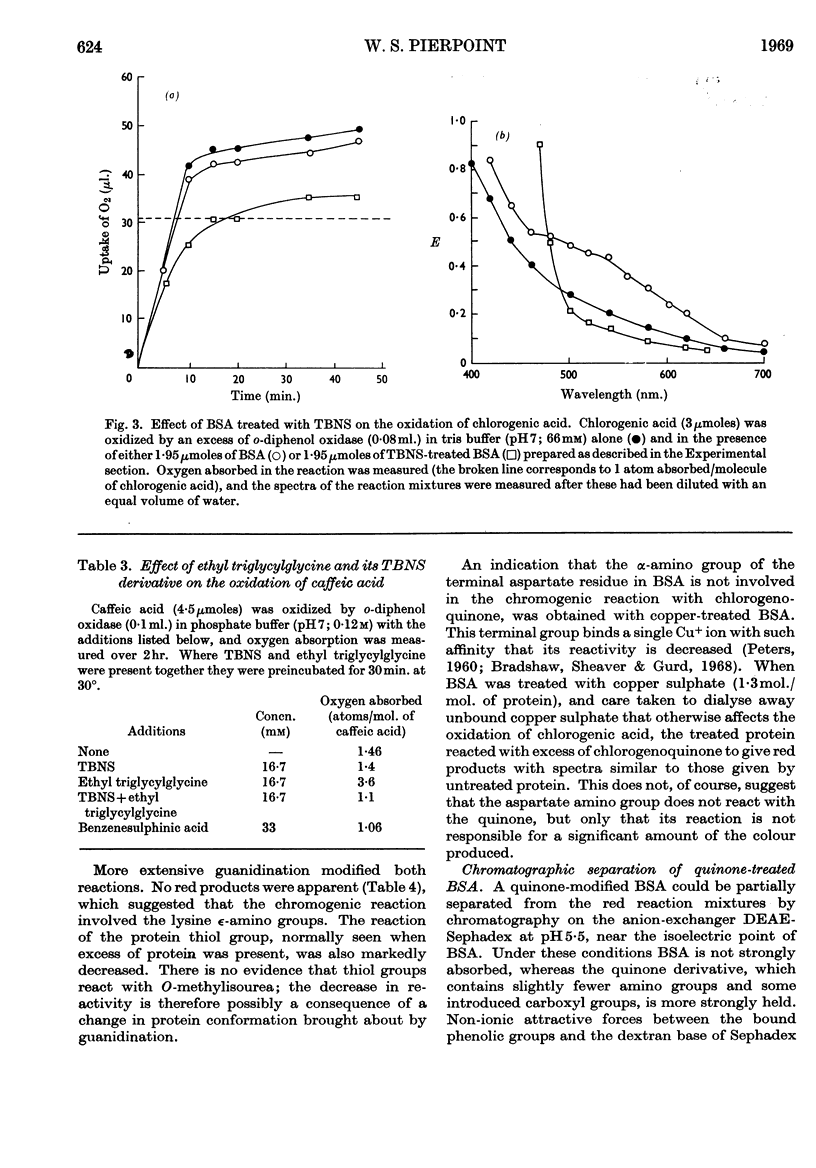
1. The reactions between chlorogenoquinone, the o-quinone formed during the oxidation of chlorogenic acid, and bovine serum albumin depend on the ratio of reactants. 2. When the serum albumin is in excess, oxygen is not absorbed and the products are colourless. This reaction probably involves the thiol group of bovine serum albumin; it does not occur with bovine serum albumin which has been treated with p-chloromercuribenzoate, iodoacetamide or Ellman's reagent. 3. When bovine serum albumin reacts with excess of chlorogenoquinone, oxygen is absorbed and the products are red. The red colour is probably formed by reaction of the lysine ∈-amino groups of bovine serum albumin, as it is prevented by treating the protein with formaldehyde, succinic anhydride or O-methylisourea. 4. Bovine serum albumin modified by a 1·5-fold (BSA-Q) and a fivefold (BSA-Q2) excess of chlorogenoquinone were separated by chromatography on DEAE-Sephadex A-50, and some of their properties observed. 5. Reaction of BSA-Q2 with fluorodinitrobenzene suggests that the terminal α-amino group, as well as lysine ∈-amino groups, are combined with chlorogenoquinone.
Full text
PDF

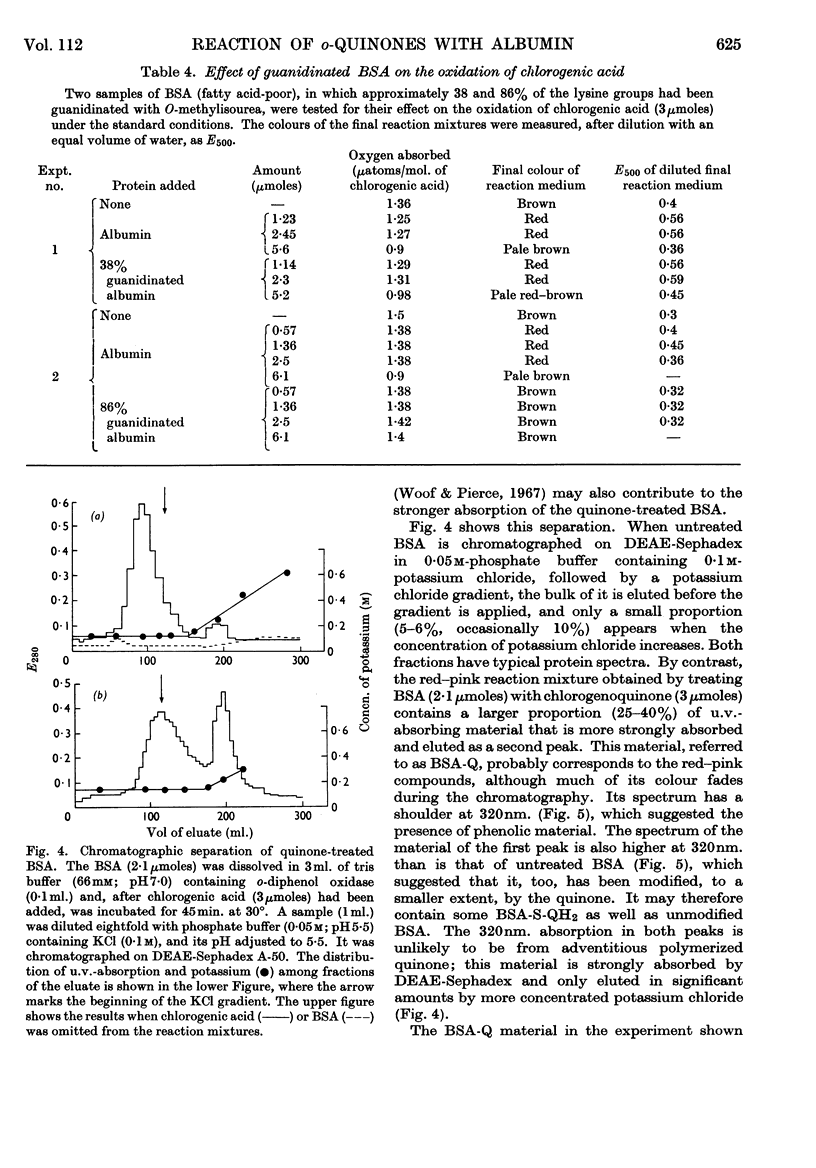
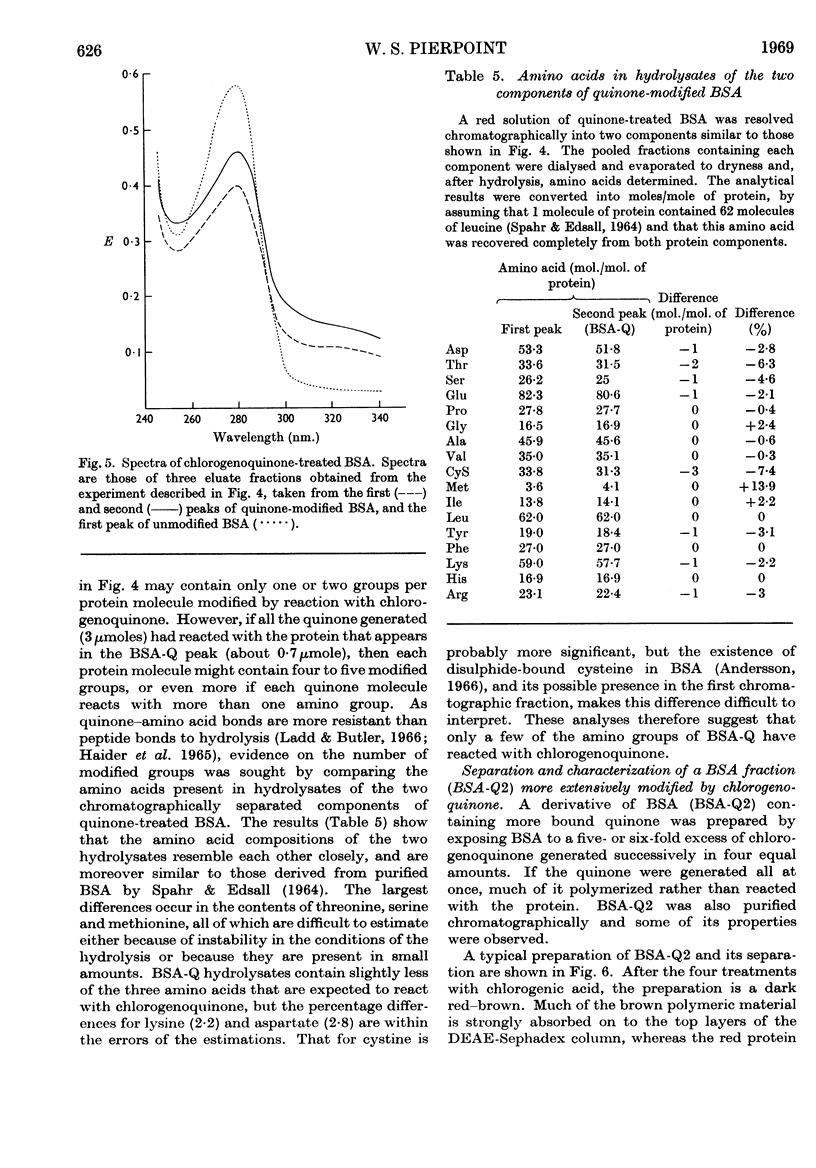
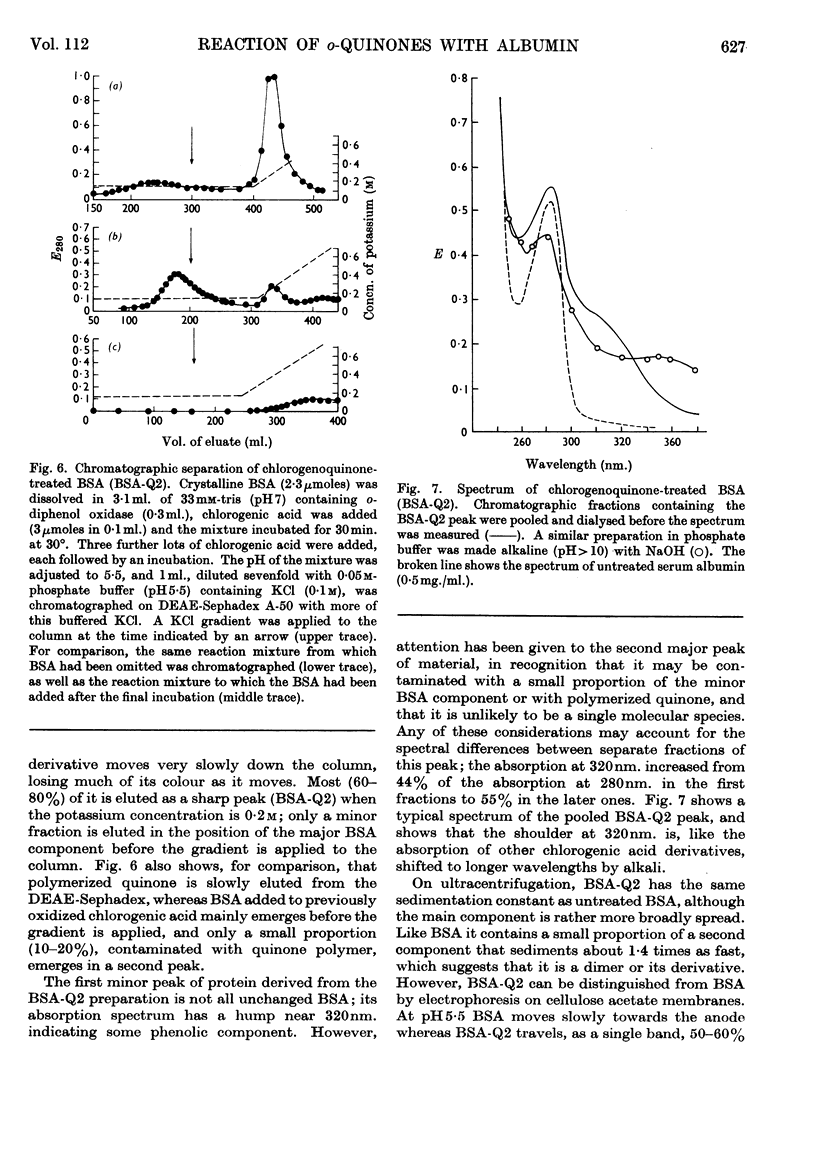
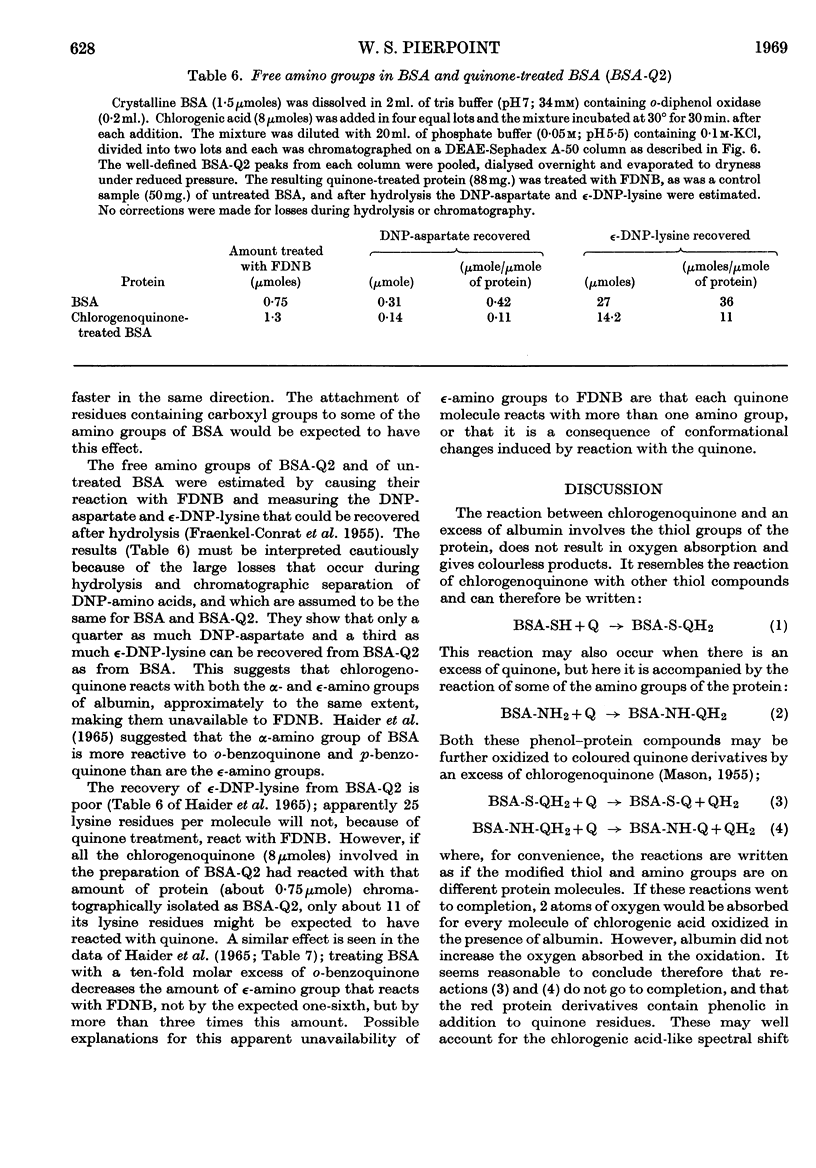

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. O. The heterogeneity of bovine serum albumin. Biochim Biophys Acta. 1966 Mar 28;117(1):115–133. doi: 10.1016/0304-4165(66)90159-0. [DOI] [PubMed] [Google Scholar]
- Bradshaw R. A., Shearer W. T., Gurd F. R. Sites of binding of copper (II) ion by peptide (1-24) of bovine serum albumin. J Biol Chem. 1968 Jul 25;243(14):3817–3825. [PubMed] [Google Scholar]
- CHERVENKA C. H., WILCOX P. E. Chemical derivatives of chymotrypsinogen. II. Reaction with O-methylisourea. J Biol Chem. 1956 Oct;222(2):635–647. [PubMed] [Google Scholar]
- Cooper E. A. On the Relations of the Phenols and their Derivatives to Proteins. A contribution to our knowledge of the Mechanism of Disinfection: Part III. The Chemical Action of Quinone upon Proteins. Biochem J. 1913 Mar;7(2):186–196. doi: 10.1042/bj0070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. The reaction of 2,4,6-trinitrobenzenesulphonic acid with amino acids, Peptides and proteins. Biochem J. 1968 Jul;108(3):383–391. doi: 10.1042/bj1080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABEEB A. F., CASSIDY H. G., SINGER S. J. Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochim Biophys Acta. 1958 Sep;29(3):587–593. doi: 10.1016/0006-3002(58)90016-7. [DOI] [PubMed] [Google Scholar]
- Little C., O'Brien P. J. Products of oxidation of a protein thiol group after reaction with various oxidizing agents. Arch Biochem Biophys. 1967 Nov;122(2):406–410. doi: 10.1016/0003-9861(67)90212-3. [DOI] [PubMed] [Google Scholar]
- Mason H. S., Peterson E. W. Melanoproteins. I. Reactions between enzyme-generated quinones and amino acids. Biochim Biophys Acta. 1965 Nov 15;111(1):134–146. doi: 10.1016/0304-4165(65)90479-4. [DOI] [PubMed] [Google Scholar]
- PETERS T., Jr Interaction of one mole of copper with the alpha amino group of bovine serum albumin. Biochim Biophys Acta. 1960 Apr 22;39:546–547. doi: 10.1016/0006-3002(60)90215-8. [DOI] [PubMed] [Google Scholar]
- Pierpoint W. S. The enzymic oxidation of chlorogenic acid and some reactions of the quinone produced. Biochem J. 1966 Feb;98(2):567–580. doi: 10.1042/bj0980567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpoint W. S. o-Quinones formed in plant extracts. Their reactions with amino acids and peptides. Biochem J. 1969 May;112(5):609–616. doi: 10.1042/bj1120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woof J. B., Pierce J. S. Separation of complex mixtures of polyhydroxy phenols on columns of Sephadex. J Chromatogr. 1967 May;28(1):94–103. doi: 10.1016/s0021-9673(01)85933-1. [DOI] [PubMed] [Google Scholar]



